Abstract
We studied the effect of metformin or placebo in a lifestyle modification program (LSM) combined with oral contraceptives (OC) on quality of life parameters measured by the PCOS questionnaire (PCOSQ) in obese adolescent women with validated PCOS.
The quality of life indicators were measured at baseline and conclusion for 5 domains on the PCOSQ, with equal improvement in scores in both placebo and Metformin groups, suggesting metformin addition does not add improvement to quality of life measures above those observed with lifestyle modification and oral contraceptive treatment.
Polycystic Ovarian Syndrome (PCOS) affects approximately 6–8% of adult women.(1) It is one of the most common endocrine disorders that affect women of reproductive age.(2) It is a heterogeneous condition characterized by chronic anovulation and androgen excess (3) or alternatively using the 2003 ASRM/ESHRE criteria including 2 of 3 criteria: 1) irregular menses, 2) clinical and/or biochemical hyperandrogenism, and 3) polycystic ovaries on ultrasound.(4)
Signs and symptoms of the disorder may appear in puberty but may not be diagnosed until well into adulthood.(5) Clinical features of PCOS include irregular menstrual cycles, hirsutism, acne, acanthosis nigricans, obesity and infertility. Any of these components could have a significant impact on an adolescent female’s quality of life given that this population is at the height of identity development and awareness of body image.(6)
Trent et. al. has previously shown that adolescents with PCOS experience a lower quality of life compared to healthy adolescents.(7) They also showed, in PCOS adolescents, BMI is a primary mediator of health-related-quality-of-life (HRQL).(8) In a Bulgarian study of 100 adult women with PCOS it was demonstrated that the greatest negative influences on quality of life were linked to hirsutism (especially under the age of 25 years old), being overweight, and infertility concerns (over age 25).(9)
Short-term treatments such as OCs are used to reduce serum androgens and improve menstrual cycles as well as acne in women with PCOS. In 2006, Hahn et al. showed that HRQL and emotional well-being improved after metformin therapy in adult women.(10)
The exact mechanism of how metformin improves HRQL is not exactly known. Possible theories include the fact that metformin has led to more regular menstrual cycles, less acne and improved weight loss in women with PCOS however a placebo effect cannot be ruled out.
In 1998, Cronin et. al. reported a health-related quality of life instrument specific to the diagnosis of PCOS (PCOSQ.(11) In 2004, the questionnaire was validated for use as a reliable instrument for measuring the health-related-quality-of-life (HRQL) in women with PCOS.(12)
To date, there have been no published data on PCOS adolescent females with respect to effects on psychological or emotional well-being after therapeutic interventions. An examination of the effect of treatment on quality of life in this age group could provide additional insight into the appropriate treatment course for this population. We report use of the PCOSQ to investigate quality of life factors for a group of obese adolescent women with PCOS as a secondary analysis during a randomized trial of metformin or placebo, with concurrent lifestyle modification (LSM) and OC treatment.
The study was a prospective, 24 week, randomized, placebo-controlled trial. The full details of the study and the metabolic and hormonal results have been previously published.(13) Subjects were administered the PCOSQ at the beginning and conclusion of the study. Eligible subjects were post-menarchal adolescent females, ages 12–18 years with PCOS by NIH diagnostic criteria as defined by irregular menses, and either clinical or biochemical evidence of hyperandrogenism, at least 20% above their ideal body weight (BMI>95 percentile). Subjects were recruited by direct advertisement in the community, or by referral from local physicians. All subjects were studied at the University of Rochester Medical Center in the Division of Reproductive Endocrinology. Both studies were approved by the internal Institutional Review Board (Research Subjects Review Board) at the University of Rochester and all subjects provided signed consent if they were 18 years of age or older or gave assent with signed consent by parent or legal guardian.
A total of 36 subjects were randomized to receive one of two treatments for 6 months: Oral Contraceptive (OC-- ethinyl estradiol + drospirenone) (Yasmin, Bayer Scherling Pharma, Berlin, Germany) with metformin (MET) (2 g/day) versus OC with placebo (PL). All subjects enrolled in the LSM. All were studied randomly at baseline without regard to time in menstrual cycle, off any treatment for at least 2 months. Subjects were otherwise healthy and all were non-smokers. Subjects were assigned by a random number table to metformin or placebo. Metformin and placebo were provided by the Investigational Drug Pharmacy service at the University of Rochester. Subjects were seen weekly for the LSM with pill counts and menstrual data collected monthly. Metformin or placebo was given divided into 4 doses with a gradual build-up at study start.
