Abstract
Co-cultures of endothelial cells (EC) and mesenchymal stem cells (MSC) in three-dimensional (3D) protein hydrogels can be used to recapitulate aspects of vasculogenesis in vitro. MSC provide paracrine signals that stimulate EC to form vessel-like structures, which mature as the MSC transition to the role of mural cells. In this study, vessel-like network formation was studied using 3D collagen/fibrin (COL/FIB) matrices seeded with embedded EC and MSC and cultured for 7 days. The EC:MSC ratio was varied from 5:1, 3:2, 1:1, 2:3 and 1:5. The matrix composition was varied at COL/FIB compositions of 100/0 (pure COL), 60/40, 50/50, 40/60 and 0/100 (pure FIB). Vasculogenesis was markedly decreased in the highest EC:MSC ratio, relative to the other cell ratios. Network formation increased with increasing fibrin content in composite materials, although the 40/60 COL/FIB and pure fibrin materials exhibited the same degree of vasculogenesis. EC and MSC were co-localized in vessel-like structures after 7 days and total cell number increased by approximately 70%. Mechanical property measurements showed an inverse correlation between matrix stiffness and network formation. The effect of matrix stiffness was further investigated using gels made with varying total protein content and by crosslinking the matrix using the dialdehyde glyoxal. This systematic series of studies demonstrates that matrix composition regulates vasculogenesis in 3D protein hydrogels, and further suggests that this effect may be caused by matrix mechanical properties. These findings have relevance to the study of neovessel formation and the development of strategies to promote vascularization in transplanted tissues.
Keywords: collagen, fibrin, vasculogenesis, stiffness, mesenchymal stem cells, endothelial cells
Introduction
Angiogenesis and vasculogenesis are biological processes that are vital to developing and regenerating tissues [1]. Vasculogenesis is the de novo formation of neovessels through the assembly of endothelial cells into a tube, followed by stabilization and maturation into a blood vessel. Angiogenesis creates new vessels via the sprouting of new capillaries from existing vasculature. When tissues are transplanted, these processes are critical in enabling the transport of nutrients and waste products and achieving integration of the transplanted tissue with the host vasculature [2, 3]. The field of tissue engineering has encountered a particular challenge in creating large tissue constructs that can be transplanted without subsequent loss of tissue viability, since the limit of effective diffusive transport has been suggested to be only 150-200 μm [4]. The interior regions of large implanted tissues therefore become necrotic due to the lack of a functional vascular supply to provide convective transport [5]. Stimulating the rapid creation of a functional vascular supply by either vasculogenesis or angiogenesis is a key to maintaining tissue viability post-implantation [6].
A number of experimental investigations have demonstrated that important aspects of vasculogenesis can be recapitulated in vitro [7, 8, 9]. Recent studies have focused on the interactions of endothelial cells (EC) with stromal cells in generating vessel-like structures [10, 11, 12]. In such co-culture systems, stromal cells have been suggested to act as pericytes that provide paracrine factors to induce the formation of vessel structures and also stabilize neovessels by providing mechanical support [13, 14]. In vivo evidence suggests that the lack of a stromal cell component leads to pathological vessel formation characterized by hyperplasia and irregular EC morphology [15, 16].
Co-cultures of EC and mesenchymal stem cells (MSC) have been used as a model of vasculogenesis and it has been shown that MSC can function as pericytes to promote vessel formation and maturation [17, 18, 19]. In this role, MSC secrete specific pro-angiogenic cytokines [20, 21] and control the permeability of neovessels through regulation of cell-cell adherens junctions [22, 23]. The ratio of EC to MSC has been shown to affect cell function and capillary morphogenesis. Lower EC:MSC ratios have been associated with higher cell metabolic activity in vitro, though higher ratios increase the proportion of CD31+ cells [24]. In vivo, lower ratios have been associated with an increased vasculogenic response [25], and functional prevascular networks have been observed in vivo at even very low (2:99) ratios [26]. Although the effect of EC:MSC ratio is not yet clear, it is likely to be one of the contributing factors in determining the rate and degree of vasculogenesis.
Vasculogenesis has been studied in a variety of extracellular matrix systems in vitro. Collagen and fibrin are of particular interest in this context because of their prominent role as wound healing proteins [27, 28]. Studies in which collagen and fibrin were combined as composite matrices have suggested that collagen delays neovessel formation in a dose-dependent manner [29]. Previous work in our lab has shown that collagen/fibrin composite materials have mechanical properties distinct from either of the pure components alone, even at equivalent total protein concentrations [30, 31]. The effects of matrix concentration and stiffness on capillary morphogenesis have recently been investigated using pure extracellular matrix components of different compositions and stiffness [32, 33, 34]. These studies have shown that the degree of vascularization can be modulated by the properties of the surrounding matrix, and have suggested an inverse relationship between matrix stiffness and the degree of neovessel formation.
