Abstract
Purpose of review
Racial disparities appear to exist in the susceptibility and severity of systemic sclerosis (SSc, scleroderma) and are responsible for a greater health burden in blacks as compared to whites. Disparities in socioeconomic status and access to health care do not sufficiently explain the observed differences in prevalence and mortality. It is important to determine if there might be a biologic basis for the racial disparities observed in SSc.
Recent findings
We present data to suggest that the increased susceptibility and severity of SSc in blacks may result in part from an imbalance of pro-fibrotic and anti-fibrotic factors. Racial differences in the expression of transforming growth factor-β1 (TGF-β1) and caveolin-1, as well as differences in the expression of hepatocyte growth factor (HGF) and PPAR-γ have been demonstrated in blacks with SSc, as well as in normal black subjects. A genetic predisposition to fibrosis may account for much of the racial disparities between black and white patients with SSc.
Summary
A better understanding of the biologic basis for the racial disparities observed in SSc may lead to improved therapies, along with the recognition that different therapies may need to be adapted for different groups of patients.
Keywords: Systemic Sclerosis, Health Disparities, TGF-β, Caveolin-1, HGF
Introduction
There is considerable evidence that the health burden of systemic sclerosis (SSc, scleroderma) is greater among blacks than whites. In the United States, blacks are more likely to develop SSc than whites and to experience greater morbidity and reduced survival [1-7]. Age of onset is earlier and the more severe diffuse cutaneous form of SSc (dcSSc) is more frequent in blacks than in whites [1,5,8]. The incidence of dcSSc among blacks is approximately 2.5 fold higher than for whites (20 cases per million per year versus 8 cases per million per year) [1]. The most recent study of SSc mortality in the United States reported death rates that peaked a decade earlier in the black population when compared with those in the white population, with age-adjusted mortality significantly higher for blacks than for whites (7.1 vs. 4.4 deaths per million population per year) [7]. For those hospitalized when under the age of 65 years, black patients are more likely to experience in-hospital death than whites (<35 yrs: 4.2 vs. 1.2%; 35-49 yrs: 4.7% vs. 2.7%; 50-64 yrs: 6.9 vs. 6.8%) [9]. Blacks are also more likely to develop severe interstitial lung disease (ILD) or pulmonary hypertension (PH), two pulmonary complications that together account for more than 50% of all SSc-related deaths [8,10,11].
The basis for the racial disparities in prevalence and outcomes remains uncertain and is not fully explained by differences in socioeconomic status or access to health care [9, 12. In this review we present results of recent studies suggesting there may be an underlying genetic predisposition that provides a biological basis for the racial disparities observed in SSc patients.
Serum Auto-Antibody Profiles and Racial Differences
Serum auto-antibodies are strongly associated with specific SSc disease manifestations, and the frequency of these various serum auto-antibodies differs among blacks and whites. Topoisomerase I and U3 RNP auto-antibodies, which tend to be associated with dcSSc and severe ILD, are seen more commonly in black patients than in white patients [4,13-15]. In contrast anti-centromere antibodies, which tend not to be associated with dcSSc nor with severe ILD, are seen with much lower frequency in black patients [16].
Such observations might suggest an immunogenetic and serological basis for the racial disparities observed among SSc patients. However, a recent study by Steen and colleagues suggests that auto-antibody status per se is not a sufficient explanation for the observed racial disparities [17]. Steen and colleagues analyzed consecutive black (n=203) and white (n=2945) SSc patients seen between 1972 and 2007, as part of the Pittsburgh Scleroderma Database. Consistent with prior studies, black patients were found to have certain auto-antibodies with significantly greater frequency than white patients (anti-topoisomerase I: 25% vs. 18%; anti-U1 RNP: 13% vs. 4%; anti-U3 RNP: 16% vs. 2%). On the other hand, certain auto-antibodies were detected with significantly less frequency in blacks than in whites (anti-centromere: 7% vs. 22%, anti-RNA polymerase III: 10% vs. 21%).
