Abstract
Alcohol consumption increases the risk of breast cancer among women in the general population, but its effect on women who carry a BRCA gene mutation is unclear. We conducted a case-control study of 1925 matched pairs of predominantly premenopausal women who carry a BRCA1 or a BRCA2 mutation. Information on current alcohol consumption was obtained from a questionnaire administered during the course of genetic counselling or at the time of enrolment. A modest inverse association between breast cancer and reported current alcohol consumption was observed among women with a BRCA1 mutation (OR = 0.82, 95% CI 0.70–0.96), but not among women with a BRCA2 mutation (OR = 1.00; 95% CI 0.71–1.41). Compared to non-drinkers, exclusive consumption of wine was associated with a significant reduction in the risk of breast cancer among BRCA1 carriers (p-trend = 0.01). Alcohol consumption does not appear to increase breast cancer risk in women carrying a BRCA gene mutation.
Keywords: BRCA1, BRCA2, Alcohol, Breast cancer, Case-control, Wine
Introduction
Inherited mutations in the BRCA1 and BRCA2 genes confer significantly increased lifetime risks of breast cancer: in a meta-analysis of 22 studies of BRCA1 and BRCA2 mutation carriers who were unselected for family history, the average cumulative risk of breast cancer up to 70 years of age was 65% (95% CI 44–78%) in BRCA1 carriers and 45% (95% CI 31–56%) in BRCA2 carriers[1] Given the high risk of breast cancer in these women, it is important to find potentially modifiable risk factors that may be integrated into a cancer prevention program.
Alcohol consumption is an established risk factor for breast cancer. Most epidemiological studies have found a dose-dependent increase in breast cancer risk beginning at consumption levels as low as one drink per day. [2–8] However, participants in these studies were women from the general population who were unlikely to carry a mutation in the BRCA1 or BRCA2 genes. The characteristics of the breast cancers that arise in women with BRCA1 or BRCA2 mutations may differ from those of women in the general population and the risk factor profile may be different as well. The objective of this study was to determine if the risk of breast cancer is influenced by alcohol consumption in women with a BRCA1 or BRCA2 mutation.
Materials and methods
Study population and design
Eligible cases and controls were identified from 54 participating centres in eight countries. All women were carriers of a BRCA1 or BRCA2 mutation. These women were participants in ongoing research protocols at the host institutions. In most cases, genetic testing was initially offered to women who had been diagnosed with breast or ovarian cancer. When a BRCA1 or BRCA2 mutation was identified in a proband, genetic testing was offered to other at-risk individuals in the family. A woman was eligible for the current study when molecular analysis established that she was a carrier of a known deleterious mutation in the BRCA1 orBRCA2 gene. Mutation detection was performed using a range of techniques, but all abnormal nucleotide sequences were confirmed by direct sequencing of DNA. Most (>95%) of the mutations identified in the study subjects were either non-sense mutations, deletions, insertions, or small frameshifts resulting in premature termination of the protein. Women with variants of unknown significance were not included. The majority of study participants received counselling and provided written informed consent for genetic testing. The institutional review boards of the host institutions approved the study.
Information on cancer history and lifestyle risk factors was available for 8192 women. Potential subjects were excluded if they had been diagnosed with ovarian cancer (1043 women) or another cancer (681 women), or if information was missing on alcohol consumption (175 women) or on another key variable (87 women). Case subjects were women with a diagnosis of invasive breast cancer. Control subjects were women who were never diagnosed with breast cancer and who were carriers of a mutation in either BRCA1 or BRCA2 (or both). After exclusions, there were 6206 eligible women, including 2707 with breast cancer (potentially eligible cases) and 3503 without breast cancer (potentially eligible controls).
Each case was matched with a single control subject according to year of birth (within one year), mutation in the same gene (BRCA1 or BRCA2), and country of residence. Within Canada, women were also matched on ethnicity (French-Canadian or non-French-Canadian). A control was eligible to be matched to a given case if the date of interview or date of prophylactic mastectomy in the matched control occurred at or after the year of breast cancer diagnosis in the case subject. A total of 1925 matched case-control pairs was generated, including 1480 pairs with BRCA1 mutations and 445 pairs with BRCA2 mutations.
