Abstract
We evaluated the use of sorafenib to overcome resistance to aromatase inhibitors (AIs) in patients with metastatic breast cancer who had disease recurrence or progression while on AIs. We performed a multi-institution phase I/II study of sorafenib and anastrozole 1 mg daily in 35 postmenopausal females with hormone receptor positive metastatic breast cancer resistant to AIs. Primary objectives were to determine the dose of sorafenib in conjunction with anastrozole and the clinical benefit rate (CBR) (complete response [CR], partial response [PR], or stable disease [SD] ≥ 24 weeks). Secondary objectives were to determine toxicity and to evaluate if response was associated with change in number of circulating endothelial cells or circulating endothelial progenitor cells. Based on the phase I portion, sorafenib 400 mg twice daily was selected as the phase II dose. Among 35 patients, 7 had SD ≥ 24 weeks, 1 had PR ≥ 24 weeks, and 14 had progressive disease (PD) ≤ 24 weeks, corresponding to a CBR of 23%. The most common adverse events (all; Grade 3/4) were fatigue (66%; 17%), diarrhea (63%; 6%), nausea (60%; 9%), and hand-foot syndrome (57%; 34%). Dose reduction occurred in 77% of the patients and 31% came off study due to toxicity. The combination of sorafenib and anastrozole demonstrated a 23% CBR in patients with hormone receptor positive, AI-resistant metastatic breast cancer, which may be attributable to the restoration of sensitivity to AIs. Toxicities occurred frequently resulting in a high rate of discontinuation.
Keywords: Breast cancer, Endocrine resistance, Aromatase inhibitor, Anastrozole, Sorafenib
Introduction
Breast cancer is the most frequently diagnosed cancer and the second most common cause of cancer deaths in females in the United States. About 6% of the female breast cancer cases are advanced stage at the time of diagnosis [1]. For early stage disease, relapses are most common within the first 5 years after treatment [2, 3]. Over the past two decades, the median breast cancer specific survival has been 23 months for all stage IV breast cancer patients and 31 months for those with hormone receptor positive disease [4].
Targeting the estrogen receptor is the oldest form of targeted therapy. Aromatase inhibitors (AIs) inhibit the synthesis of estrogens from androgens and have been used in postmenopausal breast cancer patients with hormone receptor positive (ER/PR+) disease, resulting in improvements in disease free survival rates when used in the adjuvant setting [5]. Additionally, endocrine therapy is a mainstay in the treatment of patients with ER/PR+ metastatic disease. However, the benefit of such therapy is transient, as virtually all patients will develop disease progression or recurrence while receiving AIs, raising the question of endocrine resistance. Data suggest that endocrine resistance can be due to enhanced signal transduction pathways, such as human epidermal growth factor receptor 2 (HER2) [6], and ras/raf/mitogen-activated protein kinase (MAPK) [7]. While activating mutations of ras and raf are fairly uncommon in breast cancer, activation of this pathway is seen in over 50% of breast carcinoma compared to adjacent normal breast tissue [8–10]. Thus, signal transduction inhibitors have been suggested to restore the sensitivity to endocrine therapy [11].
Sorafenib is a multiple kinase inhibitor against ras/raf/MAPK, platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptors (VEGFR-1, 2, and 3), c-KIT and FLT3 [12]. In two large randomized phase III trials in renal cell carcinoma and hepatocellular carcinoma, sorafenib was shown to significantly prolong progression-free survival (PFS) in patients with metastatic disease [13, 14]. In breast cancer, sorafenib at a dose 400 mg twice daily has little activity as monotherapy [15, 16]. Two recent randomized, placebo-controlled phase IIb studies showed that sorafenib has activity when combined with chemotherapy with a dose of 400 mg twice daily. Sorafenib increased the PFS in patients with metastatic breast cancer when combined with capecitabine [17]. It also improved time to progression in combination with paclitaxel [18]. A crosstalk between ER and growth factor pathways, including ras/raf/MAPK, makes the combination of AIs and sorafenib a potentially powerful combination to overcome endocrine resistance. Results from preclinical studies support this hypothesis, as synergistic activity of letrozole and sorafenib has been observed on breast cancer cells [19].