In the present study, we systematically examined vasculogenesis by EC-MSC co-cultures in vitro using well defined three-dimensional (3D) matrices made of collagen and fibrin. Our goal was to determine the relative role of cell ratio and matrix composition in a controlled environment that incorporated both pure and composite formulations of matrix proteins of relevance in wound healing. In particular, we varied the EC:MSC ratio and the collagen/fibrin proportion of the surrounding matrix and quantified the degree of vessel-like structure formation. The mechanical properties of the extracellular matrices were assessed using rheometry to provide measures of the stiffness of the matrix. Our findings demonstrate that collagen/fibrin matrices supported vasculogenesis in vitro, but the degree of vessel-like structure formation was dependent on matrix composition. Further experiments in which matrix mechanical properties were varied revealed a clear correlation between matrix stiffness and the degree of vasculogenesis. These studies highlight key features of the extracellular milieu that regulate neovessel formation, which may provide insight into the biological process of vasculogenesis. In addition this knowledge may be applied to promoting neovascularization of transplanted tissues.
Materials and Methods
Cell Culture
Human mesenchymal stem cells (MSC; Lonza Inc., Walkersville, MD) were cultured in Dulbecco’s modified Eagle’s medium (low glucose DMEM; Thermo Scientific; Logan, UT) supplemented with 10% MSC-qualified fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and 1% penicillin and streptomyocin (PS; Invitrogen). MSC were used between passages 6-8. Media was changed every other day.
Human umbilical vein endothelial cells (EC) were isolated from harvested umbilical cords as previously described [20]. Briefly, umbilical veins were irrigated with sterile phosphate buffered saline (PBS) and then incubated with 0.1% collagenase (Type I, Worthington Biochemical, Lakewood, NJ) at 37°C for 20 min. The digestion product was collected, the vein was washed with PBS, and the resulting suspension was centrifuged. The cell pellet was re-suspended in Endothelial Growth Medium-2 (EGM-2, Lonza) and plated into flasks. After 24 hours, the cells were washed with PBS to remove residual erythrocytes. EC were cultured in EGM-2 and used at passage 4. Culture medium was changed every other day.
Fabrication of Three-Dimensional Collagen-Fibrin Gels
Collagen (COL) and fibrin (FIB) composite gels were fabricated as previously described [31], and the process is shown schematically in Figure 1. Briefly, 4.0 mg/ml Type I COL (MP Biomedicals, Solon, OH) was dissolved in 0.02 N acetic acid and 4.0 mg/ml bovine fibrinogen (Sigma Aldrich, St. Louis, MO) was dissolved in DMEM. Cell-seeded COL/FIB composite gels were created by suspending EC and MSC in a mixture with 10% FBS, 10% 5×-concentrated DMEM (starting concentration), 5% 0.1 N NaOH, 2% bovine thrombin (0.1 UT/ml; Sigma), COL, and fibrinogen at 4°C. COL and fibrinogen volumes were varied to generate COL/FIB composite gels at mass ratios of 100/0 (pure COL), 60/40, 50/50, 40/60, and 0/100 (pure FIB) at a constant total protein concentration of 2.5 mg/ml. 1×-concentrated DMEM was used to fill the final volume to 100%. The suspension was then pipetted into a 24-well plate and allowed to gel at 37°C for 45 min. The resulting gels contained homogenously distributed EC and MSC embedded directly within the protein matrix. Cells were embedded in the gels in all five COL/FIB concentrations at EC:MSC ratios of 5:1, 3:2, 1:1, 2:3, and 1:5. The total cell concentration of all gels was kept constant at 6.0×105 cells/ml. After fabrication cell-seeded gels were cultured for 7 days at 37 °C and 5% CO2.
Fig. 1.
Schematic of composite matrix fabrication and parameters varied in experimental design.
The first experimental series examined the effects of cell ratio and relative matrix composition using the parameter described above. In subsequent studies, a cell ratio of 1:1 EC-MSC and a matrix composition of 40/60 COL/FIB were used. The effect of total protein concentration on vasculogenesis was evaluated by creating COL/FIB gels with total protein concentrations of 1.25 mg/ml, 2.5 mg/ml, and 5.0 mg/ml using a stock collagen solution of 4.0 mg/ml and stock fibrinogen solutions of 4.0 and 20.0 mg/ml. To examine the effect of matrix stiffness independently from matrix concentration, the small dialdehyde glyoxal was used to crosslink 40/60 COL/FIB composite gels. Glyoxal was added at 1.0 mM directly to the pre-gelled matrix solution containing 1:1 EC:MSC and gelation was initiated by incubating the mixture at 37°C for 45 minutes. Gels were then washed three times for 10 minutes each in PBS to remove unreacted glyoxal and medium was added.
Vasculogenesis Assay
EC were labeled with a fluorescent protein (mCherry; Clontech, Mountain View, CA) as previously described to allow visualization and quantification of vessel-like networks [17]. A retroviral expression system (Orbigen Inc., San Diego, CA) was used to achieve stable expression of the mCherry gene by EC. At days 1, 3, 5, and 7 post-fabrication, gels were imaged on a fluorescent microscope system (Olympus America Inc., Center Valley, PA). Five representative images of each sample were taken at each time point. The images were analyzed using the Angiogenesis Module in Metamorph Premier software (Molecular Devices Inc., Sunnyvale, CA) as previously described [20]. Minimum width, maximum width, and intensity over background were set to discriminate vessel-like structures within the images. The total length of formed vessel-like structures was then calculated.