Data collection and definition of variables
Case and control subjects completed a self-administered questionnaire between January, 1992 and February, 2009. The questionnaire was given to the subjects during clinical appointments at the individual study centers or was mailed to the study subject’s home at a later date. The mean time between diagnosis/censoring age and interview was 6.3 years among cases and 6.8 years among controls. The questionnaire was identical in all study centres except two and included items related to ethnicity, family history, reproductive and medical histories, menopausal status, smoking, oral contraceptives and hormone replacement therapy. Subjects were asked whether or not they currently drank alcoholic beverages and, if so, to estimate the number of drinks they consumed per week on average using pre-defined groups of 0–3, 4–9, 10–20, or 20 or more. Subjects who responded that they were not current drinkers were assigned an alcohol consumption value of ‘None’. Because subjects were asked about current drinking habits, we were unable to distinguish between ex-drinkers and never drinkers. From 2007 onwards, the questionnaire included an item for the type of alcohol regularly consumed (wine, beer, liquor, or a combination). Subjects reported their height and weight at 20, 30, and 40 years of age, as well as their maximum attained weight and the age at which they reached this weight. The most recent recorded body weight before breast cancer diagnosis was used to calculate body mass index (BMI) in the case; body weight at the corresponding age was used to determine BMI in the matched control.
Statistical methods
The distributions of continuous and categorical variables were compared between case and control subjects using Student’s t-test and the Chi-square test, respectively. Odds ratios for breast cancer were estimated with regard to current alcohol consumption (yes/no) and to the number of drinks consumed per week. Analyses were stratified by mutation (BRCA1 vs BRCA2), age at diagnosis (<50 vs ≥50 years of age) and by BMI (<25 vs ≥25). Odds ratios were estimated using conditional logistic regression. Odds ratios were also calculated by type of alcohol typically consumed. This analysis was restricted to the pairs in which either both members were non-drinkers, or if one or both of the members consumed alcohol, the type of alcohol was known. All multivariable models were adjusted for ethnicity (French-Canadian, Jewish, other white, other), menopausal status, oral contraceptive use (ever/never), hormone replacement therapy (HRT) use (ever/never), smoking (ever/never), oophorectomy, BMI (<25, 25 to <30, ≥30), and parity (0, 1, 2, 3, 4+). All analyses were done with SAS Version 9.1 (SAS Institute, Cary, NC).
Results
Cases and controls were similar with respect to age at interview, menopausal status, past use of oral contraceptives, smoking, BMI, and parity (Table 1). Controls were significantly more likely than cases to have to have had an oophorectomy (10.7% vs 8.7%, p = 0.04) and to have used hormone replacement therapy (10.8% vs 6.2%, p < 0.0001). Among menopausal women, 24.9% of the cases and 44.0% of the controls had used hormone replacement therapy. Controls had a higher mean age at menopause than cases (44.8 vs 42.7, p = 0.0001); however, among women who had undergone natural menopause, the mean age of menopause was 47.8 years for cases and was 47.4 years for controls.
Table 1.
Characteristics of study participants.
