Abstract
The ex-utero intrapartum treatment (EXIT) is one type of fetal surgery, performed before delivery while the fetus remains attached to the uteroplacental circulation. This intervention improves neonatal morbidity and mortality of certain congenital diseases. For instance, securing the airway of a fetus with congenital airway obstruction while on uteroplacental circulation prevents the hypoxemia during the establishment of an airway post-delivery. Anesthesia for fetal surgery now incorporates new knowledge of the maternal/fetal response to anesthetic agents. This chapter reviews for the EXIT procedure the effects of maternal anesthesia on fetal hemodynamics, intravenous anesthesia to supplement inhalational anesthesia in order to provide maternal-fetal hemodynamic stability during surgery, intraoperative fetal monitoring, maternal pharmacokinetics approach to study placental drug transfer and fetal pharmacokinetics to improve our understanding of the effects of maternal anesthesia on the fetus.
Introduction
Due to advances in prenatal diagnosis and imaging, fetal surgery has become an accepted treatment option for certain neonatal diseases. Fetal surgery is commonly divided into three categories: minimally invasive procedures, open mid-gestation procedures and the ex utero intrapartum treatment (EXIT). Minimally invasive techniques allow for fetal intervention without a large hysterotomy, while open midgestation and EXIT procedures require major abdominal surgery. Despite this difference, the advantage of all fetal surgery is the ability to palliate or correct the fetal disease early with fetal circulation and uteroplacental support still intact, before the disease becomes irreversible and morbidity or mortality ensues. For example, securing the airway of a fetus with congenital airway obstruction while on uteroplacental circulation will prevent the hypoxemia and risk of hypoxic organ injury that can occur as attempts are made to establish an airway post-delivery. This fetal intervention with subsequent delivery, known as ex-utero intrapartum treatment (EXIT), can greatly improve neonatal morbidity and mortality. As fetal surgical technique has improved, anesthesia for fetal surgery has advanced reflecting new knowledge of the maternal/fetal response to anesthetic agents. This chapter reviews the effects of maternal anesthesia on fetal hemodynamics during the EXIT procedure, examines new techniques in anesthesia for open fetal surgery, reviews the placental transfer of anesthetic agents and introduces the use of population pharmacokinetic-pharmacodynamic modeling for the study of fetal drug exposure.
Prior to the development of the EXIT procedure, prenatal attempts at treatment of a fetus with the diagnosis of airway obstruction were termed “Operation on Placental Support” (OOPS). The OOPS procedure was performed before umbilical cord clamping and placental delivery during cesarean section or vaginal delivery1. Unfortunately, the adequacy of uteroplacental blood flow was questionable since no attempt was made to preserve amniotic fluid volume or aggressively prevent uterine contractions during the time between exposure of the fetus and division of the umbilical cord2. For this reason, surgical time for the fetal intervention was limited to 5–20 minutes1,2. Over time, the OOPS procedure evolved in to the EXIT procedure which preserves uteroplacental circulation and allows for longer surgical times with uterine relaxation and preserved placental perfusion and gas exchange. The EXIT procedure has been reported as a successful intervention for tumors and malformations of the fetal airway, thoracic masses, and cannulation for extra corporeal membrane oxygenation (ECMO) in a fetus with unstable, life threatening disease such as congenital diaphragmatic hernia (CHD), and hypoplastic left heart syndrome3. Table 1 lists the indications for the EXIT procedure. Similar to the OOPS procedure, the EXIT procedure occurs while the fetus is attached to placental circulation and then delivery of the fetus is performed at the end of the procedure. In contrast to the OOPS, the EXIT procedure converts “emergency” surgery into a “scheduled” surgery emphasizing uterine relaxation and uteroplacental blood flow. It is possible to conduct surgery on placental support for 2.5 hours4. The EXIT procedure is one of the more commonly performed fetal surgeries today. This procedure requires a multidisciplinary team of anesthesiologists, pediatric surgeons, pediatric otolarygologists, obstetricians, cardiologists, neonatologists, echo sonographers, nurses and scrub technicians5.
Table 1.
Indications for the EXIT procedure
| Diagnosis | Surgical procedures |
|---|---|
| Congenital airway obstruction | |
|
|
|
| |
| Intrathoracic lesions | |
|
|
|
| |
| Congenital diaphragmatic hernia |
|
|
| |
| Conjoined twins |
|
New techniques in fetal anesthesia and the effect of maternal anesthesia on the fetus
Anesthetic considerations
Both cesarean section and the EXIT procedure involve two patients, the mother and the fetus, and result in the delivery of the fetus at the end of the procedure. However, from an anesthetic standpoint, the EXIT procedure is very different from a cesarean section. The goal in a cesarean section is to minimize fetal exposure to anesthetic agent and to maximize uterine tone in order to deliver a vigorous infant and prevent postpartum hemorrhage. In contrast, the goal of the EXIT procedure is to relax the uterus, to facilitate fetal surgery. This relaxation and preservation of uterine volume decreases the likelihood of uterine contractions and placental abruption, and maintains adequate uterine blood flow. Unfortunately, the depth of maternal anesthesia necessary to maintain adequate uterine relaxation can lead to maternal hypotension and uteroplacental hypoperfusion ultimately causing fetal cardiovascular insufficiency5. With the potential hemodynamic consequences of maternal anesthesia, it is critical for the anesthesia providers to maintain maternal arterial pressure within a normal range.