Confocal Imaging of EC, MSC, and Matrix Architecture
To determine the relative positions of the different cell types in 3D composite matrices, MSC were retrovirally transduced to achieve expression of GFP, using the same methods as for mCherry transfection (Orbigen Inc., San Diego, CA), and were co-embedded with mCherry labeled EC. Images were acquired using a laser scanning confocal microscope (Olympus America Inc., Center Valley, PA). Separate image scans were taken to identify the EC and MSC, and these sections were combined to determine co-localization of the cell types. Image scans were captured in a horizontal plane containing vessel-like structures. Images of the matrix architecture were obtained using confocal reflectance microcopy at a wavelength of 488 nm. These images are included in the Supplementary Data.
Cell Viability and Proliferation
Cell viability was assessed using a vital staining kit (Live/Dead®, Molecular Probes, Eugene, OR) as previously described [35]. Briefly, gels were washed three times in sterile PBS for 10 min and then incubated at 37°C for 45 min in a solution containing 4.0 μm calcein-AM and 4.0 μm ethidium homodimer-1 in PBS. After three subsequent PBS washes, gels were imaged using a laser scanning confocal microscope (Olympus). Viability was quantified using ImageJ software (National Institute of Health, Bethesda, MD).
To quantify cell proliferation during the culture period, the total DNA content of gels was determined and run against a standard curve. Gels were washed three times in phosphate buffered saline for 10 minutes per wash and were then extracted using 4.0 M guanidine hydrochloride solution. DNA analysis was performed on samples at days 0, 1, 3, and 7 of culture using a commercially available DNA assay (PicoGreen®, Invitrogen Inc.).
Gel Rheology
The mechanical properties of acellular COL/FIB composite gels were determined by gel rheometry (AR-G2, TA Instruments, New Castle, DE) [36]. Pre-mixed COL/FIB matrix solutions were loaded onto a Peltier stage precooled to 10 °C. A 20 mm steel parallel plate was used at a gap height of 1000 μm. A temperature ramp over 2 minutes was performed to raise the temperature of the system to 37°C. A time sweep was then conducted for 45 minutes at 37°C at 1% strain and at an oscillation frequency of 1 radian/second to simulate the gelation conditions used in the vasculogenesis assay. Reported storage (G’) and loss (G”) moduli were generated from the average of the last 5 minutes of the time sweeps.
Statistical Analysis
All quantitative analyses were processed using a one-way Analysis of Variance using Tukey’s post-hoc analysis. Statistical significance was set at p < 0.05. Numerical values are presented as mean +/− standard deviation; n = 3 for EC-MSC cell ratio vasculogenic assays, n = 4 for cell proliferation studies, n = 5 for all rheological data, n = 4 for protein concentration and glyoxal vasculogenic assays, and n = 4 for cell viability studies.
Results
Effect of Matrix Composition
COL/FIB composite matrices were fabricated at COL/FIB compositions of 100/0, 60/40, 50/50, 40/60, and 0/100. Figure 2 shows representative images of these materials seeded with cells at a 1:5 EC-MSC ratio (panels a-e) and examined after 7 days in culture. In general, the degree of vessel-like network formation clearly increased with increasing fibrin content in the composite gels. Figure 2f shows quantification of the total network length over time in culture. The trend for increased network development over time is evident and all five matrix compositions showed significantly higher values (p<0.05) of both total network length and the number of segments at day 7 compared to day 1. By day 7, the length for all composite matrix compositions were significantly higher than the pure collagen (100/0) group. At day 7 the pure fibrin (100/0) group had statistically higher values (p < 0.05) of network length compared to the 60/40, 50/50, and 0/100 matrix compositions. However, there was no statistical difference in either total network length or the number of segments between pure fibrin and the 40/60 matrix composition.
Fig. 2.
(a-e) Representative images of vessel-like structures in COL/FIB matrices of indicated composition at an EC:MSC ratio of 1:5. (f, g) Quantification of total network length and number of segments in each of the matrix compositions over time in culture. (*) denotes statistical significance (p < 0.05) against 100/0 (collagen), (^) denotes statistical significance against 0/100 (fibrin), # denotes statistical significance against day 1.
Figure 2g shows quantification of the number of network segments in each of the matrix compositions over time. These data closely mirror the total network length data, indicating that the vessel-like networks grew through branching and joining, as opposed to simple elongation of existing vessel segments. This trend of network growth via branching and joining of segments was evident in all of the samples analyzed (see Supplementary Data).