| Characteristic | Controls (N = 1925) | Cases (N = 1925) | p-valuea |
|---|---|---|---|
| Country of study site, N (%) | |||
| Austria | 26 (1.4) | 26 (1.4) | |
| Canada, not French-Canadian | 430 (22.3) | 430 (22.3) | |
| French-Canadian | 141 (7.3) | 141 (7.3) | |
| Israel | 62 (3.2) | 62 (3.2) | |
| Italy | 6 (0.3) | 6 (0.3) | |
| Norway | 45 (2.3) | 45 (2.3) | |
| Poland | 600 (31.2) | 600 (31.2) | |
| UK | 7 (0.4) | 7 (0.4) | |
| USA | 608 (31.6) | 608 (31.6) | |
| Mutation, N (%) | |||
| BRCA1 | 1480 (76.8) | 1480 (76.8) | |
| BRCA2 | 445 (23.1) | 445 (23.1) | |
| Mean year of birth | 1955.9 | 1955.8 | |
| Mean age at diagnosis, years | 40.3 | 40.3 | |
| Mean time between diagnosis and interview, years | 6.8 | 6.3 | |
| Mean age at interview, years | 47.0 | 46.6 | 0.13 |
| Ethnicity, N (%) | |||
| French-Canadian | 133 (6.9) | 150 (7.8) | |
| Jewish | 359 (18.7) | 284 (14.8) | |
| Other | 31 (1.6) | 55 (2.9) | |
| Other White | 1402 (72.8) | 1436 (74.6) | 0.0008 |
| Premenopausal, N (%) | |||
| Yes | 1545 (81.7) | 1526 (80.2) | 0.24 |
| Ever oral contraceptive use, N (%) | |||
| Yes | 1194 (62.3) | 1163 (60.6) | 0.29 |
| Ever smoked, N (%) | |||
| Yes | 829 (43.2) | 833 (43.3) | 0.93 |
| Oophorectomy, N (%) | |||
| Yes | 205 (10.7) | 167 (8.7) | 0.04 |
| Mean age at menopause, years | 44.8 | 42.7 | 0.0001 |
| Ever HRT use, N (%) | |||
| Yes | 206 (10.8) | 119 (6.2) | <0.0001 |
| Mean BMI | 24.1 | 24.4 | 0.07 |
| Mean parity | 2.0 | 1.9 | 0.52 |
HRT = Hormone replacement therapy; BMI = Body mass index in kg/m2.
p-value for difference between breast cancer cases and controls (based on Student’s t-test for continuous variables and Chi-square test for binary variables).
Current alcohol consumption was more commonly reported among controls than among cases (60.9% vs 57.3%, p < 0.02) (Table 2). Overall, fewer than 3% of participants consumed 10 or more drinks per week. Average alcohol consumption varied widely by country, with Israel having the lowest average consumption (99% non-drinkers) and the United Kingdom the highest (29% of those from the United Kingdom consumed 10 or more drinks per week) (Fig. 1). For some countries, the estimates were based on small numbers of women.
Table 2.
Frequency of alcohol consumption and types of alcohol regularly consumed among cases and controls.
| Measure of Alcohol Consumption | Controls (N = 1925) | Cases (N = 1925) | p-valuea |
|---|---|---|---|
| Current alcohol consumption, N (%) | |||
| Yes | 1172 (60.9) | 1102 (57.3) | 0.02 |
| Number of drinks consumed per week, N (%) | |||
| None | 753 (39.1) | 823 (42.8) | |
| 0–3 | 916 (47.6) | 849 (44.1) | |
| 4–9 | 193 (10.0) | 206 (10.7) | |
| ≥10 | 63 (3.3) | 47 (2.4) | 0.04 |
| Type of Alcohol, N (%) | Controls (N = 1141) | Cases (N = 1141) | |
| None | 612 (53.6) | 666 (58.4) | |
| Wine only | 218 (19.1) | 173 (15.2) | |
| Wine and otherb | 201 (17.6) | 211 (18.5) | |
| Otherb | 110 (9.6) | 91 (8.0) | 0.02 |
based on Chi-squared test.
Includes beer and spirits.
Fig. 1.
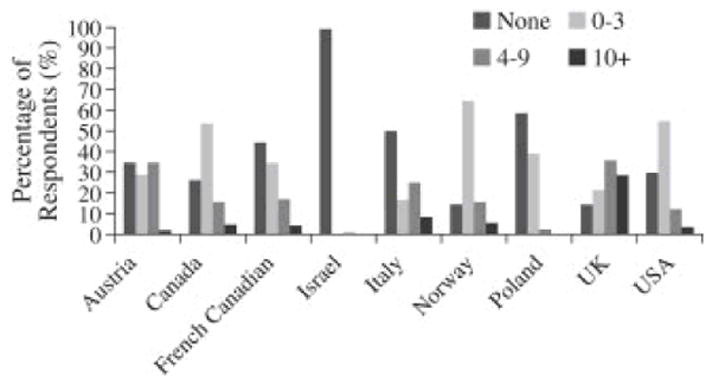
Variation in the number of drinks consumed per week by country of study site UK = United Kingdom; USA = United States of America.