Traditional anesthesia: Inhalation-based anesthetic technique
The anesthetic of choice for the EXIT procedure is general anesthesia maintained with inhalational anesthetic agents. Volatile anesthetic agents are favorable due to their uterine relaxation properties and rapid placental transfer. The requirement for inhalational anesthetic agents decreases by 30% starting in early gestation of pregnancy, allowing for a more rapid induction of general anesthesia6. Insoluble volatile agents are particularly well suited to fetal surgery as they permit rapid dose adjustments and rapid elimination. In our institution, desflurane is commonly used because of its low blood/gas partition coefficient producing a more immediate response to changes in concentration and a rapid elimination and recovery from anesthesia. These properties make desflurane the inhalational anesthetic of choice for fetal surgery. Nitrous oxide is avoided to permit high inspired oxygen delivery to the fetus7.
When the uterus is exposed during the EXIT procedure, adequate uterine relaxation responses to 2–3 MAC of inhalational anesthetic agent; a high concentration that may induce maternal and fetal hemodynamic instability, including maternal hypotension, decreased uterine blood flow, impaired utero-placental circulation, fetal hypoxia and myocardial depression. A chronically instrumented maternal-fetal sheep model is a customary model to study the effects of volatile anesthetic agents on maternal and fetal hemodynamics. Using this model, inhalational anesthesia at 1 MAC provides stable maternal and fetal hemodynamic parameters, while 1.5–2 MAC decrease maternal and fetal arterial pressure and uteroplacental blood flow8. Prolonged exposure to high concentration volatile anesthetic agents also induces fetal hypoxia and acidosis9. The placental transfer of volatile anesthetic agents and fetal anesthetic requirements has been studied in animal models. Gregory et al found that the MAC of halothane in fetal sheep is much lower than in ewes (0.33% vs. 0.69%), while the MAC of halothane in neonatal lambs is 1.15%10. When the ewes are exposed to 1 MAC (0.69%) of halothane, the blood concentration of halothane in the fetus is equal to 0.54% end-tidal concentration; which is much higher than 1 MAC (0.33%) in the fetus. These results raise concerns for anesthetic overdose in the fetus under high levels of inhalational anesthetic agents during fetal surgery.
Alternative anesthesia: Intravenous and supplemental intravenous anesthetic techniques
Due to concerns for fetal hemodynamic instability, alternatives to the high dose inhalational anesthetic technique for fetal surgery have been explored. At our institution, a propofol and remifentanil based-technique has been used for the EXIT procedure and is based on a case series demonstrating the use of total intravenous general anesthesia with propofol and remifentanil for elective cesarean section11. Interestingly, in vitro studies have shown that propofol and remifentanil decrease isolated gravid uterine muscle contractility in dose-dependent fashion12,13. Therefore, propofol and remifentanil intravenous infusions in combination with lower doses (1–1.5 MAC) of desflurane represent an alternative technique to high dose desflurane anesthesia. In a retrospective study, adequate uterine relaxation was achieved at 1.5 MAC of desflurane with a propofol and remifentanil infusion, comparable to 2.5 MAC of desflurane anesthesia alone14. After induction of anesthesia, propofol and remifentanil were infused at 150–250 mcg/kg/min and 0.2–0.5 mcg/kg/min. When the uterus was exposed, the propofol infusion rate was decreased to 50–75 mcg/kg/min while the remifentanil infusion rate remained the same. Desflurane was initiated with uterine exposure and the concentration was gradually increased to 6–9% until adequate uterine relaxation was observed. In our human fetal anesthesia study, the decrease in dose and exposure time of maternal desflurane resulted in less fetal cardiac depression, as shown by a lower incidence of moderate to severe left ventricular systolic dysfunction14.
In vitro, propofol, the only pregnancy category B sedative agent, crosses the placenta and induces vasodilation without reducing placental blood flow15. It has been used during fetoscopic surgery and EXIT procedures in patient at risk for malignant hyperthermia16. In women undergoing cesarean sections, propofol readily crosses the placenta to the fetus as shown by an umbilical to maternal venous ratio of 0.7617,18. Propofol based anesthesia also provides stable maternal and fetal hemodynamic parameters. In the pregnant sheep model, stable maternal and fetal arterial pressure, as well as stable uterine blood flow with normal fetal oxygenation and fetal acid-base status occurred after propofol 2 mg/kg followed by an infusion of 450 mcg/kg/min and 50% nitrous oxide19.
Remifentanil, an ultra-short acting opioid, also crosses the placenta and is rapidly metabolized without adverse neonatal or maternal effects. Remifentanil possesses high lipid solubility, which facilitates placental transfer and is rapidly metabolized by non-specific plasma and tissue esterases, potentially beneficial in a fetus with diminished liver and kidney function. The clearance of remifentanil is independent of body weight or age; its degradation rate is comparable in preterm and term infants20. The short context-sensitive half-life and minimal extravascular accumulation of remifentanil means the clinical effect remains independent of the duration of the infusion. Missant et al. reported fetal immobilization during minimally invasive fetoscopic surgery with maternal remifentanil infusions of 0.1 mcg/kg/min21. Kan et al. established the blood level of remifentanil in pregnant women undergoing cesarean section22. The mean ratio of remifentanil in umbilical vein: maternal artery was 0.88 and the mean ratio in umbilical artery: umbilical vein was 0.29, indicating a high level of placental transfer and fetal metabolism, and/or rapid redistribution of remifentanil making it an effective and relatively safe drug for administration during pregnancy22.