In order to better evaluate the potential role of sorafenib as a means to overcome resistance to endocrine therapy, we initiated a phase I/II study utilizing sorafenib in combination with anastrozole 1 mg daily in patients with hormone receptor positive, AI-resistant metastatic breast cancer, defined by disease recurrence or progression on an AI. We first conducted a pilot phase I study on six patients to determine the dose of sorafenib. Based on the absence of dose limiting toxicities with the dose of sorafenib 400 mg twice daily, it was chosen for the phase II study. We also conducted an exploratory biomarker analysis on circulating endothelial cells (CECs) and circulating endothelial progenitor cells (CEPs).
Patients and methods
Patients
Thirty-five patients with advanced or metastatic breast cancer were enrolled from Georgetown University Hospital, Medical Oncology Associates of Northern Virginia, Franklin Square Hospital, Yale University and Washington Cancer Institute from July 2005 through March 2009. Women with histologically confirmed estrogen receptor (ER) and/or progesterone receptor (PR) positive (defined as >1% staining by immunohistochemistry or >10 fmol/mg of protein by radio-ligand dextran-coated steroid binding assay), with a history of disease progression on at least one prior aromatase inhibitor (AI) in an adjuvant or in the metastatic setting were eligible. Patients had to have measurable disease by RECIST criteria or patients with bone only disease were eligible if they had lytic bone lesions 10 mm or larger on thin cut CT scan or MRI. Disease recurrence within 6 months of completion of adjuvant AI or progression on AI in the metastatic setting was required. Patients were postmenopausal or premenopausal on LHRH agonists. Unlimited prior endocrine therapies were allowed. A maximum of two prior chemotherapy regimens were allowed for metastatic disease. Patients had to have adequate renal, hepatic, and bone marrow function. Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2 was required. Patients with uncontrolled hypertension were excluded. All patients provided signed informed consent, and the protocol was approved by the institutional review boards (IRB). This study was supported by Avon-NCI PFP Award 3 P30CA051008-15S3 and Bayer Pharmaceutical.
Study design
This phase I/II study of sorafenib with anastrozole was conducted in patients with MBC involving a short phase I portion followed by a Simon's optimal two-stage design [20]. Therapy with sorafenib and anastrozole was given orally every day in 28 day cycles with no rest period. Patients underwent clinical and laboratory evaluation with CBC (complete blood count) and CMP (complete metabolic panel) every 4 weeks. Restaging studies including CT with IV contrast of the chest and abdomen and radionuclide bone scan were performed every 8 weeks. Treatment was continued until disease progression, unacceptable adverse events, patient withdrawal, investigator initiated termination, or subject death. For grade 2 toxicity, the daily dose of sorafenib was reduced by 200 mg increments. For grade 3 and 4 toxicities, sorafenib was held until toxicity resolved to grade 2 or lower and then sorafenib was resumed with a dose reduction as above. Patients with intolerable toxicities while taking 200 mg daily were removed from the study.
The phase I portion of the trial of two sorafenib dose levels was performed to find the maximum tolerated dose (MTD). Escalation in the phase I study followed the 3 + 3 design with exceeding of the MTD if 2 dose limiting toxicities (DLTs) were observed at the given dose during cycle 1. Dose levels 1 and 2 for sorafenib were 200 and 400 mg twice daily for the first dose, respectively, followed by 400 mg twice daily for remaining doses in both dose levels. Anastrozole was given once daily as 1 mg in both cohorts. For the phase II portion, the primary objective was to determine the clinical benefit rate (CBR) of sorafenib in combination with anastrozole. CBR was defined as the number of patients with complete response (CR), partial response (PR), or stable disease (SD) for ≥24 weeks, divided by the number of patients enrolled. Response and progression were assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) [21].