Among women with a BRCA1 mutation, current alcohol consumption was more common among controls than among cases (58.7% vs 54.4%, p = 0.02). In a multivariable model, the odds ratio for breast cancer associated with current alcohol consumption was 0.82 (95% CI 0.70–0.96), and a significant trend of decreasing risk with increasing drinks per week was observed (p-trend = 0.03) (Table 3). When analyses were restricted to the 1034 pairs in which both the case and control consumed alcohol, the trend in multivariable odds ratios was no longer significant (p-trend = 0.50), although the sample size was considerably reduced. The association was not appreciably modified by age at diagnosis or BMI.
Table 3.
Association between breast cancer risk and the number of alcoholic drinks consumed per week overall and by the type(s) of alcohol typically consumed among BRCA1 and BRCA2 mutation carriers.
| Model | Number of Alcoholic Drinks Consumed per Week
|
||||
|---|---|---|---|---|---|
| None | 0–3 | 4–9 | ≥10 | p-trend | |
| BRCA1 (n = 1480 pairs) | |||||
| Cases/controls | 675/612 | 640/706 | 135/118 | 30/44 | |
| Multivariable OR (95% CI)a | 1.00 | 0.77 (0.67–0.94) | 0.98 (0.73–1.32) | 0.55 (0.33–0.91) | 0.03 |
| BRCA1 (n = 895 pairs with information on type of alcohol consumed)b | |||||
| Exclusive wine consumers | |||||
| Cases/controls | 543/501 | 89/127 | 18/20 | 4/7 | |
| Multivariable OR (95% CI)a | 1.00 | 0.62 (0.45–0.87) | 0.82 (0.41–1.67) | 0.39 (0.11–1.45) | 0.01 |
| Wine and other alcohol typesc | |||||
| Cases/controls | 543/501 | 126/114 | 35/27 | 7/7 | |
| Multivariable OR (95% CI)a | 1.00 | 1.05 (0.78–1.42) | 1.10 (0.61–1.96) | 0.64 (0.21–1.99) | 0.79 |
| Other alcohol typesc | |||||
| Cases/controls | 543/501 | 60/80 | 10/9 | 3/3 | |
| Multivariable OR (95% CI)a | 1.00 | 0.62 (0.43–0.91) | 1.07 (0.40–2.85) | 0.70 (0.13–3.75) | 0.08 |
| BRCA2 (n = 445 pairs) | |||||
| Cases/controls | 148/141 | 209/210 | 71/75 | 16/18 | |
| Multivariable OR (95% CI)a | 1.00 | 0.97 (0.67–1.41) | 1.04 (0.67–1.63) | 1.16 (0.55–2.45) | 0.72 |
| BRCA2 (n = 246 pairs with information on type of alcohol consumed)b | |||||
| Exclusive wine consumers | |||||
| Cases/controls | 123/111 | 41/43 | 18/17 | 3/4 | |
| Multivariable OR (95% CI)a | 1.00 | 1.09 (0.60–1.95) | 1.12 (0.49–2.60) | 0.50 (0.08–3.02) | 0.92 |
| Wine and other alcohol typesc | |||||
| Cases/controls | 123/111 | 28/33 | 11/16 | 4/4 | |
| Multivariable OR (95% CI)a | 1.00 | 0.90 (0.44–1.82) | 0.81 (0.34–1.93) | 1.38 (0.31–6.15) | 0.68 |
| Other alcohol typesc | |||||
| Cases/controls | 123/111 | 12/10 | 3/8 | 3/0 | |
| Multivariable OR (95% CI)a | 1.00 | 1.19 (0.41–3.45) | 0.54 (0.12–2.34) | N/A | 0.79 |
HRT = Hormone replacement therapy; BMI = Body mass index in kg/m2.
Adjusted for ethnicity (other white, French-Canadian, Jewish, other), menopause, oral contraceptive use, HRT use, smoking, oophorectomy, BMI (<25, 25 to <30, ≥30), and parity (0, 1, 2, 3, 4+).
Non-drinkers were included if their matched pair was also a non-drinker, or if their matched pair was a drinker and had information on the type of alcohol consumed.
Includes beer and spirits.
Among women with a BRCA2 mutation, cases and controls were equally likely to consume alcohol (68.3% vs 66.7%, p = 0.62), with the multivariable odds ratio associated with current alcohol consumption estimated to be 1.00 (95% CI 0.71–1.41). The association between the number of drinks consumed per week and breast cancer risk was not significant (p-trend = 0.72), nor was it modified by restricting to pairs in which both the case and control consumed alcohol, by age at diagnosis, or by BMI.
