Abstract
In mammalian cells, the Golgi complex has an elaborate structure consisting of stacked, flattened cisternal membranes collected into a ribbon in the center of the cell. Amazingly, the flattened cisternae can rapidly dilate to accommodate large cargo as it traffics through the organelle. The mechanism by which this occurs is unknown. Exocytosis of large cargo is essential for many physiological processes, including collagen and lipoprotein secretion, and defects in the process lead to disease. In addition, enveloped viruses that bud into the endoplasmic reticulum or Golgi complex must also be transported through Golgi cisternae for secretion from the infected cell. This review summarizes our understanding of intra-Golgi transport of large cargo, and outlines current questions open for experimentation.
Keywords: Golgi complex, Cisternae, Large cargo, Exocytosis, Coronavirus
Introduction
Golgi complex structure and function
The Golgi complex plays a central role in processing and sorting of biosynthetic cargo in all eukayotic cells. In mammals, the Golgi complex consists of sets of flattened cisternal membranes arranged in stacks with associated tubules and vesicles, which are usually collected at the microtubule organizing center in a ribbon structure (Klumperman 2011). Peripheral proteins including GRASPs and golgins help maintain the stacks and ribbon, although the mechanism for maintaining the flattened cisternae is poorly understood. Cargo moves from the endoplasmic reticulum (ER) to the cis face of the Golgi and then through the cisternae before being packaged at the trans Golgi network (TGN) for export to its final destination (Farquhar and Palade 1998). Golgi processing enzymes are compartmentalized by being enriched in cis, medial, or trans regions of the Golgi, largely in the order in which they act on their substrates. Golgi structure is simpler in lower eukaryotes, from dispersed stacks in invertebrates to single stacks in some protozoa and yeasts, to isolated cisternae in budding yeast (Mowbrey and Dacks 2009). However, these less organized Golgi complexes also process and sort cargo. Thus, the complex ribbon structure is not essential for the known functions of the Golgi, and the reason mammalian cells maintain the Golgi ribbon remains an unanswered mystery in cell biology. Regardless of the complexity of its structure, the Golgi is dynamic and can accommodate and process many types of macromolecules as they pass through the organelle.
Intra-Golgi transport
The mechanism of intra-Golgi transport in mammalian cells is still debated, with cisternal maturation/progression in combination with intercisternal tubules the current favorite (reviewed in (Glick and Luini 2011)). However, a recent study from the Rothman laboratory demonstrated that cargo proteins could be transported through cisternal rims while the flat regions of cisternae remain fixed in place (Lavieu et al. 2013). These results support a newer “cisternal progenitor” model, where Rab cascades allow transport by limited fusion of sequential cisternae in specific membrane subdomains while the remainder of the organelle remains stable (Pfeffer 2010). Transport of large cargo (such as procollagen, which cannot fit into conventional transport vesicles) supports the cisternal maturation or cisternal progenitor models of intra-Golgi transport. However, a major unanswered question is how large cargo actually fits inside Golgi cisternae. In the flattened regions of cisternae, the luminal space is only about 20 nm (Klumperman 2011), much narrower than the width of cargo such as procollagen aggregates (up to 150 nm), enveloped viruses that assemble intracellularly (70–160 nm), and very low lipoprotein particles (chylomicrons) in the intestine (400–1200 nm). Even the dilated rims of the cisternae (~70 nm) are too small to accommodate these large cargos without distension.
Golgi luminal microenvironment
The microenvironment in the Golgi lumen is important for the function of the organelle. The pH of the Golgi is mildly acidic, with the greatest acidification in the TGN (pH ~6.0) (Paroutis et al. 2004). The pH is important for targeting of resident proteins and efficient function of processing enzymes, particularly the furin family of proteases. The luminal pH of acidic organelles is generated and maintained by a combination of proton transport by the v-ATPase, anion transporters that relieve the membrane potential that arises with proton accumulation, and proton leak channels that limit acidification (reviewed by (Rivinoja et al. 2011)). The anion transporter regulating Golgi pH has been elusive, although the Na+/H+ exchanger family has been reported to contribute to Golgi pH (Nakamura et al. 2005) as has the anion exchanger AE2 (Holappa et al. 2004). An anion transporter called GPHR (Golgi pH regulator) was recently shown to be essential for maintaining cisternal pH, and the absence of GPHR leads to reduced membrane traffic and morphological disruption of the Golgi complex (Maeda et al. 2008). Luminal acidification also regulates events on the cytoplasmic side of the membrane, including vesicle assembly. Weak bases (which disrupt acidification), v-ATPase inhibitors, and expression of a pH-activated proton channel all block a number of membrane trafficking steps (reviewed by (Weisz 2003)). One mechanism for how luminal pH can affect vesicular trafficking involves a subunit of the membrane sector of the v-ATPase, which has been shown to act as a pH sensor in endosomes. This subunit undergoes a conformational change as the luminal pH drops, which recruits cytoplasmic machinery leading to subsequent vesicle formation (Hurtado-Lorenzo et al. 2006). TGN acidification is also required for efficient trafficking out of the Golgi, but it is not clear how TGN pH is sensed.