Intravenous anesthetic techniques utilized during fetal surgery that do not include volatile agents rely on nitroglycerin for uterine relaxation. Fink et al. demonstrated the use of a remifentanil infusion and combined spinal-epidural anesthesia for the EXIT procedure23. In this case series, a phenylephrine infusion is immediately started at 50 mcg/min after neuraxial blockade in order to prevent maternal hypotension. A remifentanil infusion 0.1–0.2 mcg/kg/min is started 15 minutes before skin incision, to ensure a steady state without the need for bolus dosing. For uterine relaxation, a 50–100 mcg bolus of nitroglycerin is administered followed by an infusion at 50–100 mcg/min. With this technique, the fetus does not receive the anesthetic intramuscular “cocktail” of fentanyl, atropine and muscle relaxant because the remifentanil provides immobilization and analgesia. Similarly, Ioscovich et al. reported the use of remifentanil and nitroglycerin combined with endotracheal inhalational anesthesia for the EXIT procedure24. General anesthesia is induced with propofol 200 mg, remifentanil 20 mcg, rocuronium 50 mg, and then maintained with nitrous oxide 60%, oxygen 40%, a remifentanil infusion up to 0.8 mcg/kg/min and a nitroglycerin infusion at 0.06–0.3 mcg/kg/min. Finally, Rosen et al. reported the successful use of a propofol and nitroglycerin infusion to produce general anesthesia for the EXIT procedure in a patient at risk for malignant hyperthermia16. In summary, intravenous anesthesia or intravenous anesthesia supplemented with inhalational anesthesia offer a safe and effective alternative to pure inhalational general anesthesia for fetal surgery.
Update in sympathomimetic uses and fetal pain perception
Sympathomimetic agents
The fetus relies on uteroplacental blood flow during the EXIT procedure to deliver oxygen and to remove carbon dioxide from the fetus. This placental gas exchange is also influenced by the umbilical artery blood flow, which depends on fetal cardiac output and placental vascular resistance. Uterine blood flow displays no auto regulation and directly depends on maternal systolic arterial pressure and uterine tone. Volatile anesthetic agents promote uterine relaxation and vasodilation; the latter can result in maternal hypotension, impaired uteroplacental blood flow, and decreased fetal cardiac output9. Ephedrine and phenylephrine are the two most commonly used vasopressors during fetal surgery to maintain maternal arterial pressure. Ephedrine, often considered the vasopressor of choice for obstetric patients, is a mixed α and β-agonist and indirectly induces catecholamine release to increase maternal cardiac output with mild vasoconstriction. Ephedrine crosses the placenta more than phenylephrine and increases fetal heart rate and atrial natriuretic peptide levels. Phenylephrine, on the other hand, is a direct α-agonist and produces vasoconstriction. In animal models, ephedrine has been shown to increase uterine blood flow and decrease uterine vascular resistance, whereas phenylephrine decreases uterine blood flow and increases uterine vascular resistance25,26. Ephedrine has long been used in obstetric anesthesia, while phenylephrine has increased lately in popularity. Studies in humans demonstrate higher umbilical artery pH and base excess with phenylephrine compared to ephedrine but no difference in APGAR scores27,28. Neither of these vasopressors has any effect on umbilical blood flow, which may be explained by decreased α-receptors function in the umbilical-placental base29.
Fetal pain perception
The hormonal stress response can be detected in the fetus as early as 20 weeks gestation30. Even though stress hormones may not reflect pain perception, many studies demonstrate simultaneous hormonal and hemodynamic responses to invasive stimuli in the fetus. These responses can be abolished by administering an analgesic leading to the belief that the fetus perceives pain and thus requires anesthesia for surgical procedure31. The nociceptive pathway exists anatomically along with the cortical activity by mid-gestation32. Peripheral nociceptors are present throughout the fetal body by 20 weeks gestation. The spino-thalamic connections are formed at 14 weeks and completed at 20 weeks. The thalamo-cortical fibers are formed at 17 weeks and completed by 26 weeks gestation. Since the serotonin-inhibitory pathway develops after birth, it might be possible that the fetus perceives more pain than the infant33. Fetal anesthesia and analgesia can be accomplished in two ways during fetal surgery: 1) transfer of anesthetics from mother across the placenta and 2) direct injection of anesthetics into the fetus.
Significant concern for anesthesia induced neurotoxicity has been raised for fetal surgery. Neurotoxicity has been demonstrated in animal studies, whereas human studies are conflicting, some suggesting neurotoxicity after prolonged or repeated exposure to anesthesia and other studies do not34,35. Although exposure to anesthetic agents during fetal surgery may trigger neuron cell degeneration, exposure to pain during this period alters the development of brain and nociception while in turn is detrimental. Repetitive pain during the neonatal period decreases a neonate’s pain threshold and increases vulnerability to future behavioral disorders36. Therefore, administering analgesics to the fetus, especially during the third trimester when the EXIT procedure is performed, is encouraged. In our practice, intramuscular injection of an anesthetic “cocktail” (opioid, muscle relaxant and atropine) into the fetus is routinely administered during the EXIT procedure to provide analgesia, immobilization and prevention of bradycardia from surgical manipulation.