Secondary objectives were to determine toxicities of sorafenib in combination with anastrozole. Toxicities were recorded according to the NCI common toxicity criteria, version 3. Additionally, analysis of circulating endothelial cells (CECs) and circulating endothelial progenitor cells (CEPs) as putative markers for angiogenesis and response to therapy was performed. We selected CD45 negative cells to exclude blood leukocytes. CECs were defined as CD146+/CD45− and CEPs as CD133+/CD45− cells [22, 23]. They were identified through flow cytometry adjusted concentrations of CD146 and CD133 each compared to CD45. CECs and CEPs were measured prior to the first treatment and again between days 6 and 10 of the first cycle.
Statistical analysis
The phase I patients were included in the phase II response assessment based on the optimal Simon design decision rules since the differences in the first dose only were not deemed likely to change CBR. In the first stage, 12 patients were to be enrolled with continuation to 35 patients if at least 2 patients exhibited clinical benefit. The study design assumed a clinical benefit rate of 30% as significantly better than 10% with a one-sided 10% significance and 90% power. However, the treatment was considered beneficial enough for further study if at least 6 patients of the 35 (17%) derived clinical benefit.
The proportion of patients with CBR was calculated from all enrolled patients and reported. Toxicities were tabulated by symptom and grade. The progression-free survival was plotted using the method of Kaplan and Meier [24]. The percent changes in CECs and CEPs were evaluated according to response status: clinical benefit versus progressed. Differences in variability were tested using Bartlett's test of equal variances [25]. Patients removed from study before the first assessment, were excluded from CEC/CEP analyses.
Results
Thirty-five patients were enrolled in the study between July 2005 and May 2009. Details of the patient characteristics are described in Table 1. All enrolled patients had developed disease recurrence while on an AI in the adjuvant setting or disease progression while taking an AI in the metastatic setting. Clinical benefit rate (CBR) was a primary end point of the study. Table 2 presents the response status of patients. Among the 35 enrolled patients, one patient (3%) had PR as best response and seven (20%) had stable disease (SD) for more than 24 weeks, corresponding to a CBR of 23%. This exceeded the minimum number of benefited patients needed to consider this treatment promising for further investigation. Fourteen patients (40%) had progressive disease (PD) before 24 weeks.
Table 1.
Patient characteristics
| Characteristics | N (%) |
|---|---|
| Age | |
| <40 | 1 (3) |
| 40–49 | 7 (20) |
| 50–59 | 17 (49) |
| 60–69 | 5 (14) |
| 70+ | 5 (14) |
| Race/ethnicity | |
| White | 32 (91) |
| Black | 2 (6) |
| American Indian/Alaska | 1 (3) |
| Performance status | |
| 0 | 19 (54) |
| 1 | 15 (43) |
| 2 | 1 (3) |
| Menopausal status at study entry | |
| Premenopausala | 4 (11) |
| Postmenopausal | 31 (89) |
| Lesion | |
| Visceral only | 8 (23) |
| Soft tissue/bone only | 15 (43) |
| Both | 12 (34) |
| Number of prior chemotherapy regimens for metastatic disease | |
| 0 | 19 (54) |
| 1 | 11 (31) |
| 2 | 5 (14) |
| Prior anthracycline | |
| Neoadjuvant/adjuvant | 18 (51) |
| Metastatic | 2 (6) |
| Prior taxane | |
| Neoadjuvant/adjuvant | 12 (34) |
| Metastatic | 3 (9) |
| Prior anthracycline and taxane ever (Note 12 in adjuvant) | 13 (37) |
| Number of prior hormone treatments | |
| 1 | 3 (9) |
| 2 | 9 (26) |
| ≥3 | 23 (66) |
| Prior tamoxifen | |
| Neoadjuvant/adjuvant | 18 (51) |
| Metastatic | 7 (20) |
| Both | 2 (6) |
| Prior anastrozole | |
| Neoadjuvant/adjuvant | 7 (20) |
| Metastatic | 5 (14) |
| Prior letrozole | |
| Neoadjuvant/adjuvant | 3 (9) |
| Metastatic | 22 (63) |
| Prior exemestane | |
| Neoadjuvant/adjuvant | 2 (6) |
| Metastatic | 15 (43) |
Given goserelin or leuprolide
Table 2.