Notably, when black and white SSc patients within a specific auto-antibody sub-group were compared, racial differences were found to persist. For example, among SSc patients with anti-topoisomerase I antibodies, blacks tended to be younger (mean age 37 yr vs. 43 yr, p=0.005) and had pulmonary fibrosis with greater frequency (72% vs. 52%, p=0.01) and with greater severity (44% vs. 18%, p=0.001) than whites, despite there being no significant difference in disease duration at presentation. After adjustment for age, gender and disease subset, blacks with anti-topoisomerase I antibodies were 75% more likely to die within five years than whites having the same auto-antibody (hazard ratio 1.75, 95% CI 1.06-2.88, p=0.03). This important study suggests that other genetic and/or environmental influences might explain the disparities observed among black and white SSc patients. In the following sections, we will examine potential biologic pathways that may underlie racial disparities observed in SSc.
Racial Differences in TGF-β Expression
Transforming growth factor-β1 (TGF-β1) is a member of a superfamily of proteins regulating cell growth and differentiation, inflammation and immunity [18]. TGF-β1 is considered to be the master regulator of physiologic fibrogenesis and pathological fibrosis [19]. In both normal and SSc fibroblasts, TGF-β1 induces Smad2/3 activation and collagen gene transcription. In SSc dermal fibroblasts, the expression of receptors for TGF-β1 is elevated [20], and multiple abnormalities in downstream Smad signaling pathways have been identified [19].
TGF-β1 undoubtedly plays an important role in the pathogenesis of SSc. Racial differences in the expression of TGF-β1 are well documented [21], and one might speculate that differences in TGF-β1 could contribute to the greater disease susceptibility and severity observed in black SSc patients. Blacks are known to have an increased prevalence of other diseases, e.g., hypertension, focal glomerulosclerosis, diabetic nephropathy, end stage renal disease, keloids, uterine leiomyomata, sarcoidosis and glaucoma, in which TGF-β has been implicated [22-25]. For example, in blacks hypertension is one and one-half times more common and end stage renal disease is eight times more common than in whites [26]. Blacks with hypertension over-express TGF-β1, and genotyping has revealed a single-nucleotide polymorphism (SNP) at codon 10 occurring with higher frequency in blacks than in whites that is associated with higher levels of TGF-β mRNA and protein expression [27]. The same investigators also showed that peripheral blood TGF-β1 protein levels are high in blacks compared with whites, and the levels are associated with plasma renin activity, systolic and diastolic blood pressure, metabolic syndrome and microalbuminuria in blacks, but not in whites [28].
Racial Differences in Other Fibrogenic Cytokines
Interleukin-6 (IL-6) is a pro-fibrotic cytokine implicated in the pathogenesis of SSc. Although its precise role in SSc is not clear, IL-6 is over-expressed by SSc dermal fibroblasts [29,30]. IL-6 serum concentrations are increased and have been correlated with skin thickness [31,32]. Blockade of the IL-6 receptor with the monoclonal antibody tocilizumab, has been reported recently to be associated with improvement in skin score along with histological thinning of dermal collagen bundles [33].
Variants in cytokine genes, including IL-6, have shown association with SSc. Given the association with IL-6, it is interesting that normal black recipients of renal transplants have been shown to have a significantly higher probability of a particular IL-6 polymorphism (-174 G to C allele), which was shown to be associated with greater synthesis of IL-6 protein [34]. The authors suggest that differences in IL-6 production as a result of this polymorphism in the IL-6 gene may be an important factor in the racial variation in renal transplant outcomes [34].
Other cytokines and cytokine modulators with potential relevance to SSc also show racial variations. When analyzing cytokine expression in bronchoalveolar lavage fluid, we observed differences in the expression between blacks and whites for the following cytokines and cytokine modulators: hepatocyte growth factor (HGF)(vide infra), osteoprotegerin (OPG), thrombopoietin (TPO), insulin-like growth factor binding protein 3 (IGFBP-3), stem cell factor (SCF) and vascular endothelial growth factor (VEGF)[35]. Differences between blacks and whites were observed not only in SSc patients, but also in healthy control subjects (see Figure 1). Among the cytokines of potential relevance to SSc and interstitial lung disease, IGFBP-3 was over-expressed in blacks. Recently it was shown that IGFBP-3 production by lung epithelial cells mediates TGF-β-induced tenascin C production [36].
Figure 1. Cytokine array analysis of bronchoalveolar lavage (BAL) fluid from Caucasian and African American patients with systemic sclerosis (SSc) and normal controls.