Intraoperative fetal monitoring during the EXIT procedure
A fetus undergoing surgery is often critically ill. The pathophysiology of the particular fetal malformation added to immature organ function risks significant compromise. Under fetal circulation, the cardio-placental relationship is more important than the cardio-pulmonary relationship. Changes in vascular tone of the placenta tremendously affect fetal cardiac output. The right ventricle is dominant in the fetus, accounting for 60% ejection of the biventricular output. The relatively well oxygenated blood from the umbilical vein passes the foramen ovale to the left side of the heart, and then to the brain and the upper extremities. In contrast, the venous return from superior vena cava enters the right side of the heart, and then diverts to ductus arteriosus and the lower extremities. Myocardial contractility of the fetus is less than the neonate, child and adult; nevertheless, contractility can increase by inotropic drugs and increased preload (Frank-Starling law). Blood volume of the fetus is small and coagulation is less, which predispose to bleeding and transfusion with minimal blood loss. The fetal skin is thin and susceptible to heat and evaporative fluid losses, while fetal baroreceptor activity is less, and limiting vasoconstriction in response to hypovolemia. Therefore, the fetus is at high risk for hypothermia, hypovolemia and hypoperfusion. The combination of exposure to anesthesia, surgical manipulation, oxygenation and circulatory insufficiency from disease, make intensive monitoring during surgery crucial.
In additional to standard ASA monitors, invasive arterial pressure is also monitored to promptly detect and treat maternal hypotension which can endanger fetal oxygen delivery. Continuous fetal monitoring includes pulse oximetry and echocardiography. A pulse oximeter can be wrapped on the fetal hand with foil to minimize operating room bright light interference, for continuous oxygen saturation and heart rate monitoring. Normal fetal oxygen saturation is 60–70%. As such low saturation, the accuracy of pulse oximetry has not been established in the fetus; however, its accuracy been determined in animal and in vitro models37. Fetal arterial saturation less than 40% is a critical, requires prompt treatment, and often results from poor placental perfusion or compression of the umbilical cord. If the fetal heart rate decreases more than 20% of baseline, or below 140 beat/min, the umbilical cord position, uterine volume and bleeding need to be assessed. Fetal echocardiography can identify fetal heart rate, intraventricular volume, myocardial contractility, atrioventricular valve competency and ductal constriction. Fetal hemoglobin and blood gases can be obtained periodically from umbilical artery and vein by the surgeon. The normal values of the umbilical cord blood gas vary and depend on multiple such factors as gestational age and the mode of delivery38. Normal values for the fetus undergoing EXIT procedure have not been established, however, the lower limit of pH 7.10 and base excess of −12 are used38,39.
Near InfraRed Spectroscopy (NIRS) is a tool that uses an optical probe for continuous measurement of tissue oxygenation. At Near InfraRed wavelengths (650–900 nm), light can penetrate several centimeters depth in human tissue. Both NIRS and pulse oximetry measure changes in hemoglobin oxygen saturation; however pulse oxemetry identifies pulsatile component and measures arterial oxygenation whereas NIRS measures a mixed vascular oxygenation of arterial, venous and capillary blood. NIRS has been used in adult, pediatric and neonatal populations to assess cerebral oxygenation as well as somatic organ perfusion40,41. In fetal cardiac surgery animal models, NIRS correlated well with the umbilical venous oxygenation42. The use of NIRS during fetal surgery has not been reported, but it may prove valuable to monitor fetal well-being during the EXIT procedure.
Pharmacokinetic-pharmacodynamic (PK/PD) models for study of fetal pharmacology
Understanding the placental transfer of anesthetic agents
Substances cross the placenta by 5 mechanisms: passive diffusion, active transport, bulk flow, pinocytosis, and inter villous space32. Passive diffusion, the main form of placental transportation, is favored for lipid soluble, un-ionized and low molecular weight drugs. Drugs with molecular weight less than 400 kilo Dalton readily cross the placenta. For example, opioids and propofol cross the placenta whereas larger molecules such as muscle relaxant do not cross. Most of drugs are weak acids or weak bases. A weak acid will be uncharged and more lipid soluble at acid pH while a weak base will be uncharged and more lipid soluble at alkaline pH. Since maternal pH is higher than fetal pH, the weak base drug is mainly uncharged and readily diffuses pass the placenta, and then is converted to the ionized form in the fetal circulation, thus the fetal concentration of base drug is higher than the maternal concentration. This results in trapping of more basic pH drugs in the fetus, especially when fetal acidosis develops. Ion trapping of lidocaine with fetal distress during labor under paracervical block or pudendal nerve block is well-documented43. Active transport is a process involving transporters against concentration gradient. The other 3 mechanisms of transport are negligible for placental transfer. Recent studies reveal the placenta as a source of drug metabolism with the presence of various enzymes including cytochrome P-45044.
