Abstract
Human exposure to cold stimulates cutaneous vasoconstriction by activating both sympathetic reflex and locally mediated pathways. Older humans are vulnerable to hypothermia because primary aging impairs thermoregulatory cutaneous vasoconstriction. This article highlights recent findings discussing how age-related decrements in sympathetic neurotransmission contribute directly to thermoregulatory impairment, whereas changes in local cold-induced intracellular signaling suggest a more generalized age-associated vascular dysfunction.
Keywords: skin blood flow, aging, temperature regulation, adrenergic, Rho kinase, cold
INTRODUCTION
Cutaneous vasoconstriction (VC) is the initial thermoregulatory response to cold exposure, minimizing convective heat loss to the environment through distinct reflex and local pathways that work both independently and cooperatively to maximize VC. Whole-body cooling evokes sympathetic reflex VC, which is dependent on the release of norepinephrine (NE) and cotransmitters from perivascular sympathetic adrenergic nerve terminals (24,25). In contrast, localized cooling of the cutaneous blood vessels and surrounding tissue engages local (i.e., nonreflex) cold-induced VC that is mediated primarily by NE at α2-adrenoceptors (3,9,17,20) and by Rho kinase (ROCK) (29), along with a proposed passive constriction via nitric oxide (NO) withdrawal (13).
Cutaneous VC responses to cold exposure are impaired in aged skin, leading to higher skin blood flows during cold exposure and rendering older humans more susceptible to excessive heat loss and, ultimately, hypothermia (6,7,10,18,26). Indeed, recent statistics indicate that people over the age of 65 account for fully half of all cold-exposure deaths each year (4). Over the past several years, we have systematically explored the mechanisms through which this heat-conserving response becomes impaired with aging, culminating in a working model that suggests that decrements in sympathetic neurotransmission contribute directly to thermoregulatory impairment, whereas changes in local cold-induced intracellular signaling indicate a more generalized age-associated vascular dysfunction.
REFLEX VASOCONSTRICTION
Mechanisms Mediating Reflex Vasoconstriction in Young Skin
Human cutaneous blood vessels are innervated by sympathetic adrenergic vasoconstrictor nerves that participate in the reflex VC response. The 19th century French physiologist Claude Bernard, well known for his study of vasomotor nerves, originally pioneered the notion of sympathetic innervation of thermoregulatory vasculature. His conclusions laid the groundwork for later in vivo human research, which confirmed that reflex sympathetic outflow is responsible for not only resting vessel tone but also the pronounced cutaneous VC observed during cooling of the skin (21).
Cutaneous reflex vasoconstriction in humans occurs most often when mean skin temperature decreases below a thermoneutral point (~34°C) because of either convective (cold air) or conductive (cold wet clothes and cold surfaces) heat transfer to the environment. A decrease in core temperature can also bring about reflex VC, although core cooling in the absence of skin cooling only occurs under special medical circumstances, such as surgical anesthesia. Reflex VC is a graded response, where the intensity of the response mirrors the intensity of the cold stimulus until blood flow reaches a basement plateau, after which further cooling will not induce further VC. In controlled laboratory experiments, the effects of whole-body cooling (reflex VC) are isolated from the effects of local changes in skin temperature (locally mediated VC) by inducing reflex VC with whole-body cooling. Skin blood flow is then recorded at a site where local skin temperature has been artificially maintained at 34°C — that is, any VC that occurs at those warmed sites could only be attributed to reflex pathways because local VC mechanisms would not be engaged.
Sympathetic reflex VC is mediated by efferent skin sympathetic nerve signals traveling to cutaneous sympathetic axon terminals, stimulating the release of neurotransmitters and cotransmitters from perivascular nerves. Through the use of iontophoresis and intradermal microdialysis, various pharmacological agents can be delivered to localized areas of skin to identify the transmitters contributing to cutaneous reflex VC. Localized intradermal applications of bretylium tosylate, yohimbine, and propranolol (antagonists of presynaptic neurotransmitter release, α-adrenoceptors, and β-adrenoceptors, respectively) have revealed that, although the VC response to whole-body cooling is entirely dependent on the sympathetic release of transmitters, only 60% of VC is mediated by NE. These findings provide in vivo evidence supporting the participation of sympathetic cotransmitter(s) in cutaneous reflex VC to cold (24–26). Stephens and colleagues (23) provided evidence that neuropeptide Y (which is costored with NE in sympathetic axon terminals) may operate as a sympathetic cotransmitter; it is unknown whether other transmitters that are costored with NE, such as adenosine triphosphate, may participate in this response as well.