Positive controls are at positions A1, B1, C1, D1, J8, and K8. Negative controls are at positions E1, F1, and I8. Arrow indicates the postion of hepatocyte growth factor (I6). Insulin-like growth factor binding protein-3, osteoprotegerin, stem cell factor, thrombopoietin, and vascular growth factor are located at positions A7, B8, C4, B5, and C5, respectively. Note that overall BAL fluid samples from Caucasian subjects contains less of the cytokines than do the BAL fluid samples from African American subjects. Reprinted with permission from Arthritis Rheum 2007;56:2432-42 (reference 35).
It remains to be determined if racial variations in IL-6, IGFBP-3 or other cytokines play a role in the susceptibility or severity of SSc. One Italian study found no association between 22 cytokine SNPs and SSc susceptibility or severity, however the numbers of patients and controls were modest [37]. Using a multifactor dimensionality reduction model, the authors concluded there was evidence for gene-gene interaction among cytokine single-nucleotide polymorphisms, including the pro-fibrotic cytokine IL-6, as well as genes for other regulatory cytokines [37].
Racial Differences in Caveolin Expression
Our group and others have shown that a reduced level of caveolin-1 in lung fibroblasts from patients with SSc and from bleomycin-treated mice promotes collagen over-expression and lung fibrosis [38-40]. Caveolin-1 is a protein associated with plasma membrane invaginations known as caveolae and with other cellular membranes. Caveolin-1 binds to and thereby inhibits the function of kinases in several major families including PKC, MAPK, Src, and G protein [41-44]. Of note, caveolin-1 regulates signaling and cell functions induced by the major pro-fibrotic cytokine, TGF-β [45-47]. There are multiple points of intersection between TGF-β and caveolin-1 signaling. For example, TGF-β inhibits caveolin-1 expression in a variety of cell types including fibroblasts (both lung and dermal) and monocytes, suggesting that the TGF-β-rich milieu in the blood and tissues of SSc patients leads to the reduction of caveolin-1 expression and to an altered behavior of cells that promote fibrosis. Caveolin-1 also modulates TGF-β signaling in dermal fibroblasts by inhibiting Smad3 phosphorylation and its translocation to the nucleus [48-50] . Additionally, caveolin-1 regulates TGF-β signaling via its effects on the endocytosis of TGF-β ligand-receptor complexes. TGF-β receptors sort to both caveolin-1-rich lipid rafts and early endosomes [50-52]. Early endosomal internalization increases TGF-β signaling while caveolin-1-dependent internalization in lipid rafts leads to receptor degradation, thereby inhibiting TGF-β signaling [53].
We recently demonstrated that deficiency of caveolin-1 exists not only in SSc lung fibroblasts but also in other cell types, e.g., monocytes and neutrophils, from patients with SSc [54]. Furthermore, with decreased expression of caveolin-1 there was activation of ERK, JNK and p38 [54]. To evaluate the potential role of caveolin-1 in the predisposition of blacks to SSc, we compared caveolin-1 levels in monocytes from SSc patients and normal subjects. We found caveolin-1 expression in normal black subjects to be only 40% of that in normal white subjects, i.e., caveolin-1 in normal black subjects was almost as low as in monocytes from SSc patients (see Figure 2).
Figure 2. Low caveolin-1 expression in healthy African American and SSc monocytes.
Caveolin-1 levels in monocytes from healthy Caucasians, healthy African Americans (AA), and SSc patients (SSc) were quantified by Western blotting followed by densitometry using GAPDH as a loading control. The level in healthy Caucasians was set to 100 arbitrary units. The data shown represent the average ± s.e.m. from 7 healthy Caucasians, 7 healthy AAs, and 7 SSc patients. Compared to Caucasian, p < 0.01 for both AA and SSc.
We also recently demonstrated that the low level of caveolin-1 expression in SSc peripheral blood monocytes (PBM) is associated with enhanced migratory capacity toward CXCL12 (stromal-cell derived factor 1), a chemokine ligand that is highly upregulated in SSc lung tissue [55]. Furthermore, the SSc PBM phenotype characterized by a low level of caveolin-1 and enhanced migratory behavior could be induced in normal monocytes by treatment with TGF-β [55]. Given the causal relationship between low caveolin-1 and enhanced migration toward CXCL12 in SSc monocytes, together with the low caveolin-1 levels in monocytes from normal black subjects, we evaluated the ability of monocytes from normal black subjects to migrate toward CXCL12 (Figure 3). As predicted from the reduced expression of caveolin-1, migration of monocytes from normal blacks toward CXCL12 was enhanced (> 2.5 fold compared to monocytes from normal whites). Altogether, these data suggest that blacks may be predisposed to SSc and interstitial lung disease due to reduced expression of caveolin-1 which may, in turn, arise from over-expression of TGF-β (vide supra).