The LSM portion of the study consisted of a rolling group admission and support groups. Each study participant enrolled with one adult family member (parent or guardian). Weekly discussions were held on topics related to nutrition and lifestyle changes. Discussions about nutrition were led by a registered dietitian. Discussion on other components of lifestyle change were led by a licensed psychologist and included topics such as goal setting, motivation for making change, problem-solving, and stress management. This core curriculum was taught to parents and adolescents together during the 24 week study period, interspersed with individual meetings as needed to discuss concerns that may have arisen.
The second portion of the lifestyle classes was structured group exercise, which occurred weekly for 60 minutes following the support group. Goals of therapy were based on an initial caloric intake by dietary record aiming for a 500kcal/day deficit. Outside of the program, participants were encouraged to exercise for 30 minutes per day of moderate-to-intense physical activity. Compliance with an exercise regimen was monitored weekly by actigraph activity monitors.
The questionnaire was administered and supervised by a single investigator (L.K.) at study onset and conclusion. The main outcome measure was response to the PCOSQ at enrollment versus conclusion for five domains: Emotions, Weight, Menses, Body Hair, Infertility. Data were analyzed by SPSS statistical software. The changes in mean scores were compared between groups by t-test analyses. Since the groups did not vary by randomization, they were combined for correlation analysis.
The ethnicity of the subjects consisted of 75% Caucasian, 16% African American, 6% Hispanic and 3% Asian. A total of four participants did not complete the trial; two from the metformin group and two from the placebo group. One subject in each of the groups stopped metformin or placebo due to gastrointestinal complaints but each continued OC and the lifestyle program. Additionally, two subjects reduced the number of pills they took regularly in the metformin group, but took at least 1000mg/day. The majority of subjects (94%) completing the trial attended at least 75% of the lifestyle sessions. The remainder completed greater than 50%.
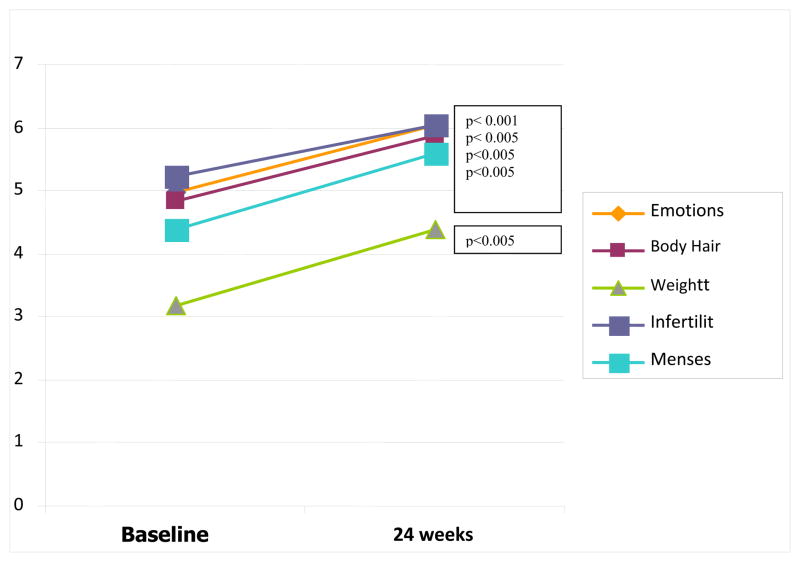
Mean BMI was 34.8 kg/m2 at baseline and BMI decreased 4% in PL (p=0.008) and 5.2% (p=0.001) in MET with no difference in weight reduction between groups. Changes in PCOSQ for both groups without regard to randomization are shown in Figure 1. Patients rated symptoms on a scale of 1 to 7, in accordance with the PCOSQ with 1 being worst (symptoms all of the time) and 7 best (symptoms none of the time). PCOSQ total scores significantly improved in both groups, across all domains, between baseline and conclusion. There was no significant difference between those treated with metformin as compared to placebo.