It is still unclear how sex hormones may effect the mechanisms driving reflex cutaneous VC to cold. There is evidence that women in the high-hormone phase of oral contraceptives exhibit the same ratio of NE to cotransmitter-mediated VC that men do, whereas women in the low-hormone placebo phase of the pill tend to rely almost entirely on NE to effect the same magnitude of VC (25). However, when normally menstruating women were tested under a similar protocol during the early follicular phase (low hormone state), there was no difference in either the magnitude of VC or the individual contributions of sympathetic transmitters (26). The disparity between these findings suggests that further work is warranted in the study of the effects of sex hormones to clarify the roles of synthetic versus endogenous female reproductive hormones on reflex vasoconstrictor function.
Mechanisms Mediating Reflex Vasoconstriction in Aged Skin
In 1977, thermoregulatory researcher K.J. Collins and colleagues (6) noted that hypothermia was an increasingly common medical concern among the elderly: “[A]ccidental hypothermia is now recognized as one of the natural hazards of old age. The problem is not simply one of unintentional accidental hypothermia resulting from a fall or accident at home and subsequent immobilization and exposure, nor one entirely associated with concurrent illness; spontaneous hypothermia also occurs among apparently fit elderly people.” Their longitudinal study investigating the aging process as it specifically pertained to thermoregulation concluded that cutaneous VC to cooling is impaired in older humans, predisposing them to hypothermia (6).
Subsequent thermoregulatory studies not only confirmed these findings but also characterized and documented this decrement in VC function in greater detail (7,10,18,26). The cumulative finding of these studies and many others — that reflex cutaneous VC is markedly impaired in aged skin, regardless of how the cold stress is induced or how skin blood flow is measured — suggests that pronounced cutaneous vasomotor dysfunction is widespread in older populations.
Cutaneous sympathetic VC is compromised in aged skin because of impaired function at multiple points along the efferent arm of the reflex (Fig. 1). Thermoregulatory control of sympathetic vasoconstrictor nerves is decreased with advancing age. Specifically, cold-induced increases in skin sympathetic nerve activity were impaired in older subjects (65–81 yr) when compared with data from young (18–29 yr) and middle-aged (38–51 yr) control subjects during whole-body cooling (11). Thus, the efferent reflex signal for cutaneous VC to cold exposure is significantly weaker in aged skin, likely contributing to a depressed axonal release of NE (10). After neurotransmitter/cotransmitter release from sympathetic axon terminals, the cutaneous vascular response to these transmitters is similarly impaired. Reflex VC in young skin is mediated by both NE (60%) and cotransmitters (40%) released from sympathetic nerves; however, the cotransmitter portion of VC is abolished in aged skin, indicating that older subjects rely entirely on NE to stimulate the reflex vascular response to cold (26). Additionally, NE-mediated VC is also significantly impaired in aged skin (up to 50%), with aged populations exhibiting a blunted response to both physiological and maximal doses of NE (10,26,28). Cumulatively, these findings suggest that the age-associated decrement in thermoregulatory reflex VC is attributable to several factors: 1) reduced efferent sympathetic signal, 2) reduced sympathetic release of NE, 3) a complete loss of functional sympathetic cotransmitters, and 4) a significant loss of both end-organ sensitivity and maximal response to NE. It is unclear how adrenoceptor-mediated signaling pathways may be affected by aging, warranting further research to determine the intracellular mechanism(s) of blunted end-organ responsiveness in the cutaneous circulation.