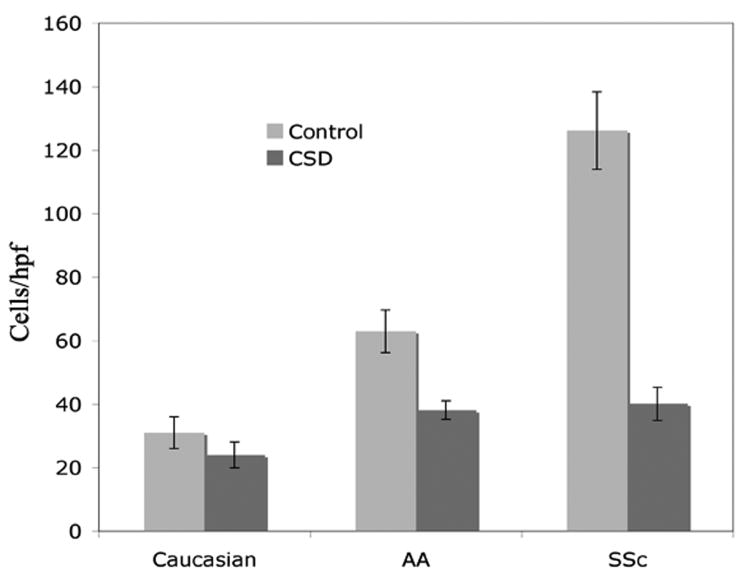
Figure 3. Hypermigration toward CXCL12 by healthy AA and SSc monocytes.
Monocyte migration was quantified using microchemotaxis chambers (Neuroprobe) with 100 ng/ml CXCL12 in the lower wells. Human monocytes (50 μl at 1 × 106 cells/ml) were placed in the upper wells in medium supplemented with 5 μM CSD or control peptide. After 3 h at 37°, the filters separating the upper and lower wells were removed, fixed, and stained with DAPI. Cells that had migrated to the lower side of the filter were counted in six high power fields per filter. The data presented are the average ± s.e.m. of experiments performed using cells from 10 healthy Caucasians, 10 healthy AA, and 10 SSc patients. Compared to Caucasian, in the absence of CSD p < 0.0001 for both AA and SSc.
Racial Differences in Expression and Function of HGF, c-MET and PPAR-γ
In addition to racial differences in TGF-β and downstream effects predisposing to fibrosis, there are racial differences in the expression of HGF, a cytokine that inhibits fibrosis. HGF is a multifunctional cytokine involved in regeneration after tissue injury that has been shown to attenuate fibrosis in a bleomycin-induced model of lung injury [56]. HGF has been shown to inhibit apoptosis in lung epithelial cells while at the same time promoting apoptotic events in lung myofibroblasts, thereby inhibiting the progression of lung fibrosis. Recent work by Hoshino et al. suggests that a SNP in the HGF promoter region may modulate the severity of interstitial lung disease by controlling the transcriptional efficiency of the HGF gene [57].
As noted above we observed racial differences in the expression of HGF, among other cytokines, in bronchoalveolar lavage fluid from both normal subjects and SSc patients [35] (Figure 1). Black SSc patients do not appear to demonstrate the same increase in HGF concentrations in bronchoalveolar lavage fluid, plasma and fibroblast culture medium as do white SSc patients [35]. We also observed that when HGF is applied to cells in vitro the anti-fibrotic effects of HGF are significantly reduced in fibroblasts from blacks [35]. Our results were consistent across different cell lines of SSc lung fibroblasts and in normal lung fibroblasts stimulated with TGF-β. Whereas HGF consistently inhibited collagen type I and connective tissue growth factor (CTGF) accumulation in lung fibroblasts from white subjects, HGF did not reduce collagen or CTGF expression in lung fibroblasts from blacks.
The biologic effects of HGF are mediated by a membrane-spanning tyrosine kinase receptor encoded by the c-Met proto-oncogene. Following binding of HGF, the c-Met receptor undergoes auto-phosphorylation at tyrosine residues in its cytoplasmic domain and initiates a cascade of signal transduction events leading to the down-regulation of collagen and CTGF expression [58]. We found these anti-fibrotic effects of HGF to be significantly reduced in lung fibroblasts from blacks, an effect that appears to be due to an impairment of c-Met receptor phosphorylation [35]. We are testing the hypothesis that a particular variant of the c-MET gene, which may be more frequent in blacks, modulates disease severity in SSc patients.