Pharmacokinetic-pharmacodynamic (PK/PD) models
Both in vitro and in vivo models have been used to study placental drug uptake and transfer and improve our understanding of pharmacokinetics (PK) and pharmacodynamics (PD) in the fetus45. In the past, PK studies in pregnancy were limited due to inability to obtain fetal blood or to draw multiple fetal blood samples. As more fetal interventions are performed, fetal pharmacokinetics has been studied by sampling from fetal cord blood before cord clamping at delivery. Tran et al. found that after intramuscular injection of 20 mcg/kg fentanyl to a fetus (range 2020–3715 g, 34–37 weeks) during the EXIT, the median serum concentration in the fetus was 14 ng/ml (range 4.3–64.0 ng/mL)46. Collins et al demonstrated plasma concentration of 7.7–13.6 ng/ml after intravenous injection of 30 mcg/kg fentanyl in neonate47. The higher and wider variation of plasma fentanyl in fetus during the EXIT procedure may due to dynamic changes in uteroplacental blood flow during surgical manipulation, immature hepatic metabolism and shunting of lung and liver blood flow under fetal circulation.
In recent years, new methods to study drug disposition including population PK/PD and physiologically-based pharmacokinetic (PBPK) modeling were developed, using quantitative mathematical modeling and limited sampling across a larger population, these techniques more accurately predict PK/PD. The following are details for the traditional pharmacokinetics and the Population Pharmacokinetics studies:
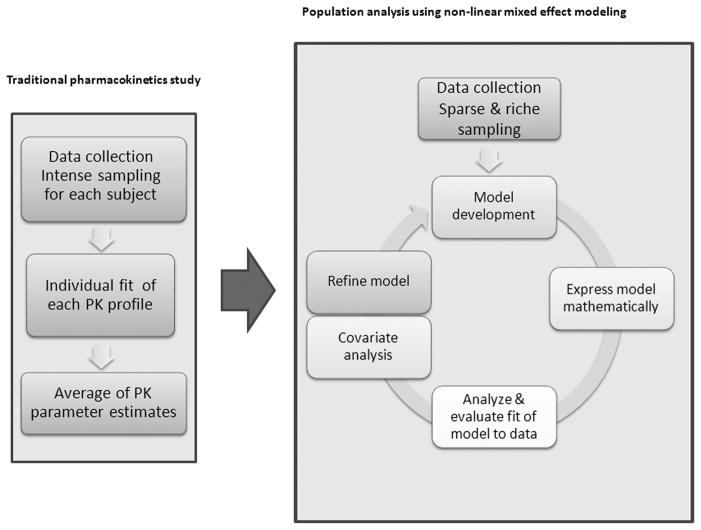
A traditional pharmacokinetic study would involve serial blood sampling for each subject in the population, fit each subject’s concentration-time profile and then calculate the population average for each PK parameter of interest. Known as the standard two-stage approach, it assigns equal weight to each sample and each subject, so outliers will have significant effects on the average value48. This approach cannot distinguish the cause of these outliers among intra-individual variability, inter-individual variability, or measurement error. This approach has been used to study maternal-fetal pharmacokinetics in an animal model49. However, multiple blood samples from each subject are required, which are not possible to get from the human fetus during surgery. When using single time point samples, the pharmacokinetics of anesthetic agent in pregnant women can only be described as a fetal: maternal ratio, which is not very accurate or useful, and changes with infusion rate and time50.
Population analysis using non-linear mixed effect modeling can analyze both population pharmacokinetics and pharmacodynamics (Pop-PK/PD). The individual finding can be compared to the any reasonable initial estimate of the population parameter values (a priori value), and then the posterior parameter values can be calculated by using the Bayesian procedure to get the maximum a posteriori probability48. This method can identify and quantify inter -individual variability, random variability (e.g. from sampling and assay error), as well as within subject variability. The inter-individual variability, which can be attributed to difference in age, weight, body surface area, and other clinical factors, can be identified through covariate analysis. After accounting for all covariates in the structured model (or the fixed effects), some variability still remains, which is attributed to the random effects. The term ‘mixed’ in non-linear mixed effect modeling refers to the combination of “fixed” and “random” effects. This modeling approach can be applied to analyze sparse samples from multiple subjects and is better suited to the maternal-fetal population where multiple blood samples cannot be obtained. Moreover, the sampling time points which minimize the number of samples while optimizing the estimation of PK parameters can be determined, particularly useful in designing human fetal-maternal PK studies.
There are several commercial software packages for non-linear mixed effect modeling. Among them, NONMEM (ICON Dev. Soln., Ellicott City, MD) is the industry standard for model development and analysis. The population model is described by using multi-compartmental PK/PD models. A typical model is composed of compartments, compartmental volumes of distribution, the rate of drug metabolism or elimination, and the rate of drug transfer between compartments. Then the covariates are examined to identify their influence on this structure model. The population pharmacodynamics model can be applied to reveal the effects of PK and covariates on pharmacodynamics clinical endpoint, such as the effect of end-tidal sevoflurane concentration on Bispectral Index monitoring51, and the effect of remifentanil on intraoperative hypotension52.