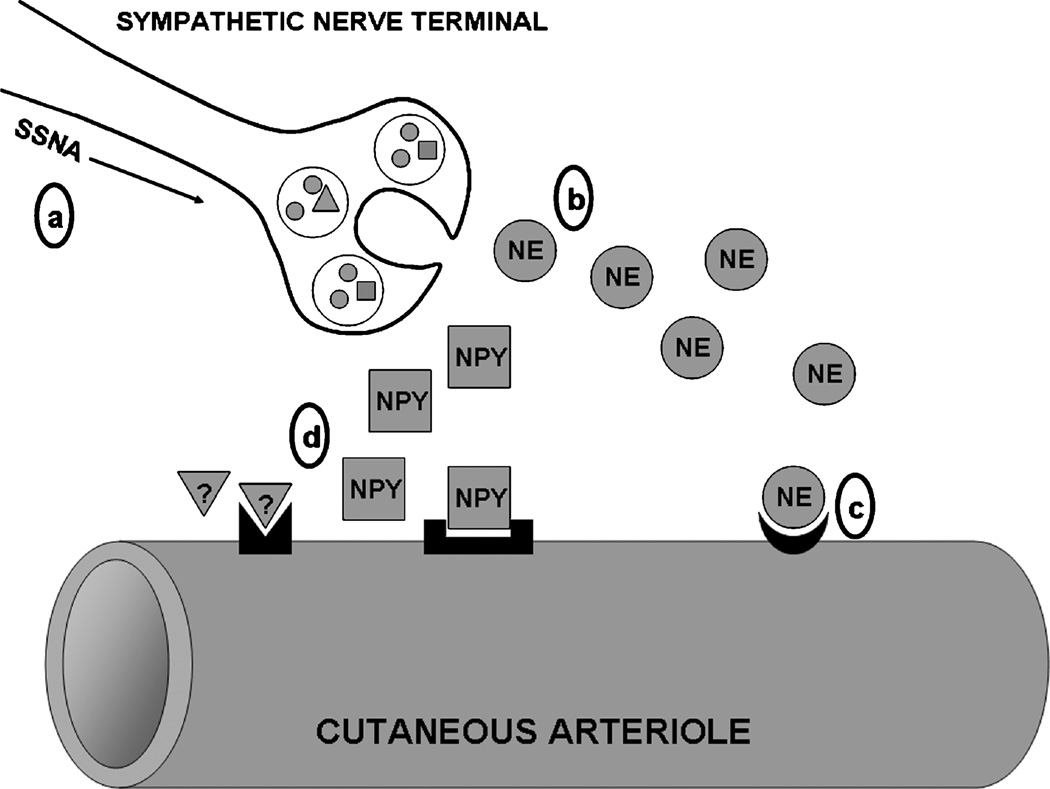
Figure 1.
Schematic representation of sites of age-associated impairment in the sympathetic reflex thermoregulatory vasoconstriction (VC) response to cold. Efferent skin sympathetic nerve activity (SSNA) is reduced (a), resulting in decreased perivascular nerve stimulation and attenuated norepinephrine (NE) release (b). End-organ adrenoceptor-mediated VC is reduced because of a decrease in both NE sensitivity and maximal response in aged vessels (c). Sympathetic cotransmitter contributions (NPY indicates neuropeptide Y; question mark, other putative unidentified cotransmitters) to VC are abolished in aged skin (d), although it is unclear whether this is due to changes in transmitter synthesis/release, receptor expression, or vascular intracellular signaling.
LOCAL VASOCONSTRICTION
Mechanisms Mediating Local Cooling-Induced Vasoconstriction in Young Skin
In contrast to reflex VC that is elicited by whole-body cooling, localized cooling of the cutaneous blood vessels and surrounding tissue engages local VC mechanisms, independent of efferent sympathetic reflex activity (9,20). During the early phase of localized skin cooling (0–10 min), VC is dependent on NE (possibly from basal sympathetic activity) acting at α2-adrenoceptors (9). Early-phase VC also seems to be dependent on intact sensory nerves (12), although the signaling pathway for this effect is still unclear. If localized cooling persists (>15 min), maintenance VC is primarily mediated by nonadrenergic and nonneuronal mechanisms, suggesting that cooling may alter signaling pathways within the vascular smooth muscle, including a down-regulation of the NO synthase (NOS) pathway (13,17,20).
