Abstract
Background
Calcium intake has been promoted due to its proposed benefit on bone health, particularly among the older population. However, concerns have been raised about the potential adverse effect of high calcium intake on cardiovascular health.
Methods
Dietary and supplemental calcium intakes were assessed at baseline (1995–96) in 388,229 men and women aged 50–71 years in the National Institutes of Health (NIH)–AARP Diet and Health Study. Supplemental calcium intake included calcium from multivitamins and individual calcium supplements. Cardiovascular disease (CVD) deaths were ascertained using the National Death Index. Multivariate Cox Proportional hazard models adjusted for demographic, lifestyle and dietary variables were used to estimate relative risks (RRs) and 95% confidence intervals (CIs).
Results
During an average of 12 years of follow-up, 7904 and 3874 CVD deaths in men and women, respectively, were identified. Supplements containing calcium were used by 51% of men and 70% of women. In men supplemental calcium intake was associated with an elevated risk of CVD death (RR>1000 vs. 0 mg/day =1.20, 95% CI: 1.05–1.36), more specifically with heart disease death (RR=1.19, 95% CI: 1.03–1.37), but not significantly with cerebrovascular disease death (RR=1.14, 95% CI: 0.81–1.61). In women, supplemental calcium intake was not associated with CVD death (RR= 1.06, 95% CI: 0.96, 1.18), heart disease death (RR=1.05, 95% CI: 0.93–1.18) or cerebrovascular disease death (RR=1.08, 95% CI: 0.87–1.33). Dietary calcium intake was not related to CVD death in either men or women.
Conclusion
Our finding suggests that high intake of supplemental calcium is associated with an excess risk of CVD death in men, but not in women. Additional studies are needed to investigate the effect of supplemental calcium use beyond bone health.
Introduction
In western countries, great emphasis has been put on calcium intake due to its proposed benefit on bone health. Calcium supplementation has become widely used, especially among the elderly. A recent study reported over 50% of older men and almost 70% of older women in the US use supplemental calcium 1. However, beyond calcium’s established role in prevention and treatment of osteoporosis, its health impact on nonskeletal outcomes, including cardiovascular health, remains largely unknown and has become increasingly contentious2–3.
Despite some earlier observational and interventional studies that suggested a protective role of calcium against cardiovascular diseases (CVD) by linking supplemental calcium intake with improved blood pressure or serum lipid profiles 4–6, recent analyses of several randomized controlled trials (RCTs) showed an increased risk of various cardiovascular events, including myocardial infarction, stroke and cardiovascular deaths, in the intervention arm with calcium supplementation 7–9. Likewise, the effects of dietary calcium intake on various cardiovascular outcomes also remain controversial, with most of the observational studies revealing inverse 10–11 or null associations 12–14. The heterogeneity of the aforementioned studies and inconsistency in their results warrant further investigation into the relation between calcium intake and cardiovascular health. Therefore, in a large cohort of US men and women, we investigated whether intakes of both dietary and supplemental calcium were associated with mortality from total cardiovascular disease, heart disease and cerebrovascular diseases.
Methods
Study population
The NIH-AARP Diet and Health Study recruited AARP members who were 50 to 71 years old and resided in one of six US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia, and Detroit, Michigan) in 1995–1996. Details of the NIH-AARP study were reported previously 15. Of 566,399 participants who satisfactorily completed baseline questionnaire, we excluded individuals whose questionnaire was completed by proxies (n=15,760), and those who had cancer except nonmelanoma skin cancer (n=51,227), self-reported heart disease (n=69,025), stroke (n=6,477), diabetes (n=30,990), or end-stage renal disease at baseline (n=447). Additionally, we excluded individuals who reported extreme intakes (>2 times the interquartile ranges of sex-specific log-transformed intake) of total energy and dietary calcium (n=4,244). The analytic cohort consisted of 219,059 men and 169,170 women. The study was approved by the National Cancer Institute Special Studies Institutional Review Board.
Mortality ascertainment
The vital status of study participants was ascertained by annual linkage to the Social Security Administration Death Master File. Cause of death information is provided by follow-up searches of the National Death Index (NDI) Plus. A previous study found that our ascertainment method yielded 95% accurate results 16. Total CVD mortality (International Classification of Diseases, Ninth Revision [ICD-9] codes 390–398, 401–404, 410–438, and 440–448 and ICD, 10th Revision [ICD-10] codes I00–I09, I10–I13, I20–I51, and I60–I78) included deaths from heart diseases, cerebrovascular diseases and other CVDs.