In addition to a proton gradient, the Golgi lumen also contains calcium and manganese; the calcium ATPase PMR1/SPCA1 is responsible for transporting both ions into the Golgi lumen (reviewed in (Van Baelen et al. 2004)). The divalent cation concentration is critical for posttranslational processing of cargo, particularly glycosylation reactions. Recently, a family of Ca2+/cation exchangers has been implicated in calcium and pH homeostasis in the Golgi (Demaegd et al. 2013). Disruption of luminal pH or calcium results in significant morphological changes in the Golgi (Sakaguchi et al. 1996; Maeda et al. 2008; Micaroni et al. 2011), suggesting that the microenvironment inside the organelle is important for its structure as well as its function.
Large exocytic cargo
Exocytosis of large cargo is essential for many physiological processes, including collagen and lipoprotein secretion, and defects in the process lead to disease. How is large cargo accommodated as it moves through the Golgi? Clearly, dilation of cisternae must occur, and intercisternal adhesion may be affected as well. The significant morphological changes that occur during passage of large cargo are completely reversible (see below). How are the dilation and other structural modifications coordinated and are Golgi structural proteins disassembled? Although work on procollagen and chylomicrons has uncovered mechanisms for incorporation into large transport vesicles for export from the ER (Malhotra and Erlmann 2011; Jin et al. 2012; Siddiqi et al. 2010), the mechanism for accommodation inside Golgi cisternae is unknown. Work on exocytosis of enveloped viruses that assemble by budding into the Golgi lumen has begun to answer some of these questions.
Procollagen aggregates
Fibrillar collagens like collagen I are synthesized in fibroblasts as procollagen monomers, which assemble in the ER lumen into a triple helix that forms a rigid rod ~300 nm in length (Leblond 1963). These rods form side-to-side aggregates in the Golgi that can be up to 150 nm in width. Once the procollagen helices are secreted, the propeptides are removed and the typical collagen fibrils form in the extracellular matrix. The export of the 300-nm long rods from the ER requires specific machinery in addition to COPII coat complexes (Venditti et al. 2012; Saito et al. 2009; Saito et al. 2011). Once in the Golgi, the aggregates remain in the cisternae as they traverse the stack (Bonfanti et al. 1998). Transport can be followed in a type of pulse-chase experiment, by reversibly inhibiting prolyl hydroxylase in the ER (which is required for posttranslational modification and subsequent assembly of the triple helix). Large swellings in cisternae can be discerned by electron microscopy as procollagen aggregates are trafficked through the Golgi complex. However, the Golgi cisternae appear to remain stacked during transport of procollagen aggregates, at least in cultured fibroblasts (Bonfanti et al. 1998).
Chylomicrons
Dietary lipids are absorbed by intestinal epithelial cells and converted to lipoproteins called chylomicrons for distribution to the rest of the body. Chylomicrons (≥400 nm in diameter) are the largest secretory cargo known in mammals. Electron micrographs from the 1970′s documenting the passage of chylomicrons through the Golgi in absorptive epithelial cells show unstacked and extremely distended cisternae (Sabesin and Frase 1977; Friedman and Cardell 1972). Yet this cargo is processed by Golgi enzymes and secreted at the basolateral surface. Once the lipids from a meal are cleared from intestinal epithelial cells, Golgi structure returns to a normal appearance, demonstrating that these massive structural alterations are reversible.
Chylomicron assembly (reviewed in (Mansbach and Siddiqi 2009)) begins in the ER of intestinal epithelia, after monoacylglycerols and free fatty acids from digested fats are absorbed at the apical surface. Lipid transfer proteins are essential for movement and transfer of these lipids into the ER lumen, where they are re-esterified into triglycerides and form lipid-rich particles also containing phospholipids and cholesterol. These particles combine with newly synthesized apolipoprotein B (apoB48) to form prechylomicrons, which are then transported to the Golgi in specialized transport vesicles (Siddiqi et al. 2010). Once in the Golgi lumen, the prechylomicrons are further processed, with addition of other apoproteins and lipids, and finally the mature chylomicrons are released at the basolateral surface into the lamina propria, where they are circulated though the lymph to be used throughout the body after lipolysis. Recently, it was shown that the Golgi GTPase ARFRP1 (Arf-related protein 1) is required for release of fully lipidated chylomicrons from the Golgi (Jaschke et al. 2012). ARFRP1 is involved in golgin recruitment to the TGN (Zahn et al. 2008; Setty et al. 2003), but it is not clear how it controls chylomicron processing. Overproduction of chylomicrons in type 2 diabetes (Xiao and Lewis 2012) is likely to prolong changes in Golgi morphology, potentially leading to stress in intestinal epithelial cells. Dissection of the mechanism by which the Golgi can accommodate and transport chylomicrons is also important for understanding chylomicron retention disease and atherosclerosis.