Figure 1. PCOSQ Scores at baseline versus conclusion for all domains (combined data presented).
Quality of Life Scores improved for all study participants regardless of randomization from study inception to completion for all variables measured on the PCOSQ: Emotions, Body Hair, Weight, Infertility and Menstruation.
There was a trend toward correlation between the reduction in BMI at study conclusion and increase in PCOSQ score at conclusion for the weight domain (r= −0.333, p= 0.06). There was a significant correlation between Ferriman-Gallway (FG) score and participants’ subjective view of hirsutism by the PCOSQ domain for hirsutism at baseline (r=−0.585, p= < 0.001).
The current study demonstrates improvement in quality of life across all variables measured for obese adolescent women enrolled in a lifestyle program with oral contraceptives. The addition of metformin offers no advantage over placebo when combined with LSM and OC. This suggests that the initiation of a treatment program including a lifestyle intervention program improves overall well-being in adolescents with PCOS, however this cannot be definitively proven by this study due to the study design.
Our study did not find a correlation between initial PCOSQ scores and success in weight loss using a lifestyle modification program, suggesting adolescent women can respond to modest LSM regardless of baseline PCOSQ parameters. This would suggest that improvements in overall quality of life can be made using lifestyle interventions and OCs, with or without metformin in combination, in obese adolescent women with PCOS irrespective of body image at the start of the intervention. There was no control group for the LSM or the OC therefore these treatments cannot be independently studied in this trial. Given, however, that there was no significant difference in PCOSQ scores between metformin and placebo groups, it does not appear that metformin treatment improves PCOSQ scores.
The trend toward correlation between improved PCOSQ and change in BMI suggests an association between improvements in weight and quality of life specifically with regard to weight in this study population. This is consistent with findings of other investigators (14, 15, 16) who found that women with PCOS seem to be most distressed about their body weight. Given the current rising prevalence of obesity in many pediatric and adolescent populations, (17) these results have implications for other populations where weight and self-esteem can be linked.
Finally, the findings of this study demonstrate that Ferriman-Gallwey scores measuring level of hirsutism in adolescents with PCOS correlate with how subjects view themselves in terms of amount of facial and body hair present. This is a validation for the current scoring measure used clinically, and may represent a good marker for impact of changes in hirsutism with treatment.
This is a small study without a true placebo group for the lifestyle program or oral contraceptive. Thus it is difficult to discern if the improvement in scores across all variables on the PCOSQ instrument is secondary to the lifestyle change program itself or to study participation and social support. Additionally, the change in PCOSQ may be secondary to repeated questionnaire administration and completion rather than a true benefit of taking part in the study.
Our findings differ from others who have looked at metformin and QOL in PCOS. (18) This could be secondary to the limited number of participants in the study, or to the age of the participants. The PCOSQ instrument itself has been validated only in adult populations. It is possible that this particular instrument lacks a domain for acne which is a significant source of distress for many adolescents and has been linked to quality of life in other populations. (19,20)
Additional areas for investigation include re-creating and re-validating the PCOSQ instrument for the adolescent population as well as conducting a study with single treatment arms to assess the independent impacts of the lifestyle intervention, metformin and the use of OCs in obese adolescent women with PCOS. It is vital that we consider quality of life measures as a significant outcome measure for future investigations in PCOS. Such data may inform investigators of possible baseline predictors of success for using a lifestyle modification program in PCOS. This may enable tailored treatment options based on the individual’s perception of her disease.
Acknowledgments
We thank Ken Edell for his assistance with data analysis.
metformin is reported for use with an off label indication, IND numbers #74, 167 and # 64,239.
Support: NIH grant K23 HD043881-01A1 (KMH) and Grant Number UL1 RR 024160 from the National Center for Research Resources (NCRR) to the University of Rochester, Rochester, NY 14620
Footnotes
The authors report no conflicts of interest.