The principal type of alcohol consumed was known for 1141 case-control pairs. Thirty-nine percent of women who drank consumed wine exclusively, ranging from 0% in the United Kingdom to 100% in Italy. Among BRCA1 carriers, consumers of wine exclusively had a reduced risk of breast cancer compared to non-drinkers (multivariable OR = 0.64, 95% CI 0.47–0.87), whereas women who did not consume wine exclusively had a risk of breast cancer that was similar to that of non-drinkers (multivariable OR = 0.89, 95% CI 0.70–1.12). A significant decreasing trend in the risk of breast cancer was seen with increasing weekly alcohol consumption for consumers of wine exclusively, but not for the other subgroups of types of alcohol consumed (Table 3). Among women with a BRCA2 mutation, the odds ratios for breast cancer associated with exclusive and non-exclusive wine consumption compared to no alcohol consumption were 1.01 (95% CI 0.61–1.69) and 0.88 (95% CI 0.53–1.48), respectively.
Discussion
In this study, we found that alcohol consumption did not increase the risk of breast cancer among women with BRCA1 or BRCA2 gene mutations. Increasing alcohol consumption was associated with a modest reduction in the risk of breast cancer among women with BRCA1 mutations. This association was restricted to consumers of wine exclusively.
Our findings are contrary to what is seen in the general population, where alcohol consumption increases breast cancer risk. This difference may be explained by the young age at diagnosis, premenopausal status, and estrogen receptor (ER) status of tumors among women in the present study. In the general population, the association between alcohol consumption and breast cancer risk is weaker among women diagnosed at a young age [2,9,10] and among premenopausal women than among post-menopausal women[2,5,11] Alcohol consumption increases the levels of circulating estrogens[12–14] and has been more strongly associated with ER-positive tumors than with ER-negative tumors,[6,9] the latter which account for approximately 80% of tumors in BRCA1 mutation carriers.[15]
A previous study of BRCA1 and BRCA2 mutation carriers less than 50 years of age from the United States, Canada, and Australia also found no increase in the risk of breast cancer associated with ever, current, increasing drink-years, or increasing number of grams per day of alcohol consumption. [16] Women were interviewed within five years of breast cancer diagnosis. Although alcohol type was considered in this study, it may have been underpowered to detect an association between breast cancer risk and wine consumption, due to the low prevalence of wine consumers (Fig. 2).
Fig. 2.
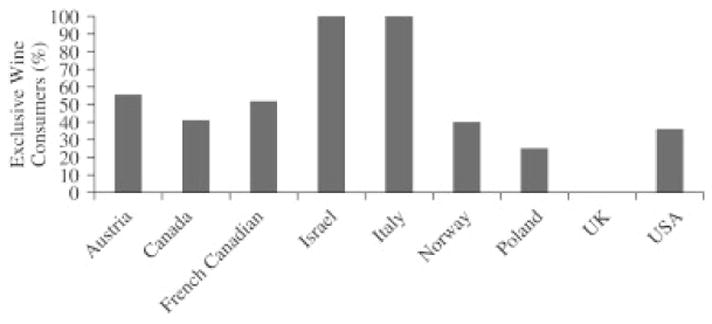
Prevalence of exclusive wine consumers among those who reported consuming alcohol by country UK, United Kingdom; USA, United States of America.