Effect of Cell Ratio
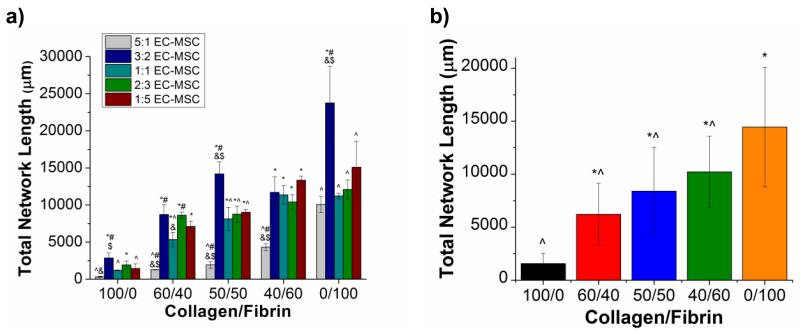
Figure 3a shows vasculogenesis data at day 7 for all five matrix compositions and all five EC:MSC ratios investigated. The trend of increasing network formation with increasing fibrin content is evident in these data as well. Within each matrix composition, the 5:1 EC:MSC ratio resulted in the the lowest values for total network length relative to all other cell ratios. In the 100/0, 50/50, and 0/100 compositions, the 3:2 EC:MSC ratio resulted in significantly greater values of total network length, compared to the other cell ratios. In general, there was no marked difference in vessel-like structure formation between the 1:1, 2:3, and 1:5 EC:MSC ratios. Figure 3b shows the total network length as a function of matrix composition, using data pooled across all cell ratios, and indicates significant differences caused by altered protein content.
Fig. 3.
(A) Total network length as a function of matrix composition at all five cell ratios investigated at day 7 in culture. (*) denotes statistical significance (p < 0.05) against the 5:1 EC:MSC cell ratio, (^) denotes statistical significance against 3:2 EC:MSC cell ratio, (#) denotes statistical significance against the 1:1 EC:MSC cell ratio, (&) denotes statistical significance against the 2:3 EC:MSC cell ratio, and ($) denotes statistical significance against the 1:5 EC:MSC cell ratio. (B) Average network length, using data from all cell ratios, as a function of matrix composition, (*) denotes statistical significance against 100/0 (collagen), (^) denotes statistical significance against 0/100 (fibrin).
A full panel of experiments was performed, which included each of the five matrix compositions seeded with each of the five cell ratios, and analyzed at each of the four time points. Figures 2 and 3 summarize the trends that were observed across all samples, and the remainder of the full data set is presented in Supplemental Figures 1-4. In the 2:3, 1:1, and 3:2 EC-MSC samples, the trends of increasing vessel formation with time and with increasing fibrin content were maintained. However, in the 5:1 EC-MSC samples the degree of vasculogenesis was markedly lower across all matrix compositions. Although vessel-like structures formed rapidly at this cell ratio, the network did not continue to expand, but instead retracted over time. In general, the pure fibrin (0/100) matrices resulted in significantly more (p<0.05) vessel-like structure formation compared to the 100/0, 60/40, and 50/50 matrix compositions. However, the 0/100 and 40/60 were similar in their behavior. Overall, it was clear that time and matrix composition had a greater effect on vasculogenesis than EC:MSC ratio.
These findings allowed us to narrow the set of matrix and cell formulations to be used in subsequent experiments. A COL/FIB composition of 40/60 was selected because it supported vasculogenesis to the same degree as pure fibrin, and both of these materials had the most robust response of the compositions tested. Similarly, an EC:MSC ratio of 1:1 was selected because this cell ratio behaved similar to the other ratios in the 40/60 matrix. The selection of a single construct formulation facilitated the extension of our studies without requiring all formulations to be examined.
Cell Interactions and Proliferation
Distinct labeling of EC and MSC using different fluorescent labels allowed the examination of the spatial relationships between the cell types in 3D COL/FIB materials, as shown in Figure 4. Panel a shows extended EC and panel b shows MSC clustering at day 7 in culture. Overlay of these images (panel c) shows very clear association of MSC with EC that suggests their role as pericytes in stabilizing vessels.
Fig. 4.
Characterization of cell-seeded 40/60 COL-FIB constructs with 1:1 EC-MSC ratio. (a-c) Co-localization of MSC and EC. (d) Cell proliferation of EC and MSC. (*) denotes statistical significance against day 0, (#) denotes statistical significance against day 7.
Figure 4d shows the total DNA extracted from cell-seeded composite matrices, which was used as a measure of cell number and proliferation over the 7 day culture period. The number of cells increased significantly day 0 (post-gelation) to day 1, and then plateaued from day 1 to 3. Significant cell proliferation was again evident between day 3 and day 6, and overall the cell content of the gels increased by about 70% over the 7 day period.
Mechanical Properties of COL/FIB Materials
To evaluate the effect of material properties on vasculogenesis, the shear moduli (storage and loss modulus) of the COL/FIB composite gels were evaluated at all five matrix compositions. Representative time sweeps are shown in Figure 5a and revealed distinct gelation curves for the pure and composite materials. Pure collagen (100/0) gelled rapidly and the modulus rose sharply, followed by a gradual decrease in modulus over time until it plateaued. Pure fibrin (0/100) had a delayed gelation response and then gelled to reach a plateau modulus. The mixed composites had intermediate behavior, which depended on their composition. The average moduli of gelled materials are presented in Figure 5b, which shows that gel stiffness as represented by the storage modulus (G’) decreased with increasing fibrin content. The 100/0 composition was significantly stiffer than the other materials. The 40/60 and the 100/0 compositions exhibited very similar mechanical properties. Figures 5c shows the correlation between total network length and matrix stiffness, and suggests an inverse linear relationship between these parameters (R2 = 0.92).