The abstract for this article was presented at the annual American Society of Reproductive Medicine (ASRM) meeting in San Francisco, CA on 10/17/08.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmina EM, Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertil Steril. 2006;86(1):S7–S8. doi: 10.1016/j.fertnstert.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Medical progress: polycystic ovary syndrome. NEJM. 1995;333(13):853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Trivax BM, Azziz R. Diagnosis of polycystic ovary syndrome. Clinical Obstetrics and Gynecology. 2007;50(1):168–177. doi: 10.1097/GRF.0b013e31802f351b. [DOI] [PubMed] [Google Scholar]
- 4.Carmina EM, Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertil Steril. 2006;86(1):S7–S8. doi: 10.1016/j.fertnstert.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Yen SSC. The polycystic ovary syndrome. Clin Endocrinol (Oxf) 1980;12:177–208. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Trent M, Rich M, Austin BS, Gordon CM. Quality of life in adolescent girls with polycystic ovary syndrome. AMA. 2002;156(6):556–560. doi: 10.1001/archpedi.156.6.556. [DOI] [PubMed] [Google Scholar]
- 7.Trent ME, Austin BS, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambulatory Pediatrics. 2005;5(2):107–111. doi: 10.1367/A04-130R.1. [DOI] [PubMed] [Google Scholar]
- 8.Trent ME, Rich M, Austin BS, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome implications for quality of life. J Ped and Adolesc Gyn. 2003;16(1):33–37. doi: 10.1016/s1083-3188(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 9.Pekhlivanov B, Kolarov G, Kavurdzhikova S, Stoikov S. Determininants of health related quality of life in women with polycystic ovary syndrome. Akusherstvo I Ginkekologiia. 2006;45(7):29–34. [PubMed] [Google Scholar]
- 10.Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, et al. Metformin treatment of polycystic ovary syndrome improves health-related quality of life, emotional distress and sexuality. Hum Reprod. 2006;10:1093. doi: 10.1093/humrep/del069. [DOI] [PubMed] [Google Scholar]
- 11.Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS) J Clin Endo & Met. 1998;83(6):1976–1987. doi: 10.1210/jcem.83.6.4990. [DOI] [PubMed] [Google Scholar]
- 12.Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ, Mulgrew NC, Shepherd KE, Wilkinson VH, Singh M, Balen A, Lashen H, Ledger WL. The polycystic ovary syndrome health related quality of life questionnaire (PCOSQ): a validation. Hum Reprod. 2004;9(2):371–377. doi: 10.1093/humrep/deh048. [DOI] [PubMed] [Google Scholar]
- 13.Hoeger KM. Obesity and lifestyle management in polycystic ovary syndrome. Clinical Obstetrics and Gynecology Clin Obst & Gyn. 2007;50(1):277–294. doi: 10.1097/GRF.0b013e31802f54c8. [DOI] [PubMed] [Google Scholar]
- 14.Trent ME, Austin BS, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Amb Ped. 2005;5(2):107–111. doi: 10.1367/A04-130R.1. [DOI] [PubMed] [Google Scholar]
- 15.Trent ME, Rich M, Austin BS, Gordon CM. Quality of life in adolescent girls with polycystic ovary syndrome. AMA. 2002;156(6):556–560. doi: 10.1001/archpedi.156.6.556. [DOI] [PubMed] [Google Scholar]
- 16.Trent ME, Upadhya K. Effects of polycystic ovary syndrome on health-related quality of life. 2008 doi: 10.1586/14737167.7.6.597. www.cdc.gov/hrqol/index.com Expert Review Pharmacoeconomics Res. [DOI] [PubMed]
- 17.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of Overweight and Obesity Among US Children, Adolescents, and Adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 18.Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, et al. Metformin treatment of polycystic ovary syndrome improves health-related quality of life, emotional distress and sexuality. Hum Reprod. 2006;10:1093. doi: 10.1093/humrep/del069. [DOI] [PubMed] [Google Scholar]
- 19.Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ, et al. The polycystic ovary syndrome health related quality of life questionnaire (PCOSQ): a validation. Hum Reprod. 2004;9(2):371–377. doi: 10.1093/humrep/deh048. [DOI] [PubMed] [Google Scholar]
- 20.Janssen OE, Hahn S, Tan S, Benson S, Elsenbruch S. Mood and Sexual Function in Polycystic Ovary Syndrome: Acne. Semin Reprod Med. 2008;26(1):45–52. doi: 10.1055/s-2007-992924. [DOI] [PubMed] [Google Scholar]