Enveloped viruses that assemble at intracellular membranes
Most well-studied enveloped viruses bud from the plasma membrane, where they are directly released from the cell. Enveloped viruses that assemble at intracellular membranes have an additional challenge in terms of release, since they must traverse the cellular secretory pathway (Griffiths and Rottier 1992). Viruses that assemble by budding into the ER include most flaviviruses such as hepatitis C virus. Besides hepatitis, flaviviruses cause different types of encephalitis (Heinz and Stiasny 2012; Shulla and Randall 2012). Viruses that assemble in Golgi membranes include coronaviruses, bunyaviruses, and rubella virus. Coronaviruses can cause severe respiratory disease in humans [severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)], bunyaviruses cause hemorrhagic fever or encephalitis, and rubella virus causes German measles (de Groot et al. 2013; Bolles et al. 2011; Elliott 2009; Banatvala and Brown 2004). The envelope proteins of these viruses are synthesized in the ER using the same machinery as host membrane proteins, and they are then targeted to the assembly compartment prior to budding. Once budded into the lumen of the ER or Golgi, the particles range from 70 to 160 nm, and dilation and even disassembly of the Golgi have been observed during infection (Ulasli et al. 2010; Gahmberg et al. 1986; Lavi et al. 1996). The advantage(s) of intracellular assembly are unknown. However, the efficient release of infectious virus appears to require modification of the secretory pathway (see below). It is likely that these viruses evolved to take advantage of normal cellular processes that allow intra-Golgi transport of large cargo in specialized cell types.
Coronaviruses: a model for exocytosis of large cargo
Coronaviruses (CoVs) acquire their envelopes from the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), also called the cis Golgi network (Klumperman et al. 1994). The three main envelope proteins (S, M, and E) are synthesized in the ER and move to the ERGIC/Golgi region where they orchestrate assembly of virus by interacting with the viral nucleocapsid [reviewed in (Hogue and Machamer 2008)] (Fig. 1). Once virions have budded into the lumen of the ERGIC, the ~120 nm particles must move through the host secretory pathway to be released from infected cells. Coronaviruses are believed to follow the constitutive secretory pathway for exocytosis, although only a few studies have addressed the release of virions. During infection, a progressive disruption of Golgi structure is observed, with swollen unstacked cisternae late in infection (Fig. 2).
Fig. 1.
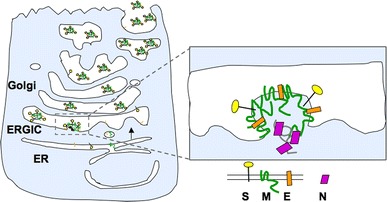
Model for coronavirus assembly in Golgi membranes. The envelope proteins (S, M, and E) are synthesized in the ER and transported to the Golgi region, where they interact with the viral RNA genome (replicated in the cytoplasm) bound to the N protein. Virions are exocytosed through the secretory pathway
Fig. 2.
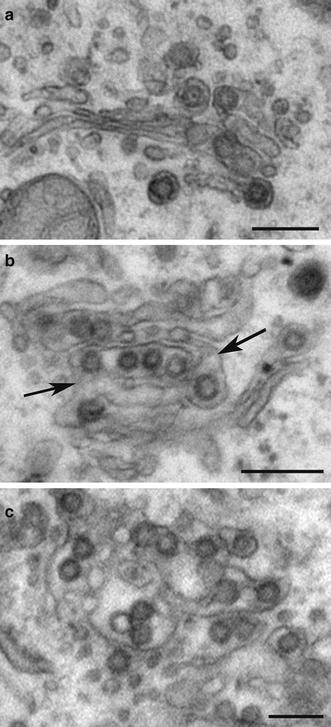
Progressive dilation of Golgi membranes during coronavirus infection. Vero cells infected with wild-type IBV for 14 h were fixed and processed for electron microscopy. a Several virions in a relatively intact Golgi stack. b Increased dilation of Golgi cisternae containing more virions, with possible intercisternal connections (arrows). c Extensive dilation of a Golgi region. Bars 200 nm
The CoV E protein and virus egress
The E protein is incorporated into CoV virions at a low level despite its robust expression in infected cells and is essential for efficient virus production (Fischer et al. 1998; Kuo and Masters 2003; Ortego et al. 2002). The E protein may induce membrane curvature or membrane scission to enhance particle production. Another role for the coronavirus E protein is in efficient release of infectious virus from cells. This role is thought to involve the population of E that is not incorporated into the viral envelope.