Fig. 5.
(a) Representative gel rheometry time sweeps for COL/FIB matrices. (b) Average storage (G’) and loss (G’) moduli for COL/FIB matrices. (*) denotes statistical significance (p < 0.05) against 100/0 (collagen), (^) denotes statistical significance against 0/100 (fibrin). (c) Correlations between formation of network formation and material stiffness.
The loss modulus (G”) was also calculated from the dynamic testing data, and generally followed the same trends as the storage modulus. This parameter provides a measure of the viscoelastic nature of the hydrogels, and reflects the amount of viscous energy dissipation under dynamic conditions. We did not include this parameter in our interpretation of network formation in different materials, since the 3D gels were cultured under static conditions, in which viscous effects are unlikely to play a major role.
Effect of Protein Concentration
The observed relationship between matrix stiffness and extent of vessel-like structure formation led us to further investigate this effect. To this end 40/60 COL/FIB gels were created at total protein concentrations of 1.25 mg/ml, 2.50 mg/mL and 5.00 mg/ml, to alter overall matrix stiffness while keeping the COL/FIB ratio constant. The rheological properties and associated vasculogenesis data at 1:1 EC:MSC for these materials are shown in Figure 6. The gelling curves in panel a show that protein concentration affects the mechanical properties of these composites. The 1.25 mg/ml gels had a storage modulus of 11±4 Pa, which increased to 56±8 Pa (p<0.03) at 2.50 mg/ml, and to 222±37 Pa (p<0.001) at 5.00 mg/ml, and the loss moduli followed a similar trend. Representative images of vasculogenesis in these materials at day 7 are shown in Figures 6c-e and network length is quantified in Figures 6f. There was no statistical difference between the 1.25 mg/ml and the 2.50 mg/ml materials at any time point. However, the 5.00 mg/ml materials exhibited dramatically decreased formation of vessel-like structures, relative to the lower concentration matrices at all time points (p<0.05).
Fig. 6.
Effect of total protein concentration on vasculogenesis in 40/60 COL-FIB matrices. (a) Representative gel rheometry time sweeps. (b) Storage and loss moduli. (c-e) Representative images on day 7 of culture. (f) Quantification of vessel-like structures. (^) denotes statistical significance (p < 0.05) against 1.25 mg/ml, (*) denotes statistical significance against 2.50 mg/ml, (#) denotes statistical significance against 5.00 mg/ml.
Effect of Glyoxal Crosslinking
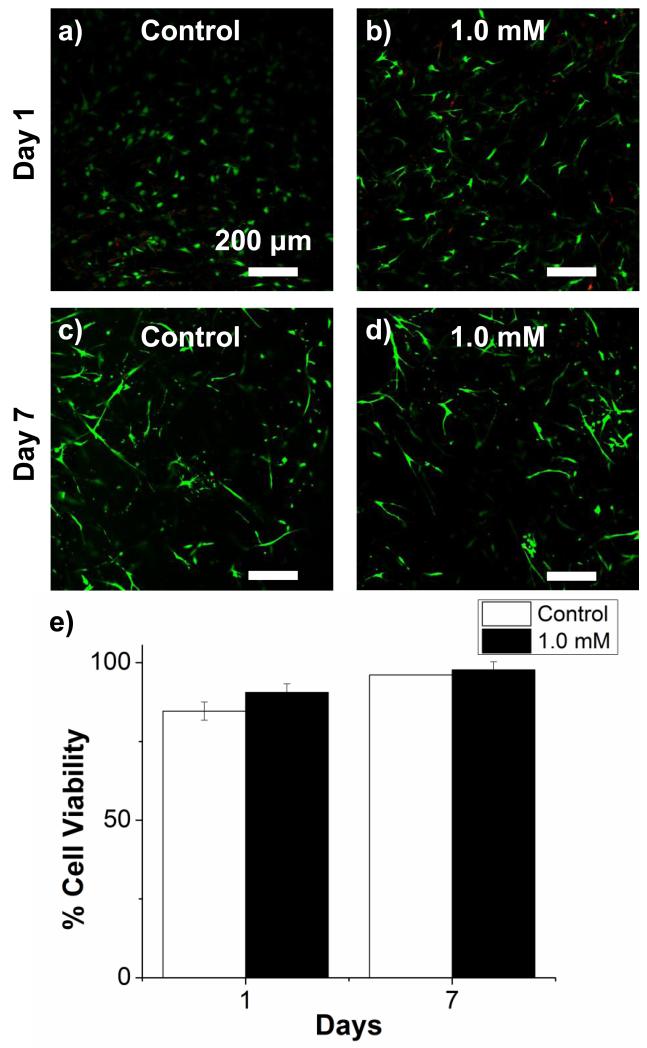
Another approach to changing the mechanical properties of protein gels without altering the overall composition is to crosslink the matrix. To this end we used glyoxal, a small dialdehyde that crosslinks free amine groups, to stiffen 40/60 COL/FIB composite gels. The rheological properties and associated vasculogenesis data for these materials seeded with 1:1 EC:MSC are shown in Figure 7. Treatment with glyoxal altered the gelation dynamics (panel 7a) and resulted in an approximately 2-fold increase in the storage moduli (panel 7b) of gels with otherwise identical composition. Vessel-like structures formed in both untreated control and crosslinked gels (panels 7c-7f), but crosslinked materials exhibited significantly lower values of both total network length by day 7 (panels 7g). Cell viability was assessed in both control and crosslinked gels, as shown in Figure 8. Viability was high in all samples at both day 1 and day 7 (panels 8a-8d), and there was no significant difference in viability between treatments at either time point (panel 8e).