In vitro models of direct cutaneous vessel cooling have implicated ROCK as a key intracellular mediator of cold-induced VC. Localized cutaneous vessel cooling stimulates the production of mitochondrial superoxide, which up-regulates the RhoA/ROCK pathway (2). ROCK, in turn, can augment VC through two distinct mechanisms: 1) inhibition of myosin light chain phosphatase (MLCP), passively permitting phosphorylation of myosin light chain in the absence of a Ca2+ influx (also referred to as “Ca2+ sensitivity”) and 2) translocation of α2C-adrenoceptors from the Golgi to the surface of the cell, leading to 5-fold increase in the adrenoceptor population available to bind with NE during cutaneous tissue cooling (1,2,5,16).
Recent in vivo work based on these in vitro findings has confirmed that ROCK participates in both adrenergic and nonadrenergic phases of locally mediated cutaneous VC, likely through α2C translocation and Ca2+ sensitivity, respectively ([15,29]; Fig. 2). This finding also provides additional support for the putative cold-mediated down-regulation of the NOS pathway (13). The Rho/ROCK and endothelial NOS (eNOS) pathways are mutually inhibitory; cyclic GMP-dependent protein kinase (PKG, a downstream product of NO metabolism) inhibits Rho activation and ROCK phosphorylation of MLCP, whereas Rho and ROCK down-regulate eNOS expression and activity (19,22), maintaining a necessary balance between dilator and constrictor influences in the vasculature (Fig. 3). A cold-mediated increase in ROCK activity would likely decrease NO production, further up-regulating ROCK, thereby strengthening the effectiveness of cold-mediated VC. However, the intricate interplay between these two signaling pathways in the cutaneous vascular response to cold requires further investigation.
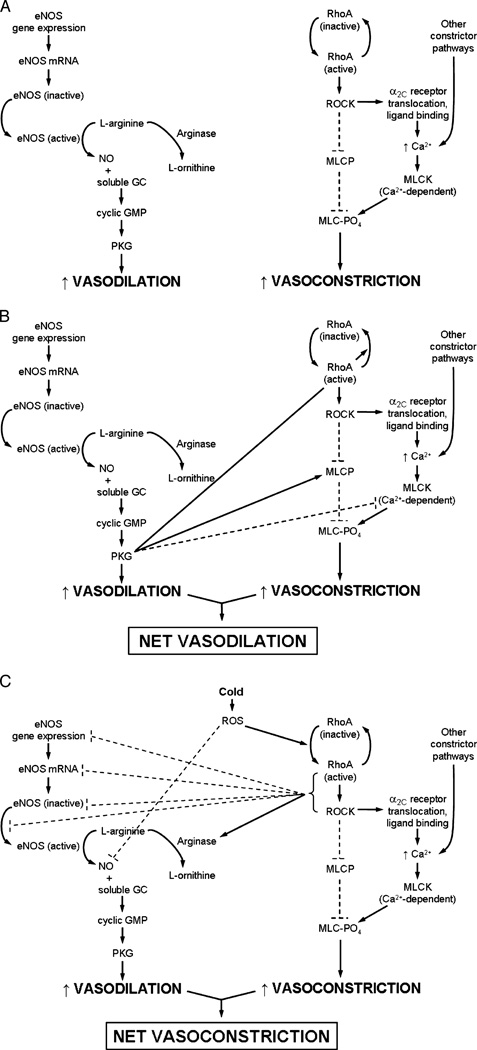
Figure 3.
Pathway schematic of endothelial nitric oxide synthase (eNOS)–mediated vasodilation and RhoA/Rho kinase (ROCK)–mediated vasoconstriction in cutaneous vessels and the putative interactions between the two pathways. A. Dilation occurs through eNOS activation, which catalyzes the conversion of l-arginine into nitric oxide (NO). Nitric oxide then binds to soluble guanylyl cyclase (GC) and increases cyclic GMP and cyclic GMP-dependent protein kinase (PKG) activity, ultimately resulting in cutaneous smooth muscle relaxation. Although arginase could potentially reduce NO production because it competes with eNOS for its substrate, arginase activity is negligible in young healthy vessels. Constriction through the RhoA/ROCK pathway occurs through RhoA/ROCK activation, which leads to the following: 1) a decrease in myosin light chain (MLC) phosphatase (MLCP) activity and 2) an increase in Ca2+-mediated MLC kinase (MLCK) activity via α2C-adrenoceptor translocation and norepinephrine binding (this pathway seems to be only active under cold conditions). Either the decrease in MLCP activity or the increase in MLCK activity leads to a net increase in MLC phosphorylation, ultimately resulting in smooth muscle contraction. B. When eNOS activity predominates in cutaneous vessels, NO drives dilation via its conventional pathway as well as through the anticonstrictor effects of PKG: directly deactivating RhoA and acting on both MLCP and MLCK to decrease the phosphorylation state of MLC. C. Cold-induced production of reactive oxygen species (ROS) activates RhoA/ROCK and leads to prolonged cutaneous constriction, which is mediated through ROCK’s established effects on MLCP and α2C translocation and possibly through its antidilator effects on the eNOS pathway. RhoA/ROCK can decrease NO bioavailability by decreasing eNOS transcription, translation, and activity and by activating arginase. It is likely that increases in ROS production may also quench NO before it can bind with soluble GC. Thus, cold- and age-induced increases in ROS production and RhoA/ROCK activity and decreases in NO production may perpetuate a cycle of proconstrictor activity by stimulating RhoA/ROCK and removing NO-mediated inhibition, respectively. Broken lines indicate inhibitory effects; continuous lines, stimulatory/activation effects.