Coronaviruse E proteins are similar in size and topology to several other viral proteins known as “viroporins,” some of which have been shown to be ion channels (Wang et al. 2011). The influenza virus M2 protein is the best-studied virus ion channel. This pH-activated proton channel is a small membrane protein that spans the bilayer with a N exo–C cyto topology, and the active channel is a homo-tetramer (Pinto et al. 1992). M2 plays an essential role in influenza virus infection, and the residues in the transmembrane domain involved in pore formation and regulation have been identified (Pinto et al. 1992; Fischer and Sansom 2002). CoV E proteins (76–109 amino acids in length) also adopt a predominantly N exo–C endo topology in the membrane (Fig. 3a) (Ruch and Machamer 2012b; Corse and Machamer 2000; Nieto-Torres et al. 2011) and are targeted to the Golgi region in infected and transfected cells (Corse and Machamer 2000; Lopez et al. 2006; Raamsman et al. 2000; Liao et al. 2006). Synthetic peptides corresponding to full-length E proteins from four different coronaviruses demonstrated cation-specific currents in planar bilayers (Wilson et al. 2004; Wilson et al. 2006). Since CoV E protein has a single transmembrane domain, it must oligomerize to produce a channel. Computational modeling and spectroscopy studies support the formation of a homo-pentamer (Pervushin et al. 2009; Torres et al. 2007; Parthasarathy et al. 2008). Although direct evidence that the CoV E protein functions as an ion channel in infected cells is lacking, it has been shown that hexamethylene amiloride blocks mouse hepatitis virus replication in an E-dependent manner (Wilson et al. 2006). In addition, different CoV E proteins can complement the growth defect of mouse hepatitis virus when the E protein gene is deleted (Kuo et al. 2007), even though their sequences are quite divergent.
Fig. 3.
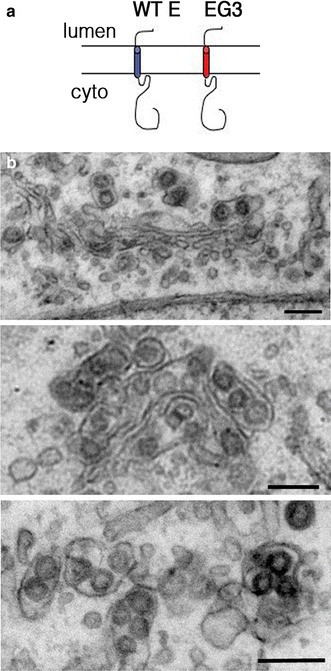
Golgi dilation occurs in the absence of CoV E protein ion channel function. a Membrane topology of IBV E and a mutant E with a heterologous transmembrane domain sequence derived from VSV-G (EG3). b Vero cells infected with IBV-EG3 were processed for electron microscopy at 14 h post-infection. The three panels show increasing dilation of Golgi cisternae similar to infection with wild-type IBV. Bars 200 nm
We use the infectious bronchitis virus (IBV) as a model CoV and discovered a role for the IBV E protein in virus release from infected cells (Machamer and Youn 2006; Ruch and Machamer 2011, 2012a). To examine the role of the putative ion channel, we replaced the transmembrane domain of E protein with a heterologous transmembrane domain (“EG3,” Fig. 3a) and generated a recombinant IBV with the EG3 sequence substituted for that of wild-type E. The mutant virus (IBV-EG3) produces virions that fail to be efficiently released from infected cells, although assembly is normal. IBV-EG3 virions accumulate in large intracellular vacuoles where they may be damaged by proteases prior to release. One idea is that the microenvironment in the lumen of the Golgi must be altered in a reversible manner to permit passage of virions. The IBV E protein might perturb the luminal pH directly as an ion channel or indirectly by interacting with a Golgi ion transporter. To test how IBV E affects cargo trafficking in the absence of infection, we overexpressed the protein from cDNA. Surprisingly, overexpression of IBV E leads to reduced trafficking of secreted and membrane cargo proteins through the Golgi, which was opposite of the expectation for a protein that seemed to enhance virus egress (Ruch and Machamer 2011). We concluded that the E-induced reduction in the rate of exocytosis was an acceptable compromise for the virus in ensuring release of infectious particles. Overexpression of IBV E also induced Golgi disassembly, although it is not known if this is a result of the trafficking defect or the cause of it. Mutation of the residues in the E transmembrane domain implicated in ion channel activity abrogates the trafficking defects and Golgi disruption, suggesting that the alterations in the Golgi correlate with efficient exocytosis of infectious virus particles (Ruch and Machamer 2012b). We concluded that the putative channel activity of the CoV E protein was required for efficient release of infectious virus from infected cells and proposed that altering the luminal microenvironment of the Golgi was essential for this process.
If the transmembrane domain of CoV E and its ion channel activity were both required for dilation of Golgi cisternae to accommodate virions, we would predict that such dilation would not occur in cells infected with IBV-EG3. Surprisingly, however, dilation does occur when the putative ion channel activity is abrogated (Fig. 3b), suggesting that the large cargo itself may be responsible. The presence of the cargo may induce rounding of flat cisternae by simply taking up space inside the lumen. Alternatively, a signal from the lumen to peripheral proteins like GRASPs may trigger disassembly that allows the rounding of cisternae. If the microenvironment alteration induced by the CoV E protein is not required for dilation of cisternae, what role does it play in efficient exocytosis of infectious virus? IBV E may alkalinize Golgi compartments to prevent damage to virions if they meet up with endosomal proteases or to promote a maturation step that could protect virions from host proteases. Alternatively, E may play an indirect role in directing virus carriers to fuse with the plasma membrane instead of the lysosomal system.