On most occasions, several drugs are administered during anesthesia. Non-linear mixed effect modeling can also describe and predict drug interactions. From the model, titration can be optimized during anesthesia. A conventional pharmacokinetics study assumes no change in condition in each individual. In reality, there are many physiologic changes during anesthetic along with changing in age and gestational age or with disease progression. A physiologically-based pharmacokinetic (PBPK) modeling accounts for the physiologically realistic compartmental structures and the changes in physiology and pharmacology during pregnancy for better model prediction53,54. Various gestational age dependent physiological changes have been integrated into the PBPK model, such as maternal body weight, organ volume or weight, organ blood flow, cardiac output, alveolar ventilation, placental volume or weight, fetal volume or weight, and placental blood flow. The reliability of the PBPK modeling depends on the accurate knowledge in physiological and pharmacological changes during pregnancy. This highly informative new technique is increasingly used to predict drug effects in pregnancy.
Conclusion
The EXIT procedure is a successful surgical technique for treatment of congenital fetal anomalies before delivery. Uterine relaxation, maintenance of utero-placental perfusion, maternal and fetal anesthesia, and prevention of maternal and fetal cardiovascular compromise are the key points to consider during the EXIT procedure. Use of intravenous anesthesia to supplement inhalational anesthesia lowers dose of the latter, and improves maternal-fetal hemodynamic stability compare to inhalational anesthesia alone. A better understanding of placental drug uptake and transfer and fetal pharmacokinetics/pharmacodynamics will improve fetal anesthesia. Non-linear mixed effect modeling and PBPK analysis are emerging new tools to understand maternal-fetal pharmacokinetics and pharmacodynamics to improve maternal anesthesia on the fetus.
Figure 1.
Flow diagram depicting the traditional two-stage pharmacokinetic approach (left) in comparison to population analysis using mixed-effect modeling (right). Traditional pharmacokinetic studies provide the mean of the population PK parameters after fitting each full concentration-time profile using non-linear regression. Population modeling identifies the population average of PK parameters of interest (fixed effects), their relationship with demographic and clinical factors (covariates), as well as the inter-individual and inter-occasion variability and residual error (random effects). The model continues to be refined until the final model or the “best fitting” model has been identified.
Objectives.
Discuss new techniques in fetal anesthesia and the effect of maternal anesthesia on fetal hemodynamic
Discuss sympathomimetic uses and fetal pain perception.
Introduce new techniques for intraoperative fetal monitoring
Introduce use of Pharmacokinetics Population modeling for study of fetal pharmacology.
Contributor Information
Pornswan Ngamprasertwong, Email: pornswan.ngamprasertwong@cchmc.org, Department of Anesthesia, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
Alexander A Vinks, Email: sander.vinks@cchmc.org, Division of Clinical Pharmacology, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
Anne Boat, Email: anne.boat@cchmc.org, Department of Anesthesia, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
References
- 1.Skarsgard ED, Chitkara U, Krane EJ, Riley ET, Halamek LP, Dedo HH. The OOPS procedure (operation on placental support): in utero airway management of the fetus with prenatally diagnosed tracheal obstruction. J Pediatr Surg. 1996;31:826–8. doi: 10.1016/s0022-3468(96)90144-x. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PJ, Urken ML, Alvarez M, et al. New approach to the management of airway obstruction in “high risk” neonates. Arch Otolaryngol Head Neck Surg. 1992;118:306–9. doi: 10.1001/archotol.1992.01880030094019. [DOI] [PubMed] [Google Scholar]
- 3.Liechty KW. Ex-utero intrapartum therapy. Semin Fetal Neonatal Med. 2010;15:34–9. doi: 10.1016/j.siny.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Hirose S, Sydorak RM, Tsao K, et al. Spectrum of intrapartum management strategies for giant fetal cervical teratoma. J Pediatr Surg. 2003;38:446–50. doi: 10.1053/jpsu.2003.50077. discussion -50. [DOI] [PubMed] [Google Scholar]
- 5.Marwan A, Crombleholme TM. The EXIT procedure: principles, pitfalls, and progress. Semin Pediatr Surg. 2006;15:107–15. doi: 10.1053/j.sempedsurg.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Gin T, Chan MT. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829–32. doi: 10.1097/00000542-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gaiser RR, Kurth CD. Anesthetic considerations for fetal surgery. Semin Perinatol. 1999;23:507–14. doi: 10.1016/s0146-0005(99)80029-9. [DOI] [PubMed] [Google Scholar]