Studies investigating sex differences in this response indicate that women rely more heavily on α2-adrenoceptors to achieve local cold-induced VC compared with men (3). A recent study by Eid et al. (8) offers an explanation for this phenomenon: estrogen, in the absence of cold, is a potent activator of α2C-adrenoceptor expression in vascular smooth muscle cells, and estrogen exposure during cutaneous vessel cooling further augments cold-induced VC. The notion of an estrogen-dependent augmentation of cold-induced α2Cadrenoceptor–mediated VC also complements medical epidemiology figures that indicate an increased incidence of Raynaud phenomenon in women of childbearing age and postmenopausal women taking estrogen replacement therapy (8).
Mechanisms Mediating Local Cooling-Induced Vasoconstriction in Aged Skin
In contrast to the marked decrement in reflex cutaneous VC that accompanies aging, the magnitude of local cold-induced VC is unaffected by age (27,30). However, the balance of the underlying mechanisms that drive this response shifts with age, becoming less adrenergic and more dependent on ROCK signaling. The depressed cutaneous adrenergic response to a local cold stimulus in aged skin parallels similar findings in reflex VC (whole-body cold stimulus) and exogenously administered NE (26,28), whereas the overall local VC response to cold remains functionally unchanged because of an apparent compensatory increase in ROCK-mediated VC (30).
Although the magnitude of local thermoregulatory responses to cold does not undergo significant change with aging, this increased dependence on Rho/ROCK signaling in a healthy aged population provides insight into the signaling changes that arise in conjunction with the development of cardiovascular disease in older humans. Greater dependence on the Rho/ROCK pathway with aging parallels the up-regulation of the Rho/ROCK pathway that is seen in several age-associated vascular pathologies, including atherosclerosis, systemic hypertension, pulmonary hypertension, vascular remodeling, coronary and cerebral vasospasm, erectile dysfunction, and diabetes (19). These similar findings in healthy aged and clinical populations suggest that augmented ROCK-mediated VC may be, at least in part, a function of aging per se rather than the diseases associated with aging. Thus, although advancing age does not affect the local cooling response from a thermoregulatory standpoint (i.e., magnitude of cutaneous constrictor activity is maintained in the face of cold stress), it is associated with preclinical proconstrictor changes in signaling, suggesting that aging may serve as a prelude to more serious clinical vascular pathologies.
In addition to using local skin cooling as a thermoregulatory stimulus, it is likely that cutaneous vascular responses to local cooling may also be useful as an indirect measure of vascular (particularly endothelial) health. Because the Rho/ROCK and eNOS pathways are mutually inhibitory, a disruption of the healthy balance between the two systems can result in disproportionate dilation or constriction. In the context of human aging, age-associated increases in oxidant stress (which can both directly activate Rho and quench NO) and arginase activity (which limits NO production by preferentially metabolizing l-arginine, the substrate for eNOS) cumulatively result in reduced NO bioavailability ([14]; Fig. 3). The consequent reduction in NO metabolism and PKG activity may sufficiently disinhibit Rho and ROCK so as to create an ideal signaling environment for unchecked vasoconstriction. Although these interactions between eNOS and Rho/ROCK pathways require further testing in vivo, it is likely that age-related decrements in endothelial function may be reflected in Rho/ROCK contributions to local cold-mediated VC.