Release of other enveloped viruses that assemble intracellularly
The putative coronavirus ion channel activity supplied by the E protein is required for efficient release of infectious virus from cells. What about the other classes of enveloped viruses that bud into the ER or Golgi? Hepatitis C virus (HCV) encodes a cation channel called p7 that plays an important role in virus release after budding into the ER lumen (Wozniak et al. 2010). This small membrane protein (63 amino acids) forms an unusual flowerlike hexamer in the membrane (Ouyang et al. 2013) with a structure very different from the influenza M2 proton channel (Pinto et al. 1992) and the proposed coronavirus E channel (Torres et al. 2007). HCV p7 is proposed to neutralize acidic compartments via equilibration of proton gradients, thus protecting intracellular virions during egress (Wozniak et al. 2010).
Bunyaviruses and rubella virus have not been reported to encode ion channels. However, the Golgi complex is disrupted in cells infected with the Uukuniemi bunyavirus (Gahmberg et al. 1986). The disrupted Golgi can still transport and process cargo (Gahmberg et al. 1986), similar to the coronavirus findings. Cells infected with rubella virus also show some Golgi rearrangements, but this has not been studied in terms of virus egress (Risco et al. 2003).
Potential mechanisms for accommodation of large cargo in Golgi cisternae
Signaling the presence of cargo
A bolus of cargo arriving at the cis Golgi has been to shown to activate Src family kinase (SFK) signaling (Pulvirenti et al. 2008; Giannotta et al. 2012). The experimental conditions that deliver a large cargo bolus include releasing a block in ER export of the vesicular stomatitis virus G protein (a temperature-sensitive mutant) or reversing the inhibition of procollagen folding in the ER. The current model for the role of SFK signaling in cargo traffic includes the following steps (Giannotta et al. 2012): (1) a cargo bolus arriving from the ER carries with it some level of ER chaperones (KDEL proteins), (2) the KDEL proteins bind the KDEL receptor, which in turn activates Gαq/11, leading to local SFK activation at the Golgi, possibly through local Ca2+ efflux after phospholipase C activation, and (3) SFK phosphorylation of trafficking machinery, including dynamin-2 (Weller et al. 2010), which ensures efficient transport. Whether constitutive delivery of cargo requires SFK signaling is an open question, as is the requirement for transport of large spherical cargo including chylomicrons or enveloped viruses.
Dilation of cisternae
Given the rapid rate of dilation of Golgi cisternae in enterocytes after fat absorption (Sabesin and Frase 1977; Hamilton et al. 1998), it is unlikely that new membrane synthesis accounts for the structural alterations as large cargo enters the Golgi. Instead, it is probable that the volume increase is due to rearrangement of the flat cisternae into more spherical shapes. A significant volume increase can occur by dilation without changing the surface area of a flat cisterna (Derganc et al. 2006). Dilation could involve influx of osmotically active ions followed by water influx, loss of intraluminal connections that keep cisternae flat, and/or remodeling of GRASPs and golgins that maintain Golgi structure from the cytoplasmic face. There is precedent for rapid Golgi dilation in cells treated with the Na+/H+ ionophore monensin, where alkalinization of the Golgi lumen results in swollen Golgi cisternae within minutes, starting on the trans face and subsequently involving the entire organelle (Dinter and Berger 1998). Other perturbations that impact Golgi luminal pH (expression of the influenza M2 proton channel or depletion of the Golgi pH regulator (GPHR) anion channel) also result in swollen Golgi cisternae (Sakaguchi et al. 1996; Maeda et al. 2008), suggesting that pH is a good indicator of ionic imbalance leading to water influx. The dilation could also involve SFK signaling but perhaps require a more robust level than stimulated by “small” cargo.
Disassembly of peripheral Golgi structural proteins
GRASP65 and GRASP55 are known to mediate cisternal stacking (Ramirez and Lowe 2009). GRASP disassembly might be an early change during cisternal dilation, since it appears that cisternae come unstacked in intestinal cells producing chylomicrons and in CoV-infected cells. The status of GRASP assembly has not yet been examined in cells producing large cargo. In mitotic cells, GRASP disassembly is mediated by phosphorylation: GRASP55 is phosphorylated through the MAP kinase pathway, and GRASP65 is phosphorylated by Plk and Cdc2 (Xiang and Wang 2010; Wang et al. 2003). However, GRASP65 can also be phosphorylated by ERK2 during interphase (Yoshimura et al. 2005), which is required for establishment of cell polarity by Golgi remodeling during cell migration (Bisel et al. 2008). This suggests that remodeling of Golgi membranes during interphase requires GRASP disassembly. It will be important to assess GRASP disassembly during passage of large cargo through the Golgi, but it is unlikely that this is the sole mechanism of cisternal dilation. Depletion of both GRASP65 and GRASP55 by RNAi results in complete unstacking, but the Golgi cisternae retain a fairly normal shape (Xiang and Wang 2010).