Response to therapy
| Best response status | N (%) |
|---|---|
| Clinical benefit (CB)a | 8 (23) |
| Partial response (PR) | 1 (3) |
| Stable disease ≥24 weeks (SD) | 7 (20) |
| Progressive disease (PD) | 14 (40) |
| Off study without confirmation of CB or PD | 13 (37) |
CB was defined as patients with complete response (CR), partial response (PR) or stable disease (SD) for ≥24 weeks
There were 27 (77%) patients with sorafenib dose reductions, of whom 11 (31%) went off study due to toxicity prior to 24 weeks. The most common AEs (all; Grade 3/4) were fatigue (66%; 17%), diarrhea (63%; 6%), nausea (60%; 9%), and hand-foot syndrome (57%; 34%). Grade 3/4 hypertension occurred in 11%. Table 3 presents these and additional symptoms. Mean daily doses of sorafenib were 556 mg for all patients and 545 mg for patients who derived clinical benefit. Therefore, the potential for treatment benefit with this drug combination was not limited to those able to tolerate the intended dose of sorafenib. This was demonstrated by a patient who remained on this study with stable disease for 70 weeks, 63 weeks of which with a sorafenib dose of 200 mg daily. Another patient, who achieved partial response, remained on a sorafenib dose of 200 mg twice daily for 40 weeks.
Table 3.
Adverse events
| Adverse events | Grade 1 or 2 | Grade 3 or 4 |
|---|---|---|
| Hand-foot syndrome | 8 (23%) | 12 (34%) |
| Fatigue | 17 (49%) | 6 (17%) |
| Rash | 13 (37%) | 4 (11%) |
| Emesis | 10 (29%) | 4 (11%) |
| Hypertension | 7 (20%) | 4 (11%) |
| Nausea | 18 (51%) | 3 (9%) |
| Arthralgias | 6 (17%) | 3 (9%) |
| Diarrhea | 20 (57%) | 2 (6%) |
| Dehydration | 1 (3%) | 2 (6%) |
| Infection | 1 (3%) | 2 (6%) |
| Anorexia | 10 (29%) | 1 (3%) |
| Headache | 8 (23%) | 1 (3%) |
| Mucositis | 7 (20%) | 1 (3%) |
| Elevated liver function tests | 6 (17%) | 1 (3%) |
| Rigors/chills | 4 (11%) | 1 (3%) |
| Joint function | 3 (9%) | 1 (3%) |
| Neutropenia | 2 (6%) | 1 (3%) |
| Dyspnea | 2 (6%) | 1 (3%) |
| Acne | 1 (3%) | 1 (3%) |
| Hypotension | 1 (3%) | 1 (3%) |
| Hypophosphatemia | 0 | 1 (3%) |
| Thrombosis | 0 | 1 (3%) |
| Urticaria | 0 | 1 (3%) |
| Pleural effusion | 0 | 1 (3%) |
| Alopecia | 18 (51%) | 0 |
| Pain—other | 17 (48%) | 0 |
| Constipation | 10 (29%) | 0 |
| Pruritus/itching | 10 (29%) | 0 |
| Sensory neuropathy | 9 (26%) | 0 |
| Weight loss | 9 (26%) | 0 |
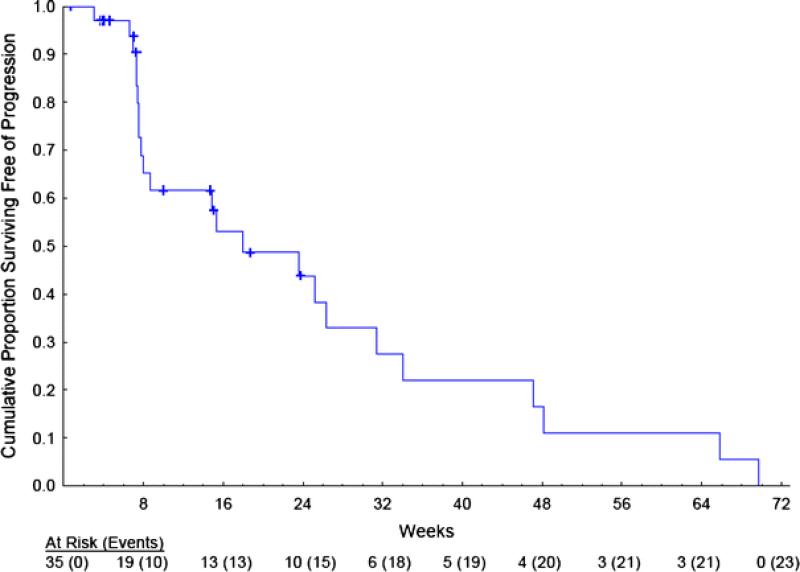
Progression-free survival (PFS) is presented in Fig. 1. The median PFS was 18 weeks, with 44% (Standard Error (SE) = 10%) and 11% (7%) surviving free of progression at 24 and 52 weeks, respectively.