Fig. 7.
Effect of glyoxal crosslinking on vasculogenesis in 40/60 COL/FIB matrices. (a) Representative gel rheometry time sweeps. (b) Storage and loss moduli. (c-f) Representative images on day 7 of culture. (g) Quantification of vessel-like structures. (*) denotes statistical significance (p < 0.05) against control.
Fig. 8.
Cell viability in 3D constructs after glyoxal treatment. (a-d) Representative images on days 1 and 7 of culture. (e) Quantification of cell viability.
Discussion
This series of studies examined the effects of material composition, EC:MSC ratio, and matrix stiffness on vasculogenesis in a well-defined, three-dimensional in vitro model. The relative amounts of collagen and fibrin in the matrices were shown to have a marked effect on the formation of vessel-like networks in COL/FIB materials. In particular, the degree of vasculogenesis clearly increased in a dose-dependent manner with increasing fibrin content. While it is well established that both the extracellular matrix and the presence of stromal cells can modulate vasculogenesis, the interaction between these factors and in particular the effects of matrix type and density on the vasculogenic response are not fully understood. Earlier studies that used collagen and fibrin in vasculogenesis assays showed that these proteins can affect neovessel formation [30, 15, 37], however the properties of the matrix that produce these effects are still being elucidated. The present study used compositionally defined 3D composite materials to further contribute to our understanding of how these biologically active and structurally important wound healing proteins can regulate neovessel formation.
The communication between EC and stromal cells is an area of increasing importance in a variety of fields [20, 24], and therefore we examined the effect of varying the EC:MSC ratio on the degree of vasculogenesis. The chosen EC:MSC fractions were varied from 1:5 to 5:1 to cover a relatively wide range of ratios, while the total cell concentration was kept constant. Interestingly, the group with the highest relative fraction of EC (5:1) exhibited the lowest vasculogenic response over a week in culture in all five of the tested matrix compositions. Although this cell ratio supported rapid formation of vessel-like structures initially, the total length and number of vessel-like structures decreased over time in culture. This behavior contrasts with the other (lower) EC:MSC ratios examined, which exhibited steadily increasing network length over time. These results highlight the importance of pericyte-like cells in promoting and stabilizing nascent structures. In addition, clear co-localization of EC and MSC was observed when both cell types were labeled and imaged, further suggesting peri-endothelial interaction between the two cell types. These associations between endothelial and stromal cells have been shown to be important for stable neovessel formation in other recent studies [14, 17].
The 40/60 COL/FIB material seeded with 1:1 EC:MSC was selected for further study because it exhibited the highest degree of vasculogenesis. This composition was statistically the same as pure fibrin in this regard, however in some applications a composite matrix may have advantages over a pure material. For example, the use of composite matrices has become more common in tissue engineering applications in order to harness both the mechanical and biochemical properties of such materials [30, 38]. Assessment of DNA content in these composite materials over time showed that cell number increased initially, followed by a plateau phase, and a subsequent second phase of cell growth. Cell proliferation is important to the vascular extension and maturation process. In this study, we were not able to distinguish between DNA contributed by EC and that contributed by MSC. However, the initial growth phase may reflect proliferation of EC, which have been shown to be capable of rapid division in response to mitogen stimulation [39]. The later growth phase may reflect either continued EC division to extend vessels or may be due to proliferation of MSC, which may grow more slowly as they establish pericyte function [40].
Rheological analysis showed that the mechanical properties of the COL/FIB composite materials varied with composition. In particular, the pure collagen materials were the stiffest and the pure fibrin materials were the least stiff, with the stiffness of composite materials falling in between the pure materials. A clear inverse relationship existed between matrix stiffness and degree of vessel-like structure formation. These findings agree with those of other recent studies that have examined the role of matrix compliance in the formation of neovasculature in vitro and in vivo [33, 34], and which have suggested that vasculogenesis is inhibited in stiffer matrices. In the present study, we further investigated the role of matrix stiffness in modulating vasculogenesis by performing studies on 40/60 COL/FIB materials fabricated at a range of total protein concentrations. The resulting materials varied in stiffness in direct relation to the total protein content. The stiffest materials (5.00 mg/ml) exhibited about a 4-fold increase in storage modulus relative to the standard 2.50 mg/ml materials, and showed a correspondingly decreased vasculogenic response. In contrast, the least stiff materials (1.25 mg/mL) exhibited a storage modulus only about one fifth of the standard 2.5 mg/ml materials, but showed no statistical difference in the formation of vessel-like structures. These results provide further evidence that matrix stiffness may be involved in modulating vasculogenesis, but also suggest that there may be a minimum stiffness below which the effect is no longer evident.