Directions for the future
It is clear that many questions remain in terms of understanding Golgi dynamics and large cargo trafficking. Many of these questions hinge on our poor understanding of how Golgi cisternae normally remain flat. The open questions include (1) Is rod-shaped cargo (procollagen aggregates) handled differently than spherical cargo such as chylomicrons? (2) Does dilation of cisternae require osmotically active ion transport and water influx or can large cargo itself induce the internal or peripheral structural changes required for accommodation of the cargo? (3) Does dilation reduce efficiency of cargo processing? (4) Does large cargo traverse ministacks (induced by microtubule depolymerization) at the same rate as in an intact Golgi ribbon? (5) Is SFK signaling more robust for large cargo than for small cargo? (6) Do enveloped viruses that bud into the Golgi need to activate SFK signaling independently of the normal mechanism (KDEL protein leak) since they are not trafficked from the ER? Many of these questions can be answered with experimental systems currently available.
A final question involves reversal of the significant morphological changes in Golgi membranes once the large cargo has moved past the Golgi complex. If cisternal maturation were the sole mechanism of transport, then recovery would be accomplished as new cisterna lacking the cargo form on the cis face. If, however, at least part of the stack were stable (Lavieu et al. 2013), the reduction in volume and flattening of the cisternae would have to be a more active process. Presumably, this would involve reversing the processes that induced the dilation in the first place. Answering this question and those above will go a long way toward understanding how large cargo is accommodated during trafficking through the Golgi complex, and how defects in the process contribute to disease. Importantly, these answers will also lead to a better understanding of the mechanism for maintaining flattened Golgi cisternae as well as the advantage of this structure for the cell.
Acknowledgments
The work in the author’s laboratory is supported by the National Institutes of Health (GM42522). I thank Travis Ruch and Jan Hoh for helpful discussions, and Catherine Gilbert and Jason Westerbeck for useful comments on the manuscript.
References
- Banatvala JE, Brown DW. Rubella. Lancet. 2004;363(9415):1127–1137. doi: 10.1016/S0140-6736(04)15897-2. [DOI] [PubMed] [Google Scholar]
- Bisel B, Wang Y, Wei JH, Xiang Y, Tang D, Miron-Mendoza M, Yoshimura S, Nakamura N, Seemann J. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182(5):837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M, Donaldson E, Baric R. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr Opin Virol. 2011;1(6):624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA, Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95(7):993–1003. doi: 10.1016/S0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Corse E, Machamer CE. Infectious bronchitis virus E protein is targeted to the Golgi complex and directs release of virus-like particles. J Virol. 2000;74(9):4319–4326. doi: 10.1128/JVI.74.9.4319-4326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish Z, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J. Middle east respiratory syndrome coronavirus (MERS-CoV); Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D, Foulquier F, Colinet AS, Gremillon L, Legrand D, Mariot P, Peiter E, Van Schaftingen E, Matthijs G, Morsomme P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci USA. 2013;110(17):6859–6864. doi: 10.1073/pnas.1219871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derganc J, Mironov AA, Svetina S. Physical factors that affect the number and size of Golgi cisternae. Traffic. 2006;7(1):85–96. doi: 10.1111/j.1600-0854.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Dinter A, Berger EG. Golgi-disturbing agents. Histochem Cell Biol. 1998;109(5–6):571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- Elliott RM. Bunyaviruses and climate change. Clin Microbiol Infect. 2009;15(6):510–517. doi: 10.1111/j.1469-0691.2009.02849.x. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8(1):2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer WB, Sansom MS. Viral ion channels: structure and function. Biochim Biophys Acta. 2002;1561(1):27–45. doi: 10.1016/S0304-4157(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Fischer F, Stegen CF, Masters PS, Samsonoff WA. Analysis of constructed E gene mutants of mouse hepatitis virus confirms a pivotal role for E protein in coronavirus assembly. J Virol. 1998;72(10):7885–7894. doi: 10.1128/jvi.72.10.7885-7894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HI, Cardell RR., Jr Effects of puromycin on the structure of rat intestinal epithelial cells during fat absorption. J Cell Biol. 1972;52(1):15–40. doi: 10.1083/jcb.52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg N, Kuismanen E, Keranen S, Pettersson RF. Uukuniemi virus glycoproteins accumulate in and cause morphological changes of the Golgi complex in the absence of virus maturation. J Virol. 1986;57(3):899–906. doi: 10.1128/jvi.57.3.899-906.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta M, Ruggiero C, Grossi M, Cancino J, Capitani M, Pulvirenti T, Consoli GM, Geraci C, Fanelli F, Luini A, Sallese M. The KDEL receptor couples to Galphaq/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 2012;31(13):2869–2881. doi: 10.1038/emboj.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3(11):a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992;3(5):367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39(8):1543–1557. [PubMed] [Google Scholar]
- Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30(29):4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- Hogue BG, Machamer CE. Coronavirus structural proteins and virus assembly. Nidoviruses. Washington, DC: ASM Press; 2008. [Google Scholar]
- Holappa K, Munoz MT, Egea G, Kellokumpu S. The AE2 anion exchanger is necessary for the structural integrity of the Golgi apparatus in mammalian cells. FEBS Lett. 2004;564(1–2):97–103. doi: 10.1016/S0014-5793(04)00315-1. [DOI] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8(2):124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- Jaschke A, Chung B, Hesse D, Kluge R, Zahn C, Moser M, Petzke KJ, Brigelius-Flohe R, Puchkov D, Koepsell H, Heeren J, Joost HG, Schurmann A. The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium. Hum Mol Genet. 2012;21(14):3128–3142. doi: 10.1093/hmg/dds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482(7386):495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J (2011) Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol 3(7):a005181 [DOI] [PMC free article] [PubMed]
- Klumperman J, Locker JK, Meijer A, Horzinek MC, Geuze HJ, Rottier PJ. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J Virol. 1994;68(10):6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L, Masters PS. The small envelope protein E is not essential for murine coronavirus replication. J Virol. 2003;77(8):4597–4608. doi: 10.1128/JVI.77.8.4597-4608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L, Hurst KR, Masters PS. Exceptional flexibility in the sequence requirements for coronavirus small envelope protein function. J Virol. 2007;81(5):2249–2262. doi: 10.1128/JVI.01577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E, Wang Q, Weiss SR, Gonatas NK. Syncytia formation induced by coronavirus infection is associated with fragmentation and rearrangement of the Golgi apparatus. Virology. 1996;221(2):325–334. doi: 10.1006/viro.1996.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Zheng H, Rothman JE. Stapled Golgi cisternae remain in place as cargo passes through the stack. Elife. 2013;2:e00558. doi: 10.7554/eLife.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP. Elaboration of dentinal collagen in odontoblasts as shown by radioautography after injection of labelled glycine and proline. Ann Histochim. 1963;8:43–50. [PubMed] [Google Scholar]
- Liao Y, Yuan Q, Torres J, Tam JP, Liu DX. Biochemical and functional characterization of the membrane association and membrane permeabilizing activity of the severe acute respiratory syndrome coronavirus envelope protein. Virology. 2006;349(2):264–275. doi: 10.1016/j.virol.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LA, Jones A, Arndt WD, Hogue BG. Subcellular localization of SARS-CoV structural proteins. Adv Exp Med Biol. 2006;581:297–300. doi: 10.1007/978-0-387-33012-9_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Youn S. The transmembrane domain of the infectious bronchitis virus E protein is required for efficient virus release. Adv Exp Med Biol. 2006;581:193–198. doi: 10.1007/978-0-387-33012-9_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Ide T, Koike M, Uchiyama Y, Kinoshita T. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat Cell Biol. 2008;10(10):1135–1145. doi: 10.1038/ncb1773. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Erlmann P. Protein export at the ER: loading big collagens into COPII carriers. EMBO J. 2011;30(17):3475–3480. doi: 10.1038/emboj.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu Rev Physiol. 2009;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micaroni M, Perinetti G, Berrie CP, Mironov AA. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic. 2011;11(10):1315–1333. doi: 10.1111/j.1600-0854.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583(23):3738–3745. doi: 10.1016/j.febslet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na +/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280(2):1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Nieto-Torres JL, Dediego ML, Alvarez E, Jimenez-Guardeno JM, Regla-Nava JA, Llorente M, Kremer L, Shuo S, Enjuanes L. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology. 2011;415(2):69–82. doi: 10.1016/j.virol.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Escors D, Laude H, Enjuanes L. Generation of a replication-competent, propagation-deficient virus vector based on the transmissible gastroenteritis coronavirus genome. J Virol. 2002;76(22):11518–11529. doi: 10.1128/JVI.76.22.11518-11529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang B, Xie S, Berardi MJ, Zhao X, Dev J, Yu W, Sun B, Chou JJ. Unusual architecture of the p7 channel from hepatitis C virus. Nature. 2013;498(7455):521–525. doi: 10.1038/nature12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P, Touret N, Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- Parthasarathy K, Ng L, Lin X, Liu DX, Pervushin K, Gong X, Torres J. Structural flexibility of the pentameric SARS coronavirus envelope protein ion channel. Biophys J. 2008;95(6):L39–L41. doi: 10.1529/biophysj.108.133041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K, Tan E, Parthasarathy K, Lin X, Jiang FL, Yu D, Vararattanavech A, Soong TW, Liu DX, Torres J. Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 2009;5(7):e1000511. doi: 10.1371/journal.ppat.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. How the Golgi works: a cisternal progenitor model. Proc Natl Acad Sci USA. 2010;107(46):19614–19618. doi: 10.1073/pnas.1011016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69(3):517–528. doi: 10.1016/0092-8674(92)90452-I. [DOI] [PubMed] [Google Scholar]