HGF expression is induced by peroxisome proliferator-activated receptor-gamma (PPAR-γ). Wei et al. recently reported that PPAR-γ is diminished in skin and lung tissues from patients with SSc, as well as in fibroblasts explanted from lesional skin [59]. These investigators postulated that excessive TGF-β activity in SSc accounts for impaired PPAR-γ function which, in turn, contributes to unchecked fibroblast activation and progressive fibrosis [59]. Our recent studies support this observation and also suggest there may be racial differences in PPAR-γ expression, with reduced expression of PPAR-γ in lung fibroblasts from black SSc patients as compared with white SSc patients [60].
Recently, Li et al. demonstrated that PPAR-γ binds to the peroxisome proliferator response element in the HGF promoter region of renal mesangial cells, thereby inducing HGF mRNA expression and protein secretion [61]. We have demonstrated that treatment with a PPAR-γ agonist, rosiglitazone, results in increased HGF protein and phosphorylation of the c-Met receptor tyrosine kinase in lung fibroblasts from white SSc patients, which was accompanied by an increase in expression of MMP-1 and a decrease in expression of collagen type I [60]. Treatment of fibroblasts from black SSc patients with rosiglitazone, however, did not affect c-Met phosphorylation [60]. Blocking the c-Met receptor with neutralizing anti-c-Met antibody abolished the effects of rosiglitazone on collagen in lung fibroblasts derived from white SSc patients, while augmenting the expression of the c-Met receptor in lung fibroblasts from black SSc patients restored the anti-fibrotic effects of rosiglitazone [60].
Conclusion
For many years it has been recognized that SSc occurs more frequently in blacks than in whites, and that blacks with SSc tend to have earlier disease onset and worse outcomes than whites. These racial disparities cannot be explained completely by socioeconomic factors, access to healthcare, or by differences in auto-antibody status. Recent studies support the notion that blacks may have a genetic predisposition to diseases that are marked by fibrosis, including SSc. We provide evidence to suggest that the biological basis predisposing to fibrosis may involve both an over-expression of pro-fibrotic factors, e.g., TGF-β and IL-6, and an under-expression of anti-fibrotic factors, e.g., caveolin-1, HGF and PPAR-γ. These differences in gene expression between whites and blacks are likely to be due, at least partially, to genetic differences between these ethnicities. A large whole exome sequencing study in black SSc patients planned to begin soon promises to identify the genetic basis for these, as well as other new disease susceptibility and severity genes. In addition to genetic variation, it will also be important to understand the role of epigenetic and environmental risk factors in contributing to disease. A better understanding of the factors underpinning the imbalance of pro-fibrotic and anti-fibrotic factors will hopefully lead to improved therapies for SSc, along with the recognition that different therapies may need to be adapted for different groups of patients.
Key points.
The health burden for systemic sclerosis (SSc, scleroderma) is greater among blacks than whites.
SSc in blacks is characterized by earlier age of onset, higher likelihood of diffuse SSc with severe interstitial lung disease and greater mortality.
Differences in auto-antibody profiles, e.g., increased prevalence of anti-topoisomerase and decreased prevalence of anti-centromere antibodies, do not account for the greater severity of SSc and increased mortality in blacks.
Racial differences in the expression levels of various pro-fibrotic and anti-fibrotic factors might predispose blacks to fibrosing diseases such as SSc.
A genetic predisposition to higher expression levels of TGF-β1 and lower expression levels of caveolin-1, PPAR-γ and HGF in blacks compared with whites may contribute to the racial disparities observed in SSc.