CONCLUSIONS
Healthy human aging leads to compromised vascular thermoregulatory VC to cold, predisposing older humans to hypothermia. This impaired response is predominately due to changes in sympathetic reflex VC, where the efferent sympathetic signal, axonal release of NE, contribution of cotransmitters, and adrenergic sensitivity are all significantly attenuated with aging. Additionally, it is possible that adrenoceptor-mediated effects are blunted because of changes in intracellular signaling pathways, although this hypothesis requires further investigation both in vitro and in vivo. The effects of aging on locally driven VC responses to skin cooling are more subtle; the absolute magnitude of the response remains unchanged in healthy aged populations, whereas the mechanisms driving the response become increasingly dependent on intracellular pathways that are associated with vascular disease. In this context, aging itself is associated with preclinical proconstrictor changes in signaling, suggesting that aging may serve as a prelude to more serious clinical vascular pathologies. Further research is warranted to explore the interaction between decreased NO bioavailability and increased ROCK-mediated VC to more fully characterize the development of age-associated microvascular dysfunction.
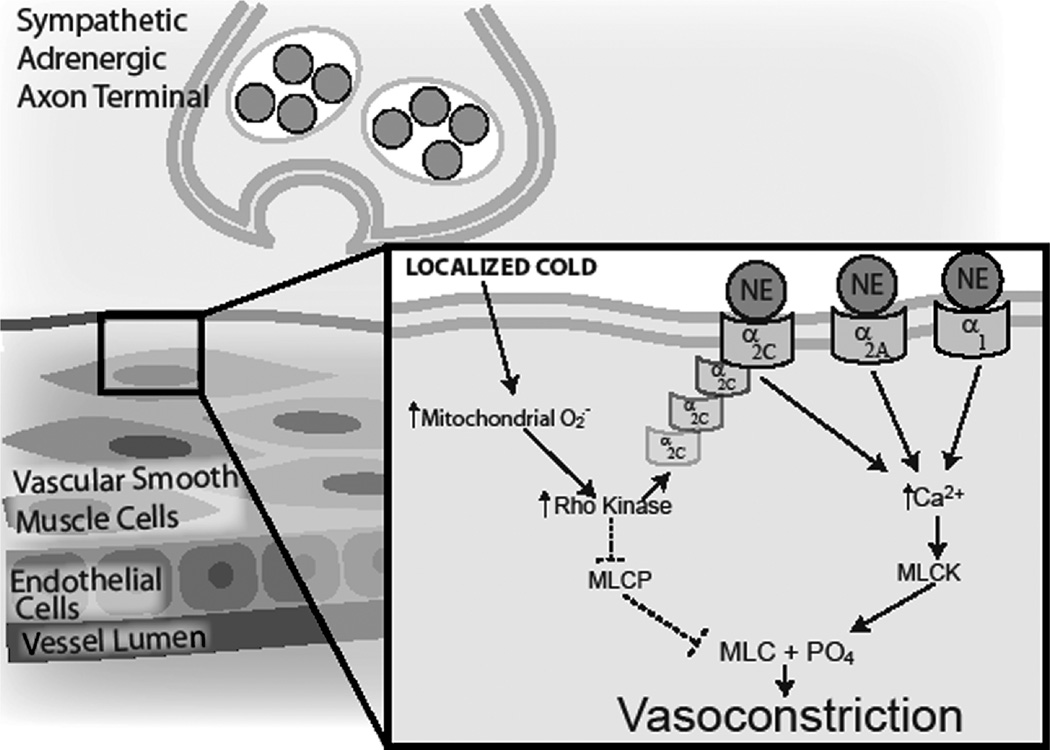
Figure 2.
Schematic model of the role of Rho kinase in local cold-induced vasoconstriction (VC). Localized cooling of cutaneous vessels and surrounding tissue stimulates the production of mitochondrial superoxide. Superoxide (O2−) activates RhoA and Rho kinase, which can stimulate VC through two distinct pathways: 1) translocation of α2C-adrenoceptors from intracellular storage to the cell membrane, joining α2A - and α1-adrenoceptors to bind norepinephrine (NE), which leads to increased intracellular [Ca2+] and Ca2+-dependent VC through phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK), and 2) inhibition of myosin light chain phosphatase (MLCP) permits extant MLC phosphorylation to remain, thereby stimulating VC in the absence of an increase in intracellular [Ca2+]. Broken lines indicate inhibitory effects; continuous lines, stimulatory/activation effects.