Fig. 1.
Progression-free survival. Censored observations are noted with a “+”
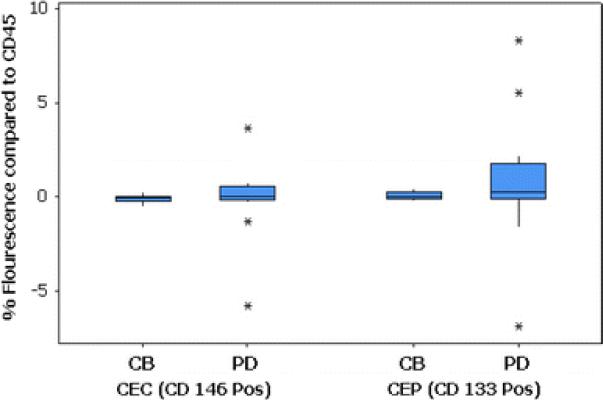
Blood specimens for CECs and CEPs were available for 14 patients. Analysis of CECs and CEPs by flow cytometry showed increased variability among patients with PD. There was no change noted in either CD146 values, CD133 values, or CD146 and CD133 values combined among those with benefit. Figure 2 presents the boxplots of the differences for each measure according to patient outcome. All differences are centered around 0, so no test of differences was performed. The variability is consistently greater among patients with PD compared to those with clinical benefit (P < 0.001 for each). The scientific rationale for such a finding is unclear, but it appears that a fluctuation in either CECs (CD146+/CD45−) or CEPs (CD133+/CD45−) may be detrimental to patient outcome. Further investigation of these relationships may be of interest.
Fig. 2.
Differences before and after therapy in CEC and CEP levels by treatment outcome. Treatment outcome is classified as clinical benefit (CB) and progressive disease (PD)
Discussion
In this study, the addition of sorafenib to anastrozole was associated with an encouraging 23% CBR in patients with ER/PR+ metastatic breast cancer resistant to or progressing while on prior therapy with an AI. This patient population had a high disease burden, demonstrated by 57% of the patients with visceral disease. These patients were also heavily pretreated, as 46% had received 1–2 prior chemotherapy regimens and 66% had received 3 or more prior hormonal therapies. The benefit observed is unlikely due to a direct anti-tumor effect of sorafenib, as a recent phase II trial of single agent sorafenib in metastatic breast cancer was stopped early due to low activity [16], and another phase II trial showed only 13% CBR [15]. The benefit may be due to restoration of sensitivity to AIs, by blocking the crosstalk between ER and growth factor pathways with sorafenib. A recent study combining letrozole and sorafenib on breast cancer cell lines suggested that the anti-proliferative effects of sorafenib involved the inhibition of mammalian target of rapamycin Complex 1 (mTORC1). Furthermore, the synergistic inhibition of cell proliferation was attributed to an enhanced accumulation of cells in the G0/G1 phase and down-regulation of cell cycle regulatory proteins c-myc, cyclin D1, and phospho-Rb [19].