Acknowledgments
The authors gratefully acknowledge Dr. Paula Ramos for review of the manuscript and the following sponsors of our research: Scleroderma Foundation, National Center For Research Resources (UL1RR029882), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant R03 AR056767, NIAMS K01 AR054143, NIAMS P60 AR049459-05, NIAMS K01 AR051052-05, USARMY/USAMRAA grant W81XWH-11-1-0508, National Heart, Lung and Blood Institute grant R01 HL73718, and National Center for Complementary and Alternative Medicine grant R21 AT004450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Funding Support: This work was supported by grants from the Scleroderma Foundation, the National Center For Research Resources (UL1RR029882), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant R03 AR056767, NIAMS K01 AR054143, NIAMS P60 AR049459-05, NIAMS K01 AR051052-05, USARMY/USAMRAA grant W81XWH-11-1-0508, National Heart, Lung and Blood Institute grant R01 HL73718, and National Center for Complementary and Alternative Medicine grant R21 AT004450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 2.Laing TJ, Gillespie BW, Toth MB, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997;40:734–42. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979-1998. Eur J Epidemiol. 2005;20:855–61. doi: 10.1007/s10654-005-2210-5. [DOI] [PubMed] [Google Scholar]
- 4.McNearney TA, Reveille JD, Fischbach M, et al. Arthritis Rheum. 2007;57:318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 5.Nietert PJ, Mitchell HC, Bolster MB, et al. Racial variation in clinical and immunological manifestations of systemic sclerosis. J Rheumatol. 2006;33:263–8. [PubMed] [Google Scholar]
- 6.Greidinger EL, Flaherty KT, White B, et al. African-American race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest. 1998;114:801–7. doi: 10.1378/chest.114.3.801. [DOI] [PubMed] [Google Scholar]
- 7*.Mendoza F, Derk CT. Systemic sclerosis mortality in the United States 1999-2002: Implications for patient care. J Clin Rheumatol. 2007;13:187–92. doi: 10.1097/RHU.0b013e318124a89e. Nashid M, Khanna PP, Furst DE, et al. Gender and ethnicity differences in patients with diffuse systemic sclerosis – analysis from three large randomized clinical trials Rheumatology 2011;50:335-42. The authors retrospectively analyzed data from SSc patients (n=495) enrolled in three separate randomized clinical trials (RCT's) for various gender and ethnic differences. Among the three ethnic groups, Caucasians were older, African-Americans had lower The authors gratefully acknowledge Dr. Paula Ramos for review of the manuscript and the following sponsors of our research: Scleroderma Foundation, National Center For Research Resources (UL1RR029882), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant R03 AR056767, NIAMS K01 AR054143, NIAMS P60 AR049459-05, NIAMS K01 AR051052-05, USARMY/USAMRAA grant W81XWH-11-1-0508, National Heart, Lung and Blood Institute grant R01 HL73718, and National Center for Complementary and Alternative Medicine grant R21 AT004450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. [DOI] [PubMed] [Google Scholar]
- 8.FVC% predicted, and Hispanics had greater tender joint counts. In these three RCT's, gender and ethnicity did not influence the disease course.
- 9.Nietert PJ, Silver RM, Mitchell HC, et al. Demographic and clinical factors associated with in-hospital death among patients with systemic sclerosis. J Rheumatol. 2005;32:1888–92. [PubMed] [Google Scholar]
- 10.Beall AD, Nietert PJ, Taylor MH, et al. Ethnic disparities among patients with pulmonary hypertension associated with systemic sclerosis. J Rheumatol. 2007;34:1277–82. [PubMed] [Google Scholar]
- 11.Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunologic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 12.Nietert PJ, Silver RM. Patterns of hospital admissions and emergency room visits among patients with scleroderma in South Carolina, USA. J Rheumatol. 2003;30:1238–43. [PubMed] [Google Scholar]
- 13.Steen VD, Powell DT, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;32:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 14.Reveille JD, Durban E, Goldstein R, et al. Racial differences in the frequencies of scleroderma related autoantibodies. Arthritis Rheum. 1992;35:216–8. doi: 10.1002/art.1780350215. [DOI] [PubMed] [Google Scholar]
- 15.Kuwana M, Kaburaki J, Arnett FC, et al. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999;42:465–74. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Steen VD, Ziegler GL, Rodnan GP, Medsger TA., Jr Clinical an laboratory associations of anticentromere antibody in patients with progressive systemic sclerosis. Arthritis Rheum. 1984;27:125–31. doi: 10.1002/art.1780270202. [DOI] [PubMed] [Google Scholar]