References
- 1.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ. Res. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H243–H250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 3.Cankar K, Finderle Z, Strucl M. The role of alpha1- and alpha2-adrenoceptors in gender differences in cutaneous LD flux response to local cooling. Microvasc. Res. 2004;68:126–131. doi: 10.1016/j.mvr.2001.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Hypothermia-related deaths — United States 2003–2004. M.M.W.R. Morb. Mortal. Wkly. Rep. 2005;54:173–175. [PubMed] [Google Scholar]
- 5.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2c)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 6.Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC, Woodward PM. Accidental hypothermia and impaired temperature homeostasis in the elderly. Br. Med. J. 1977;1:353–356. doi: 10.1136/bmj.1.6057.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeGroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2007;292:R103–R108. doi: 10.1152/ajpregu.00074.2006. [DOI] [PubMed] [Google Scholar]
- 8.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of alpha2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1955–H1961. doi: 10.1152/ajpheart.00306.2007. [DOI] [PubMed] [Google Scholar]
- 9.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. alpha-Adrenoceptors and cold-induced vasoconstriction in human finger skin. Am. J. Physiol. 1988;255(5 Pt 2):H1000–H1003. doi: 10.1152/ajpheart.1988.255.5.H1000. [DOI] [PubMed] [Google Scholar]
- 10.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in skin. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000;279:R349–R354. doi: 10.1152/ajpregu.2000.279.1.R349. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell’Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–735. doi: 10.1161/01.CIR.0000081769.02847.A1. [DOI] [PubMed] [Google Scholar]
- 12.Hodges GJ, Traeger JA, III, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H784–H789. doi: 10.1152/ajpheart.00323.2007. [DOI] [PubMed] [Google Scholar]
- 13.Hodges GA, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J. Physiol. 2006;574(Pt 3):849–857. doi: 10.1113/jphysiol.2006.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc. Sport Sci. Rev. 2007;35:119–125. doi: 10.1097/jes.0b013e3180a02f85. [DOI] [PubMed] [Google Scholar]
- 15.Honda M, Suzuki M, Nakayama K, Ishikawa T. Role of alpha(2C)-adrenoceptors in the reductions of skin blood flow induced by local cooling in mice. Br. J. Pharmacol. 2007;152:91–100. doi: 10.1038/sj.bjp.0707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol. Pharmacol. 2001;60:1195–1200. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1573–H1579. doi: 10.1152/ajpheart.00849.2004. [DOI] [PubMed] [Google Scholar]
- 18.Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J. Appl. Physiol. 1996;80:512–515. doi: 10.1152/jappl.1996.80.2.512. [DOI] [PubMed] [Google Scholar]
- 19.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am. J. Physiol. Cell. Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am. J. Physiol. Heart Circ. Physiol. 1993;265(3 Pt 2):H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 21.Pickering GW. The vasomotor regulation of heat loss from the human skin in relation to external temperature. Heart. 1930;16:115–135. [Google Scholar]
- 22.Somlyo AV. Cyclic GMP regulation of myosin phosphatase: a new piece for the puzzle? Circ. Res. 2007;101:645–647. doi: 10.1161/CIRCRESAHA.107.161893. [DOI] [PubMed] [Google Scholar]
- 23.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- 24.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1496–H1504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- 25.Stephens DP, Bennet LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H264–H272. doi: 10.1152/ajpheart.2002.282.1.H264. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J. Physiol. 2004;558(Pt 2):697–704. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson CS, Holowatz LA, Kenney WL. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J. Physiol. 2005;564(Pt 1):313–319. doi: 10.1113/jphysiol.2004.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2005;288:R1108–R1113. doi: 10.1152/ajpregu.00839.2004. [DOI] [PubMed] [Google Scholar]
- 29.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1700–H1705. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 30.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase–mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H30–H36. doi: 10.1152/ajpheart.00152.2007. [DOI] [PubMed] [Google Scholar]