- Pulvirenti T, Giannotta M, Capestrano M, Capitani M, Pisanu A, Polishchuk RS, San Pietro E, Beznoussenko GV, Mironov AA, Turacchio G, Hsu VW, Sallese M, Luini A. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008;10(8):912–922. doi: 10.1038/ncb1751. [DOI] [PubMed] [Google Scholar]
- Raamsman MJ, Locker JK, de Hooge A, de Vries AA, Griffiths G, Vennema H, Rottier PJ. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J Virol. 2000;74(5):2333–2342. doi: 10.1128/JVI.74.5.2333-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20(7):770–779. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Risco C, Carrascosa JL, Frey TK. Structural maturation of rubella virus in the Golgi complex. Virology. 2003;312(2):261–269. doi: 10.1016/S0042-6822(03)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivinoja A, Pujol FM, Hassinen A, Kellokumpu S. Golgi pH, its regulation and roles in human disease. Ann Med. 2011;44(6):542–554. doi: 10.3109/07853890.2011.579150. [DOI] [PubMed] [Google Scholar]
- Ruch TR, Machamer CE. The hydrophobic domain of infectious bronchitis virus E protein alters the host secretory pathway and is important for release of infectious virus. J Virol. 2011;85(2):675–685. doi: 10.1128/JVI.01570-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch TR, Machamer CE. The coronavirus E protein: assembly and beyond. Viruses. 2012;4(3):363–382. doi: 10.3390/v4030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch TR, Machamer CE. A single polar residue and distinct membrane topologies impact the function of the infectious bronchitis coronavirus E protein. PLoS Pathog. 2012;8(5):e1002674. doi: 10.1371/journal.ppat.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesin SM, Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18(4):496–511. [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136(5):891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22(13):2301–2308. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Leser GP, Lamb RA. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol. 1996;133(4):733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Shin ME, Yoshino A, Marks MS, Burd CG. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr Biol. 2003;13(5):401–404. doi: 10.1016/S0960-9822(03)00089-7. [DOI] [PubMed] [Google Scholar]
- Shulla A, Randall G. Hepatitis C virus-host interactions, replication, and viral assembly. Curr Opin Virol. 2012;2(6):725–732. doi: 10.1016/j.coviro.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM., 2nd A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res. 2010;51(7):1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Maheswari U, Parthasarathy K, Ng L, Liu DX, Gong X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007;16(9):2065–2071. doi: 10.1110/ps.062730007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulasli M, Verheije MH, de Haan CA, Reggiori F. Qualitative and quantitative ultrastructural analysis of the membrane rearrangements induced by coronavirus. Cell Microbiol. 2010;12(6):844–861. doi: 10.1111/j.1462-5822.2010.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742(1–3):103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A, Zelante L, Piemontese MR, Notarangelo A, Malhotra V, Vertel BM, Wilson C, Matteis MA. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337(6102):1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22(13):3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xie S, Sun B. Viral proteins function as ion channels. Biochim Biophys Acta (1808) 2011;2:510–515. doi: 10.1016/j.bbamem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz OA. Acidification and protein traffic. Int Rev Cytol. 2003;226:259–319. doi: 10.1016/S0074-7696(03)01005-2. [DOI] [PubMed] [Google Scholar]
- Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, McNiven MA. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc Natl Acad Sci USA. 2010;107(13):5863–5868. doi: 10.1073/pnas.0915123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, McKinlay C, Gage P, Ewart G. SARS coronavirus E protein forms cation-selective ion channels. Virology. 2004;330(1):322–331. doi: 10.1016/j.virol.2004.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Gage P, Ewart G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology. 2006;353(2):294–306. doi: 10.1016/j.virol.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak AL, Griffin S, Rowlands D, Harris M, Yi M, Lemon SM, Weinman SA. Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production. PLoS Pathog. 2010;6(9):e1001087. doi: 10.1371/journal.ppat.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Lewis GF. Regulation of chylomicron production in humans. Biochim Biophys Acta (1821) 2012;5:736–746. doi: 10.1016/j.bbalip.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Yoshioka K, Barr FA, Lowe M, Nakayama K, Ohkuma S, Nakamura N. Convergence of cell cycle regulation and growth factor signals on GRASP65. J Biol Chem. 2005;280(24):23048–23056. doi: 10.1074/jbc.M502442200. [DOI] [PubMed] [Google Scholar]
- Zahn C, Jaschke A, Weiske J, Hommel A, Hesse D, Augustin R, Lu L, Hong W, Florian S, Scheepers A, Joost HG, Huber O, Schurmann A. ADP-ribosylation factor-like GTPase ARFRP1 is required for trans-Golgi to plasma membrane trafficking of E-cadherin. J Biol Chem. 2008;283(40):27179–27188. doi: 10.1074/jbc.M802108200. [DOI] [PubMed] [Google Scholar]