- 17**.Steen VD, Domsic RT, Lucas M, et al. A clinical and serologic comparison of African-American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012 doi: 10.1002/art.34482. in press. This is the largest comparative study of SSc clinical and serological features in black and white patients. Blacks were shown to have greater disease severity, especially interstitial lung disease, compared with whites, which persisted even when adjusted for auto-antibody status (anti-topoisomerase and anti-U1RNP) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Eng J Med. 2000;342:1350–58. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 19*.Wei J, Bhattacharyya S, Tourtellotte WG, Varga J. Fibrosis in systemic sclerosis: Emerging concepts and implications for targeted therapy. Autoimm Rev. 2011:267–75. doi: 10.1016/j.autrev.2010.09.015. In this recent review, the authors discuss the various cells, molecules and signaling pathways implicated in fibrosis. A better understanding of the complex nature of fibrosis in SSc may ultimately lead to targeted and more effective therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami T, Ihn H, Xu W, et al. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J Invest Dermatol. 1998;110:47–51. doi: 10.1046/j.1523-1747.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- 21.Eiser AR. Does over-expression of transforming growth factor-beta account for the increased morbidity in African-Americans?: Possible clinical study and therapeutic implications. Med Hypoth. 2010;75:418–21. doi: 10.1016/j.mehy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Wen Y, Polan ML, et al. Increased expression of latent TGF-beta binding protein-1 and fibrillin-1 in human uterine leiomyomata. Mol Hum Reprod. 2007;13:343–9. doi: 10.1093/molehr/gam007. [DOI] [PubMed] [Google Scholar]
- 23.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-beta. Expert Rev Mol Med. 2003;5:1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 24.Jonth AC, Silveira L, Fingerlin TE, et al. TGF-beta 1 variants in chronic beryllium disease and sarcoidosis. J Immunol. 2007;179:4255–62. doi: 10.4049/jimmunol.179.6.4255. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–7. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 26.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–8. [PubMed] [Google Scholar]
- 27.Suthanthiran M, Baogui L, Song JO, et al. Transforming growth factor beta1 hyper-expression in African-American hypertension: a novel mediator of hypertension and/or target organ damage. Proc Nat Acad Sci. 2000;97:3479–84. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suthanthiran M, Gerber LM, Schwartz JE, et al. Circulating transforming growth factor beta1 levels and the risk for kidney disease in African Americans. Kidney Intern. 2009;76:72–80. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurram M, Pahwa S, Frieri M. Augmented interleukin-6 secretion in collagen-stimulated peripheral blood mononuclear cells from patients with systemic sclerosis. Ann Allergy. 1994;73:493–6. [PubMed] [Google Scholar]
- 30.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis of scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19:1207–11. [PubMed] [Google Scholar]
- 31.Hasegawa M, Sato S, Fujimoto M, et al. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J Rheumatol. 1998;25:308–13. [PubMed] [Google Scholar]
- 32.Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140–6. doi: 10.1016/s0923-1811(01)00128-1. [DOI] [PubMed] [Google Scholar]
- 33.Shima Y, Kuwahara Y, Murota H, et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatol. 2010;49:2408–12. doi: 10.1093/rheumatology/keq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchings A, Guay-Woodford L, Thomas JM, et al. Association of cytokine single nucleotide polymorphisms with B7 costimulatory molecules in kidney allograft recipients. Pediat Transplant. 2002;6:69–77. doi: 10.1034/j.1399-3046.2002.1o444.x. [DOI] [PubMed] [Google Scholar]
- 35.Bogatkevich GS, Ludwicka-Bradley A, Highland KB, et al. Impairment of the antifibrotic effect of hepatocyte growth factor in lung fibroblasts from African Americans: possible role in systemic sclerosis. Arthritis Rheum. 2007;56:2432–42. doi: 10.1002/art.22713. [DOI] [PubMed] [Google Scholar]
- 36*.Brossett VeraldiKL, Pilewski JM, et al. Localized expression of tenascin in systemic sclerosis-associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum. 2012;64:272–80. doi: 10.1002/art.30647. IGFBP-3, which is overexpressed in fibrotic lungs (particularly in black SSc patients, see reference 35), is shown in this study to induce production of TN-C by subepithelial fibroblasts. The increased lung tissue levels of TN-C parallel the levels detected in the sera of SSc patients with pulmonary fibrosis, suggesting that TN-C may be a useful biomarker for SSc-related pulmonary fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beretta L, Cappiello F, Moore JH, et al. Ability of epistatic interactions of cytokine single-nucleotide polymorphisms to predict susceptibility to disease subsets in systemic sclerosis patients. Arthritis Rheum. 2008;59:974–83. doi: 10.1002/art.23836. [DOI] [PubMed] [Google Scholar]