Some studies among women in the general population have found a null or even protective effect of wine consumption on breast cancer risk.[6,17,18] This effect has been attributed to polyphenols in wine, and particularly to resveratrol, a phytoalexin produced by grape vines in response to injury and found in red wine.[19–21] In vitro, resveratrol inhibits tumor initiation and progression by inducing cell cycle arrest and apoptosis.[20,22,23] One pathway by which this occurs is mediated by BRCA1. As a phytoestrogen, resveratrol binds to the estrogen receptor and up-regulates transcription of BRCA1 and BRCA1-associated proteins in human breast cancer cell lines.[23–24] In mouse models, resveratrol is a potent inhibitor of the initiation and progression of BRCA1 mutant cancer, suggesting that resveratrol may be a potential chemopreventive agent for women with BRCA1 mutations.[22]
The major strength of this study is the large sample size of women with known BRCA mutations. It provides one of the rare assessments of the association between alcohol consumption and breast cancer risk among women with BRCA gene mutations. However, our study has several limitations, including the possibility of recall bias and under-reporting of alcohol consumption. Participants in this study reported current alcohol consumption patterns, which may not reflect exposure prior to diagnosis. We did not have information on alcohol consumption patterns earlier in life. However, recent prospective, population-based evidence suggests that alcohol consumption patterns do not change appreciably following breast cancer diagnosis.[25] Furthermore, if the present results were driven by a reduction in alcohol consumption following breast cancer diagnosis, we would expect to see a protective effect among BRCA2 mutation carriers as well. Using non-drinkers as the referent group may have induced a spurious association between alcohol consumption and breast cancer risk, as non-drinkers may differ systematically from the rest of the population in other behaviours associated with breast cancer risk. Our analysis restricted to drinkers only did not find an association between alcohol consumption and breast cancer risk among BRCA1 mutation carriers, but may have been underpowered to do so. In particular, there were few study subjects who reported a high level of consumption of wine. This study is also limited by the crude measures of alcohol consumption, and no information was available on red versus white wine consumption. The study population was young and predominantly premenopausal; this may influence the generalizability of the results to older BRCA1 andBRCA2 carriers.
If breast cancer patients who consumed alcohol experienced reduced survival, compared to non-drinkers, the present analysis may be susceptible to survivor bias, which could explain the inverse association observed among BRCA1 mutation carriers. To assess the possibility of survival bias, we restricted analyses to the 794 BRCA1 and 247 BRCA2 pairs in which the case had completed the questionnaire within five years of breast cancer diagnosis. The odds ratio associated with current alcohol consumption was 0.73 (95% CI 0.58–0.92) for BRCA1 carriers and 0.87 (95% CI 0.54–1.34) for BRCA2 carriers, similar to results for the entire study population. Furthermore, if survival bias was an explanation for the results, we would have expected to see an inverse association with alcohol consumption among BRCA2 mutation carriers as inBRCA1 carriers. Lastly, in two studies that have investigated the relationship between breast cancer survival and alcohol consumption, no association was found. [26–27]
In conclusion, we found that alcohol consumption did not increase the risk of breast cancer among women with a BRCA1 or BRCA2 mutation. Increasing consumption of wine was associated with a modest reduction in the risk of breast cancer among women with a BRCA1 mutation. The possible protective effect of wine found in this study is in accordance with some, but not all studies of women in the general population and provides support for the potential role of resveratrol in BRCA1-associated breast cancer chemoprevention. Further studies in this area are needed to confirm this apparent protective effect of wine consumption against breast cancer among women with a BRCA1 mutation. These studies should include large numbers of BRCAmutation carriers followed over time for incident breast cancers. Detailed alcohol consumption information should be collected, including adistinction between red and white wine consumption.
Acknowledgments
Role of the funding source
The funding sources had no input in the study design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
This research was supported by the Canadian Breast Cancer Research Alliance. D. Krewski holds the Natural Sciences and Engineering Research Council of Canada Chair in Risk Science at the University of Ottawa. J. Little holds a Canada Research Chair in Human Genome Epidemiology.
Footnotes
Ethics statement
This study has been approved by the appropriate ethical committees related to the institutions in which it was performed. Subjects gave informed consent.
Conflict of interest statement
The authors have no conflicts of interest.
Other members of the Hereditary Breast Cancer Clinical Study Group: Barry Rosen, Olufunmilayo Olopade, Fergus Couch, Ruth Gershoni-Baruch, Teresa Wagner, Howard Saal, Wendy Meschino, Amber Trivedi, Dawna Gilchrist, Charis Eng, Jeffrey Weitzel, Wendy McKinnon, Marie Wood, Barbara Pasini, Michael Osborne, Boris Pasche, Taya Fallen, Beth Karlan, Raluca N Kurz, Edmond Lemire, Jane Mclennan, Gareth Evans, Tomas Byrski, Tomas Huzarski, Lee Shulman, Eitan Friedman, Mary Daly, Judy Garber, Andrea Eisen, Louise Bordeleau, Carey Cullinane, Dana Zakalik, Ophira Ginsburg, Rochelle Demsky, Seema Panchal.