The proportions of patients with dose reductions (77%) and discontinuation (37%) secondary to toxicities were surprisingly high in this study. Although none of the side effects observed in this study represents a new toxicity for these agents, the incidence of adverse events was greater than what would be expected through the use of each agent alone at standard doses. In a study of single agent sorafenib, grade 1 toxicities were seen in 28%, grade 2 in 43%, and grade 3 in 19% of the patients [15]. Given the mean dose of 556 mg for all patients, we conclude that the sorafenib dose of 400 mg twice daily appears too high to be used in combination with anastrozole, and was unfortunately not reflected by the phase I portion of our study. There was no apparent difference between the baseline characteristics of the patient population in the phase I portion and the subsequently enrolled patients. There was no correlation between the mean sorafenib dose for each patient and their response. A dose as low as 200 to 400 mg sorafenib daily in combination with anastrozole may be effective in restoring the sensitivity to aromatase inhibitor. High rates of toxicities have previously been reported when combining sorafenib with other targeted therapies, such as bevacizumab. Interestingly, in addition to dose reductions, intermittent dosing of antiangiogenic agents, has been suggested to overcome the toxicities with combination therapy [26].
Developing blood-based predictive biomarkers is important for patient selection and prediction of treatment response. Circulating endothelial progenitor cells (CEPs), measured by the expression of CD133, have successfully been used to select patients with non-small cell lung cancer who might benefit from combined therapy with sorafenib and erlotinib [27]. High baseline number of circulating endothelial cells (CECs) was also a strong predictive marker for clinical response in a study on metronomic bevacizumab plus chemotherapy in advanced breast cancer patients [28]. Unfortunately, we were unable to show an association between CECs or CEPs and clinical response. However, interestingly, those who responded exhibited strong stability of CEC and CEP levels. It is possible, that flow cytometry is not the optimal method for detecting changes in the CEC/CEP cells. PCR-based methods have been shown to be more sensitive, but less specific than flow cytometry for the detection of CEC/CEP cells [23]. Additionally, measuring CECs and CEPs at different time points may have given more accurate information about the response. Novel predictive biomarkers of angiogenesis that have been described include vascular endothelial growth factor A (VEGF-A) levels, basic fibroblast growth factor (b-FGF), and IL-8 gene SNPs, and these could be incorporated into future studies of sorafenib [28, 29].
In summary, the combination of sorafenib and anastrozole produced an encouraging CBR of 23%, suggesting that sorafenib may be able to restore sensitivity to hormone therapy and, therefore, delay the initiation of chemotherapy in some patients. The combination was associated with significant toxicity, and future trials examining lower doses of sorafenib are warranted. In order to identify the patients who would potentially benefit from addition of sorafenib, novel translational biomarkers are needed.