Increasing the total protein concentration in gel matrices does not alter the stiffness independently of all other factors that may affect cell function, since increased protein content can also lead to changes in adhesive ligand density and porosity of the matrices. Analysis of matrix architecture using confocal reflectance microscopy verified that the matrix architecture varied with both changing matrix composition and concentration (see Supplemental Figure 5). In an attempt to further isolate the effects of stiffness the protein matrices were crosslinked with glyoxal, a small dialdehyde that we have used previously to increase the mechanical properties of protein matrices without adversely affecting the viability of embedded cells [35]. Glyoxal crosslinking induced a two-fold increase in storage modulus in the 40/60 COL/FIB matrix composition with a resulting decrease in total network length at day 7. However confocal reflectance imaging showed that the matrix structure was also changed by crosslinking, though the effect was relatively modest. Viability staining confirmed that the decrease in vasculogenic response was not due to cell death. These data further support the idea that increased matrix stiffness results in decreased vessel-like structure formation, though the effect of crosslinking on stiffness may not have been completely independent of matrix architecture.
The cell proliferation assays used in this study measured total cell number in 3D matrices over time, but were based on DNA content and therefore did not distinguish between EC and MSC. The mechanism of network formation in 3D COL/FIB materials could therefore not be fully elucidated, but it is likely that connected EC networks developed by a combination of cell elongation and cell proliferation. Images of networks clearly showed elongated EC, in contrast to a rounded EC morphology in materials that did not support robust network formation. While overall cell number increased over time in EC-MSC constructs, our data suggest that cell proliferation was not the sole contributor to network formation in our system. Cell number in COL/FIB matrices with varying protein concentration (Supplemental Figure 6A) showed that higher concentration materials resulted in more cell proliferation, but also exhibited reduced network formation. Similarly, cell number in glyoxal-treated constructs (Supplemental Figures 6B and 6C) was practically unaffected by crosslinking, whereas the degree of network formation was decreased in crosslinked matrices. In general, the degree of cell proliferation did not correlate strongly with network formation.
This study has demonstrated that matrix composition is a potent modulator of vasculogenic activity in 3D matrices containing embedded EC and MSC. In particular, fibrin-containing matrices were permissive of a robust vasculogenic response, whereas matrices with a high collagen Type I content resulted in decreased vessel-like structure formation. Importantly, composites could be created that maintained strong vasculogenic activity while also containing both collagen and fibrin, which may be beneficial in some applications. The ratio of EC:MSC was not a strong modulator of the vasculogenic response, although high EC:MSC ratios (5:1) resulted in unstable vessel formation. The clear correlation between matrix stiffness and the vasculogenic response suggested that mechanical properties may be important in modulating vessel formation, and follow-up experiments in which stiffness was varied provided supporting evidence for this idea. However, the experimental treatments used in this study also altered the matrix architecture, which may have lead to changes in mass transport, ligand density, or other parameters that could modulate vasculogenesis. Taken together, these results are relevant to the variety of in vitro systems that have been developed to study angiogenesis and vasculogenesis, and in particular those that rely on 3D protein matrices to simulate the tissue environment. In addition, these data are relevant to efforts to understand and promote vasculogenesis in engineered tissues, which is a key challenge in the transplantation of larger structures.
Supplementary Material
Acknowledgements
This work was supported in part by a National Science Foundation Graduate Research Fellowship (to RRR), National Heart, Lung and Blood Institute grant HL085339 (to AJP), as well as by the University of Michigan Cardiovascular Center Summer Research Fellowship (to AWP).
Footnotes
Conflicts of Interests
The authors declare that they have no conflict of interest.