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer-collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009 Mar 4;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 4.Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5:73–82. doi: 10.1007/BF01830729. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studie. JAMA. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol. 2007;165:667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 7.Tjonneland A, Christensen J, Olsen A, Stripp C, Thomsen BL, Overvad K, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2007;18:361–373. doi: 10.1007/s10552-006-0112-9. [DOI] [PubMed] [Google Scholar]
- 8.Ellison RC, Zhang Y, McLennan CE, Rothman KJ. Exploring the relation of alcohol consumption to risk of breast cancer. Am J Epidemiol. 2001;154:740–747. doi: 10.1093/aje/154.8.740. [DOI] [PubMed] [Google Scholar]
- 9.McDonald JA, Mandel MG, Marchbanks PA, Folger SG, Daling JR, Ursin G, et al. Alcohol exposure and breast cancer: results of the women’s contraceptive and reproductive experiences study. Cancer Epidemiol Biomarkers Prev. 2004;13:2106–2116. [PubMed] [Google Scholar]
- 10.Kropp S, Becher H, Nieters A, Chang-Claude J. Low-to-moderate alcohol consumption and breast cancer risk by age 50 years among women in Germany. Am J Epidemiol. 2001;154:624–634. doi: 10.1093/aje/154.7.624. [DOI] [PubMed] [Google Scholar]
- 11.Longnecker MP, Newcomb PA, Mittendorf R, Greenberg ER, Clapp RW, Bogdan GF, et al. Risk of breast cancer in relation to lifetime alcohol consumption. J Natl Cancer Inst. 1995;87:923–929. doi: 10.1093/jnci/87.12.923. [DOI] [PubMed] [Google Scholar]
- 12.Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722–727. doi: 10.1093/jnci/85.9.722. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldi S, Peeters PH, Bezemer ID, Dossus L, Biessy C, Sacerdote C, et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control. 2006;17:1033–1043. doi: 10.1007/s10552-006-0041-7. [DOI] [PubMed] [Google Scholar]
- 14.Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, et al. Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93:710–715. doi: 10.1093/jnci/93.9.710. [DOI] [PubMed] [Google Scholar]
- 15.Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 16.McGuire V, John EM, Felberg A, Haile RW, Boyd NF, Thomas DC, et al. No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA1 and BRCA2 mutations ages <50 years. Cancer Epidemiol Biomarkers Prev. 2006;15:1565–1567. doi: 10.1158/1055-9965.EPI-06-0323. [DOI] [PubMed] [Google Scholar]
- 17.Bessaoud F, Daures JP. Patterns of alcohol (especially wine) consumption and breast cancer risk: a case-control study among a population in Southern France. Ann Epidemiol. 2008;18:467–475. doi: 10.1016/j.annepidem.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Newcomb PA, Nichols HB, Beasley JM, Egan K, Titus-Ernstoff L, Hampton JM, et al. No difference between red wine or white wine consumption and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1007–1010. doi: 10.1158/1055-9965.EPI-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchini F, Vainio H. Wine and resveratrol: mechanisms of cancer prevention? Eur J Cancer Prev. 2003;12:417–425. doi: 10.1097/00008469-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006 Apr;7(4):423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 21.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 22.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Corre L, Fustier P, Chalabi N, Bignon YJ, Bernard-Gallon D. Effects of resveratrol on the expression of a panel of genes interacting with the BRCA1 on cosuppressor in human breast cell lines. Clin Chim Acta. 2004;344:115–121. doi: 10.1016/j.cccn.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Fustier P, Le Corre L, Chalabi N, Vissac-Sabatier C, Communal Y, Bignon YJ, et al. Resveratrol increases BRCA1 and BRCA2 mRNA expression in breast tumour cell lines. Br J Cancer. 2003;89:168–172. doi: 10.1038/sj.bjc.6600983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skeie G, Hjartaker A, Braaten T, Lund E. Dietary change among breast and colorectal cancer survivors and cancer-free women in the Norwegian Women and Cancer cohort study. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9390-3. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–835. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]