- 8.Okutomi T, Whittington RA, Stein DJ, Morishima HO. Comparison of the effects of sevoflurane and isoflurane anesthesia on the maternal-fetal unit in sheep. J Anesth. 2009;23:392–8. doi: 10.1007/s00540-009-0763-2. [DOI] [PubMed] [Google Scholar]
- 9.Palahniuk RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–72. doi: 10.1097/00000542-197411000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Gregory GA, Wade JG, Beihl DR, Ong BY, Sitar DS. Fetal anesthetic requirement (MAC) for halothane. Anesth Analg. 1983;62:9–14. [PubMed] [Google Scholar]
- 11.Van de Velde M, Teunkens A, Kuypers M, Dewinter T, Vandermeersch E. General anaesthesia with target controlled infusion of propofol for planned caesarean section: maternal and neonatal effects of a remifentanil-based technique. Int J Obstet Anesth. 2004;13:153–8. doi: 10.1016/j.ijoa.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Thind AS, Turner RJ. In vitro effects of propofol on gravid human myometrium. Anaesth Intensive Care. 2008;36:802–6. doi: 10.1177/0310057X0803600609. [DOI] [PubMed] [Google Scholar]
- 13.Kayacan N, Ertugrul F, Arici G, Karsli B, Akar M, Erman M. In vitro effects of opioids on pregnant uterine muscle. Adv Ther. 2007;24:368–75. doi: 10.1007/BF02849906. [DOI] [PubMed] [Google Scholar]
- 14.Boat A, Mahmoud M, Michelfelder EC, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 2010;20:748–56. doi: 10.1111/j.1460-9592.2010.03350.x. [DOI] [PubMed] [Google Scholar]
- 15.Soares de Moura R, Silva GA, Tano T, Resende AC. Effect of propofol on human fetal placental circulation. Int J Obstet Anesth. 2009 doi: 10.1016/j.ijoa.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Rosen MA, Andreae MH, Cameron AG. Nitroglycerin for fetal surgery: fetoscopy and ex utero intrapartum treatment procedure with malignant hyperthermia precautions. Anesthesia and analgesia. 2003;96:698–700. doi: 10.1213/01.ANE.0000049686.20464.3B. table of contents. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Alcaraz A, Quintana MB, Laguarda M. Placental transfer and neonatal effects of propofol in caesarean section. J Clin Pharm Ther. 1998;23:19–23. [PubMed] [Google Scholar]
- 18.Dailland P, Cockshott ID, Lirzin JD, et al. Intravenous propofol during cesarean section: placental transfer, concentrations in breast milk, and neonatal effects. A preliminary study. Anesthesiology. 1989;71:827–34. doi: 10.1097/00000542-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Alon E, Ball RH, Gillie MH, Parer JT, Rosen MA, Shnider SM. Effects of propofol and thiopental on maternal and fetal cardiovascular and acid-base variables in the pregnant ewe. Anesthesiology. 1993;78:562–76. doi: 10.1097/00000542-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Welzing L, Ebenfeld S, Dlugay V, Wiesen MH, Roth B, Mueller C. Remifentanil degradation in umbilical cord blood of preterm infants. Anesthesiology. 2011;114:570–7. doi: 10.1097/ALN.0b013e318204e043. [DOI] [PubMed] [Google Scholar]
- 21.Missant C, Van Schoubroeck D, Deprest J, Devlieger R, Teunkens A, Van de Velde M. Remifentanil for foetal immobilisation and maternal sedation during endoscopic treatment of twin-to-twin transfusion syndrome: a preliminary dose-finding study. Acta Anaesthesiol Belg. 2004;55:239–44. [PubMed] [Google Scholar]
- 22.Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–74. doi: 10.1097/00000542-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Fink RJ, Allen TK, Habib AS. Remifentanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. Br J Anaesth. 2011;106:851–5. doi: 10.1093/bja/aer097. [DOI] [PubMed] [Google Scholar]
- 24.Ioscovich A, Shen O, Sichel JY, et al. Remifentanil-nitroglycerin combination as an anesthetic support for ex utero intrapartum treatment (EXIT) procedure. J Clin Anesth. 2011;23:142–4. doi: 10.1016/j.jclinane.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Erkinaro T, Kavasmaa T, Pakkila M, et al. Ephedrine and phenylephrine for the treatment of maternal hypotension in a chronic sheep model of increased placental vascular resistance. Br J Anaesth. 2006;96:231–7. doi: 10.1093/bja/aei305. [DOI] [PubMed] [Google Scholar]
- 26.Erkinaro T, Makikallio K, Kavasmaa T, Alahuhta S, Rasanen J. Effects of ephedrine and phenylephrine on uterine and placental circulations and fetal outcome following fetal hypoxaemia and epidural-induced hypotension in a sheep model. Br J Anaesth. 2004;93:825–32. doi: 10.1093/bja/aeh273. [DOI] [PubMed] [Google Scholar]
- 27.Ngan Kee WD, Khaw KS, Lau TK, Ng FF, Chui K, Ng KL. Randomised double-blinded comparison of phenylephrine vs ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective Caesarean section*. Anaesthesia. 2008;63:1319–26. doi: 10.1111/j.1365-2044.2008.05635.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DG, Robson SC, Redfern N, Hughes D, Boys RJ. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61–5. doi: 10.1093/bja/76.1.61. [DOI] [PubMed] [Google Scholar]
- 29.Alahuhta S, Rasanen J, Jouppila P, Jouppila R, Hollmen AI. Ephedrine and phenylephrine for avoiding maternal hypotension due to spinal anaesthesia for caesarean section. Effects on uteroplacental and fetal haemodynamics. Int J Obstet Anesth. 1992;1:129–34. doi: 10.1016/0959-289x(92)90016-w. [DOI] [PubMed] [Google Scholar]