References
- 1.Jain RK. Molecular regulation of vessel maturation. Nature Medicine. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;5110;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan DL, Vunjak-Novakovic G. Engineering Complex Tissues. Tissue Engineering. 2006;12(12):3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews. 2011;63(4-5):300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Santos MI, Reis RL. Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges. Macromolecular Bioscience. 2010;10(1):12–27. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 6.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization Strategies for Tissue Engineering. Tissue Engineering: Part B. 2009;15(3):353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vailhe B, Vittet D, Feige JJ. In vitro models of vasculogenesis and angiogenesis. Laboratory Investigation. 2001;81(4):439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- 8.Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. International Review of Cell and Molecular Biology. 2011;288:101–165. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods in Enzymology. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd B, Jay S, Saltzman W, Tellides G, Pober J. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Engineering Part A. 2009;15:165–173. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au P, Tam J, Fukumura D, Jain R. Bone marrow derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanaati S, Fuchs S, Webber MJ, Orth C, Barbeck M, Gomes ME, Reis RL, Kirkpatrick CJ. Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth of endothelial cells in co-culture with primary osteoblasts. Journal of Tissue Engineering and Regenerative Medicine. 2011;5(6):e136–143. doi: 10.1002/term.373. [DOI] [PubMed] [Google Scholar]
- 13.Merfeld-Clauss S, Gollahalli N, March K, Traktuev D. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Engineering Part A. 2010;16:2953–2966. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of Pericytes Lead to Endothelial Hyperplasia and Abnormal Vascular Morphogenesis. The Journal of Cell Biology. 2001;153(3):543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Current Opinion in Genetics and Development. 2005;15(1):102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC, Putnam AJ. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Experimental Cell Research. 2010;316(5):813–825. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorrell JM, Baber MA, Caplan AI. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Engineering Part A. 2009;15(7):1751–1761. doi: 10.1089/ten.tea.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy GP, Ahsan T, O’Brien T, Barry F, Nerem RM. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. 2009;15(9):2459–2470. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 20.Ghajar CM, Blevins KS, Hughes CCW, George SC, Putnam AJ. Mesenchymal Stem Cells Enhance Angiogenesis in Mechanically Viable Prevascularized Tissues via Early Matrix Metalloproteinase Upregulation. Tissue Engineering. 2006;12(10):2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 21.Gruber R, Kandler B, Holzmann P, Vogele-Kadletz M, Losert U, Fischer MB, Watzek G. Bone Marrow Stromal Cells Provide a Local Environment That Favors Migration and Formation of Tubular Structures of Endothelial Cells. Tissue Engineering. 2005;11(5):896–903. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- 22.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, Cox CS, Retz MS, Holcomb JB, Dash PK. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells and Development. 2011;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, van den Beucken JJJP, Yang F, Both SK, Cui F-Z, Pan J, Jansen JA. Coculture of Osteoblasts and Endothelial Cells: Optimization of Culture Medium and Cell Ratio. Tissue Engineering Part C: Methods. 2011;17(3):349–357. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 25.Melero-Martin JM, De Obaldia ME, Kang S-Y, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering Robust and Functional Vascular Networks In Vivo With Human Adult and Cord Blood-Derived Progenitor Cells. Circulation Research. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouwkema J, De Boer J, Van Blitterswijk CA. Endothelial Cells Assemble into a 3-Dimensional Prevascular Network in a Bone Tissue Engineering Construct. Tissue Engineering. 2006;12(9):2685–2693. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich F, Lelkes PI. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis. 2006;9(3):111–125. doi: 10.1007/s10456-006-9037-x. [DOI] [PubMed] [Google Scholar]
- 28.Martineau L, Doillon CJ. Angiogenic response of endothelial cells seeded dispersed versus on beads in fibrin gels. Angiogenesis. 2007;10:269–277. doi: 10.1007/s10456-007-9079-8. [DOI] [PubMed] [Google Scholar]
- 29.Kroon ME, van Schie MLJ, van der Vecht B, van Hinsbergh VWM, Koolwijk P. Collagen type I retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis. 2002;5:257–265. doi: 10.1023/a:1024540701634. [DOI] [PubMed] [Google Scholar]
- 30.Rowe SL, Stegemann J. Interpenetrating Collagen-Fibrin Composite Matrices with Varying Protein Contents and Ratios. Biomacromolecules. 2006;7:2942–2948. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe SL, Stegemann JP. Microstructure and Mechanics of Collagen-Fibrin Matrices Polymerized Using Ancrod Snake Venom Enzyme. Journal of Biomechanical Engineering. 2009;131(6):061012. doi: 10.1115/1.3128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvascular Research. 2010;80(1):23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kniazeva E, Kachgal S, Putnam AJ. Effects of Extracellular Matrix Density and Mesenchymal Stem Cells on NeovascularizationIn Vivo. Tissue Engineering Part A. 2011;17(7-8):905–914. doi: 10.1089/ten.tea.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. Journal of Tissue Engineering and Regenerative Medicine. 2011;5(4):e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Stegemann JP. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomaterialia. 2011;7(6):2410–2417. doi: 10.1016/j.actbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotlarchyk MA, Shreim SG, Alvarez-Elizondo MB, Estrada LC, Singh R, Valdevit L, Kniazeva E, Gratton E, Putnam AJ, Botvinick EL. Concentration Independent Modulation of Local Micromechanics in a Fibrin Gel. PLOS One. 2011;6(5):e20201. doi: 10.1371/journal.pone.0020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collen A, Koolwijk P, Kroon ME, Van Hinsbergh VWM. Influence of fibrin structure on the formation and maintenance of capillary-like tubules by human microvascular endothelial cells. Angiogenesis. 1998;2:153–165. doi: 10.1023/a:1009240522808. [DOI] [PubMed] [Google Scholar]
- 38.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomaterialia. 2011;7(6):2401–9. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Bala K, Ambwani K, Gohil NK. Effect of different mitogens and serum concentration on HUVEC morphology and characteristics: Implication on use of higher passage cells. Tissue and Cell. 2011;43(4):216–222. doi: 10.1016/j.tice.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Rao RR, He J, Leach JK. Biomineralized composite substrates increase gene expression with nonviral delivery. Journal of Biomedical Materials Research Part A. 2010;94(2):344–354. doi: 10.1002/jbm.a.32690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.