- 38.Tourkina E, Richard M, Gooz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 39.Tourkina E, Gooz P, Pannu J, et al. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 40.Del Galdo F, Sotgia F, de Almeida CJ, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couet J, Li S, Okamoto T, et al. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 42.Oka N, Yamamoto M, Schwencke C, et al. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–21. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- 43.Rybin VO, Xu X, Steinberg SF. Activated protein kinase C isoforms target to cardiomyocyte caveolae : stimulation of local protein phosphorylation. Circ Res. 1999;84:980–8. doi: 10.1161/01.res.84.9.980. [DOI] [PubMed] [Google Scholar]
- 44.Tourkina E, Gooz P, Pannu J, et al. Opposing effects of protein kinase C alpha and protein kinase C epsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 45.Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–38. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 47.Le Saux J, Teeters K, Miyasato SK, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283:5760–8. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 48.Yan SR, Fumagalli L, Berton G. Activation of SRC family kinases in human neutrophils. Evidence that p58C-FGR and p53/56LYN redistributed to a Triton X-100-insoluble cytoskeletal fraction, also enriched in the caveolar protein caveolin, display an enhanced kinase activity. FEBS Lett. 1996;380:198–203. doi: 10.1016/0014-5793(96)00029-4. [DOI] [PubMed] [Google Scholar]
- 49.Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:293S–298S. doi: 10.1378/chest.122.6_suppl.293s. [DOI] [PubMed] [Google Scholar]
- 50.Trojanowska M. Noncanonical transforming growth factor beta signaling in scleroderma fibrosis. Curr Opin Rheumatol. 2009;21:623–9. doi: 10.1097/BOR.0b013e32833038ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 52.Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713–19. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Galdo F, Sotgia F, de Almeida CJ, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tourkina E, Richard M, Oates J, et al. Caveolin-1 regulates leucocyte behavior in fibrotic lung disease. Ann Rheum Dis. 2010;69:1220–26. doi: 10.1136/ard.2009.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Tourkina E, Bonner M, Oates JC, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis & Tissue Repair. 2011;4:15. doi: 10.1186/1755-1536-4-15. This study demonstrates for the first time that peripheral blood monocytes (PBM) from normal black subjects show enhanced migration toward CXCL12, similar to SSc patients. PBM from normal blacks have low caveolin-1 levels, as seen in SSc patients and in normal PBM after exposure to TGF-β. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno S, Matssumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–2. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 57*.Hishino K, Satoh T, Kawaguchi Y, Kuwana M. Association of hepatocyte growth factor polymorphism with severity of interstitial lung disease in Japanese patients with systemic sclerosis. Arthritis Rheum. 2011;63:2465–72. doi: 10.1002/art.30415. This paper confirms an earlier observation implicating differences in hepatocyte growth factor (HGF) expression in SSc-ILD and shows that in Japanese patients a SNP in the HGF promoter region may modulate the severity of interstitial lung disease by controlling the transcriptional efficiency of the HGF gene. [DOI] [PubMed] [Google Scholar]
- 58.Bogatkevich GS, Ludwicka-Bradley A, Highland KB, et al. Down-regulation of collagen and connective tissue growth factor expression with hepatocyte growth factor in lung fibroblasts from white scleroderma patients via two signaling pathways. Arthritis Rheum. 2007;56:3468–77. doi: 10.1002/art.22874. [DOI] [PubMed] [Google Scholar]
- 59.Wei J, Ghosh AK, Sargent JL, et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5:e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Bogatkevich GS, Highland KB, Akter T, Silver RM. The PPARγ agonist rosiglitazone is antifibrotic for scleroderma lung fibroblasts: Mechanisms of action and differential racial effects. Pulm Med. 2012:1–9. doi: 10.1155/2012/545172. Article ID 545172. This paper confirms earlier suggestions of a role for PPARγ in SSc by demonstrating that PPARγ agonists modulate important fibrogenic events in lung fibroblasts. Additional foundation for the biologic differences between lung fibroblasts from white and black patients is provided, which might explain the observed differences among these racial groups in terms of severity and mortality from SSc-ILD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Wen X, Spataro BC, et al. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic actions of peroxisome proliferator-activated receptor-γ agonists. J Am Soc Nephrol. 2006;17:54–6. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]