- 30.Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–9. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 31.Littleford J. Effects on the fetus and newborn of maternal analgesia and anesthesia: a review. Can J Anaesth. 2004;51:586–609. doi: 10.1007/BF03018403. [DOI] [PubMed] [Google Scholar]
- 32.Myers LB, Cohen D, Galinkin J, Gaiser R, Kurth CD. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569–78. doi: 10.1046/j.1460-9592.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 33.Van de Velde M, Jani J, De Buck F, Deprest J. Fetal pain perception and pain management. Semin Fetal Neonatal Med. 2006;11:232–6. doi: 10.1016/j.siny.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 35.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter FL, Wolf CM, Miller JP. Procedural pain in newborn infants: the influence of intensity and development. Pediatrics. 1999;104:e13. doi: 10.1542/peds.104.1.e13. [DOI] [PubMed] [Google Scholar]
- 37.Nijland R, Jongsma HW, Nijhuis JG, Oeseburg B. Accuracy of fetal pulse oximetry and pitfalls in measurements. Eur J Obstet Gynecol Reprod Biol. 1997;72 (Suppl):S21–7. doi: 10.1016/s0301-2115(97)02714-0. [DOI] [PubMed] [Google Scholar]
- 38.Helwig JT, Parer JT, Kilpatrick SJ, Laros RK., Jr Umbilical cord blood acid-base state: what is normal? Am J Obstet Gynecol. 1996;174:1807–12. doi: 10.1016/s0002-9378(96)70214-4. discussion 12–4. [DOI] [PubMed] [Google Scholar]
- 39.Ross MG, Gala R. Use of umbilical artery base excess: algorithm for the timing of hypoxic injury. Am J Obstet Gynecol. 2002;187:1–9. doi: 10.1067/mob.2002.123204. [DOI] [PubMed] [Google Scholar]
- 40.Highton D, Elwell C, Smith M. Noninvasive cerebral oximetry: is there light at the end of the tunnel? Curr Opin Anaesthesiol. 2010;23:576–81. doi: 10.1097/aco.0b013e32833e1536. [DOI] [PubMed] [Google Scholar]
- 41.Giliberti P, Mondi V, Conforti A, et al. Near infrared spectroscopy in newborns with surgical disease. J Matern Fetal Neona. 2011;24 (Suppl 1):56–8. doi: 10.3109/14767058.2011.607673. [DOI] [PubMed] [Google Scholar]
- 42.Reed CA, Baker RS, Lam CT, et al. Application of near-infrared spectroscopy during fetal cardiac surgery. J Surg Res. 2011;171:159–63. doi: 10.1016/j.jss.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Bozynski ME, Rubarth LB, Patel JA. Lidocaine toxicity after maternal pudendal anesthesia in a term infant with fetal distress. Am J Perinatol. 1987;4:164–6. doi: 10.1055/s-2007-999764. [DOI] [PubMed] [Google Scholar]
- 44.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 45.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- 46.Tran KM, Maxwell LG, Cohen DE, et al. Quantification of Serum Fentanyl Concentrations from Umbilical Cord Blood During Ex Utero Intrapartum Therapy. Anesth Analg. 2011 doi: 10.1213/ANE.0b013e3182378d21. [DOI] [PubMed] [Google Scholar]
- 47.Collins C, Koren G, Crean P, Klein J, Roy WL, MacLeod SM. Fentanyl pharmacokinetics and hemodynamic effects in preterm infants during ligation of patent ductus arteriosus. Anesth Analg. 1985;64:1078–80. [PubMed] [Google Scholar]
- 48.Heeremans EH, Proost JH, Eleveld DJ, Absalom AR, Struys MM. Population pharmacokinetics and pharmacodynamics in anesthesia, intensive care and pain medicine. Curr Opin Anaesthesiol. 2010;23:479–84. doi: 10.1097/ACO.0b013e32833a1d2f. [DOI] [PubMed] [Google Scholar]
- 49.Andaluz A, Trasserras O, Garcia F. Maternal and fetal effects of propofol anaesthesia in the pregnant ewe. Vet J. 2005;170:77–83. doi: 10.1016/j.tvjl.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Coonen JB, Marcus MA, Joosten EA, et al. Transplacental transfer of remifentanil in the pregnant ewe. Br J Pharmacol. 2010;161:1472–6. doi: 10.1111/j.1476-5381.2010.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortinez LI, Troconiz IF, Fuentes R, et al. The influence of age on the dynamic relationship between end-tidal sevoflurane concentrations and bispectral index. Anesth Analg. 2008;107:1566–72. doi: 10.1213/ane.0b013e318181f013. [DOI] [PubMed] [Google Scholar]
- 52.Standing JF, Hammer GB, Sam WJ, Drover DR. Pharmacokinetic-pharmacodynamic modeling of the hypotensive effect of remifentanil in infants undergoing cranioplasty. Paediatr Anaesth. 2010;20:7–18. doi: 10.1111/j.1460-9592.2009.03174.x. [DOI] [PubMed] [Google Scholar]
- 53.Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Physiological, anatomical and metabolic changes with gestational age during normal pregnancy; A database for parameters required in physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2012 doi: 10.2165/11597440-000000000-00000. in press. [DOI] [PubMed] [Google Scholar]
- 54.Lu A, Abduljalil K, Jamei M, Johnson TN, Soltani H, Rostami-Hodjegan A. Physiologically-based pharmacokinetic (PBPK) models for assessing the kinetics of xenobiotics during pregnancy: Achievements and shortcomings. Current drug Metabolism. 2012 doi: 10.2174/138920012800840374. In press. [DOI] [PubMed] [Google Scholar]