Acknowledgments
Supported by Avon-National Cancer Institute (NCI) Progress for Patients (PFP) Award 3 P30CA051008-15S3, Bayer Pharmaceutical, and the Clinical Research Management Office, the Flow Cytometry and the Biostatistics & Bioinformatics Shared Resources of Lombardi Comprehensive Cancer Center. The authors gratefully acknowledge the invaluable participation of study patients as well as the research staff Julie Castle, Alissa Mun, Jean Flack, Susie Park, Bernadette Trujillo, Carol Hatch, Mary Steimer, Lois Ravage, Jeannie Kluytenaar, Laurie Thomas, Lauren Callahan, and Jennifer O'Malley. This study was presented in part at the San Antonio Breast Cancer Symposium in 2009 and at 2008 Breast Cancer Symposium, American Society of Clinical Oncology.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I, Tsimelzon A, Weiss H, et al. Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res Treat. 2005;89:173–178. doi: 10.1007/s10549-004-1722-0. [DOI] [PubMed] [Google Scholar]
- 3.Jatoi I, Chen BE, Anderson WF, et al. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25:1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26:4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlebowski R, Cuzick J, Amakye D, et al. Clinical perspectives on the utility of aromatase inhibitors for the adjuvant treatment of breast cancer. Breast. 2009;18(suppl 2):S1–S11. doi: 10.1016/S0960-9776(09)70002-5. [DOI] [PubMed] [Google Scholar]
- 6.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-Her2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 8.Adeyinka A, Nui Y, Cherlet T, et al. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002;8:1747–1753. [PubMed] [Google Scholar]
- 9.Callans LS, Naama H, Khandelwal M, et al. Raf-1 protein expression in human breast cancer cells. Ann Surg Oncol. 1995;2:38–42. doi: 10.1007/BF02303700. [DOI] [PubMed] [Google Scholar]
- 10.Von Lintig FC, Dreilinger AD, Varki NM, et al. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51–62. doi: 10.1023/a:1006491619920. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi G, Loibl S, Zamagni C, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anti-Cancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 16.Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial No. 336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baselga J, Grupo Español de Estudio Tratamiento y Otras Estrategias Experimentales en Tumores Sólidos. Roché H, et al. SOLTI-0701: a double-blind, randomized, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with capecitabine (CAP) in patients (pts) with locally advanced (adv) or metastatic (met) breast cancer (Bc).. Presented at the 15th European Cancer Organization (ECCO) and 34th European Society for Medical Oncology (ESMO) Multidisciplinary Congress; Berlin, Germany. September; 2009. pp. 20–24. 2009. [Google Scholar]
- 18.Gradishar WJ, Kaklamani V, Sahoo TP, et al. A double-blind, randomized, placebo-controlled, phase IIb study evaluating the efficacy and safety of sorafenib in combination with paclitaxel as first-line therapy in patients with locally recurrent or metastatic breast cancer.. Presented at the San Antonio Breast Cancer Symposium; San Antonio, TX. December; 2009. pp. 8–12. 2009. [Google Scholar]
- 19.Bonelli MA, Fumarola C, Alfieri RR, et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-009-0714-5. doi:10.1007/s10549-009-0714-5. [DOI] [PubMed] [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso P, Antoniotti P, Quarna J, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 23.Steurer M, Kern J, Zitt M, et al. Quantification of circulating endothelial and progenitor cells: comparison of quantitative PCR and four-channel flow cytometry. BMC Res Notes. 2008;1:71. doi: 10.1186/1756-0500-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Snedecor G, Cochran W. Statistical methods. Iowa State University Press; Ames Iowa: 1989. pp. 251–252. [Google Scholar]
- 26.Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495–499. doi: 10.1038/sj.bjc.6605514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vroling L, Lind JS, de Haas RR, et al. CD133+ circulating haematopoietic progenitor cells predict for response to sorafenib plus erlotinib in non-small cell lung cancer patients. Br J Cancer. 2009:1–8. doi: 10.1038/sj.bjc.6605477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calleri A, Bono A, Bagnardi V, et al. Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res. 2009;15:7652–7657. doi: 10.1158/1078-0432.CCR-09-1493. [DOI] [PubMed] [Google Scholar]
- 29.Schultheis AM, Lurje G, Rhodes KE, et al. Polymorphisms and clinical outcomes in recurrent ovarian cancer treated with cyclophoshamide and bevacizumab. Clin Cancer Res. 2008;14:7554–7563. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]