Calcium intake and risk factor assessment
At baseline, dietary intakes were assessed with a self-administered 124-item food-frequency questionnaire (FFQ), an earlier version of the Diet History Questionnaire developed at the National Cancer Institute17. Participants reported their usual frequency of intake and portion size during the past year. The food items, portion sizes, and nutrient database were constructed using the US Department of Agriculture’s 1994–1996 Continuing Survey of Food Intakes by Individuals 18. The questionnaire also asked participants about the frequencies (never, <1 per week, 1–3 times per week, 4–6 times per week, or every day) and dosage of individual calcium supplements, including calcium-containing antacids (eg, Tums). In addition, participants reported the frequencies and types of multivitamin intake (“stress-tab type”, “therapeutic or theragran type”, and “one-a-day type”). Calcium intake was estimated from foods only (dietary calcium), from supplements only (supplemental calcium), including both individual calcium supplement and calcium-containing multivitamins (“therapeutic or theragran type” and “one-a-day type”) and from both sources (total calcium). Dietary calcium intake was adjusted for total energy intake using the residual method 19. The FFQ used in our study was calibrated against two non-consecutive 24-hour dietary recalls in a subgroup of participants 20, with an energy-adjusted correlation coefficient of dietary calcium intake of 0.63 in men and 0.64 in women.
The baseline questionnaire also asked about demographic characteristics, anthropometric measurements, medical history, and other lifestyle factors. A subsequent questionnaire mailed within 6 months the baseline collected further information on diagnosis of hypertension and hypercholesterolemia as well as the use of medications such as non-steroidal anti-inflammatory drugs.
Statistical analysis
Relative risks (RR) and 2-sided 95% confidence intervals (CI) were estimated with the Cox proportional hazards model, using SAS (SAS Institute, Cary, North Carolina). Person-years of follow-up time were calculated from the baseline until the date of death, or the end of follow-up (December 31, 2008), whichever came sooner. We evaluated and confirmed the proportional hazards assumption for the main exposures by including interaction terms with time and using the Wald χ2 procedure to test if coefficients equaled zero.
There was a significant interaction by sex (p=0.001), therefore we conducted analysis and report results separately for men and women. Intakes of dietary and total calcium were categorized into sex-specific quintiles. Test for linear trend were performed using the median value in each quintile or category.
Multivariate models were adjusted for potential confounders, including age, race/ethnicity, education, marital status, self-reported health status, body mass index (BMI), physical activity, smoking status, smoking dose, years since quitting smoking, and intakes of alcohol, fruit and vegetable, red meat, whole grain, fat and total energy. Menopausal hormone therapy use was adjusted in women. Supplemental and dietary calcium intakes were mutually adjusted. For each covariate, missing values (generally <5%) were put in the reference group. Assigning missing values into separate groups did not change the results materially. We also examined the potentially non-linear relationship between total calcium intake and risk of total CVD mortality using non-parametric regression analyses21–22. A likelihood ratio test was used to compare the model with both the linear and the cubic spline terms with the model with the linear term only.
Results
During 3,549,364 person-years of follow-up, we identified 7,904 CVD deaths in men and 3,874 CVD deaths in women. Overall, 23% of men and 56% of women took individual calcium supplements and 56% of men and 58% of women took multivitamins containing calcium. Compared with participants in the lowest quintile of dietary calcium intake, or nonusers of calcium supplement, those in the highest quintile or supplement users were more likely to be non-Hispanic white, to have a college education, to have self-rated their health as being excellent, to be physically active, to use multivitamins, and to have higher intakes of fruits and vegetables, and whole grains, but they were less likely to smoke or have a history of hypertension, and had lower consumption of alcohol, red meat and total fat. Compared to women who were non-users, women who used calcium supplement had lower BMI and were more likely to use menopausal hormone therapy (table 1).
Table 1.
Selected Characteristics of Study Participants by Categories of Dietary and Supplemental Calcium Intakes *
| Variable a | Dietary Calcium | Supplemental Calcium | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
|
| ||||||||
| Q1 | Q5 | Q1 | Q5 | Non-user | User | Non-user | User | |
| Age at baseline | 61.3 | 62.0 | 61.2 | 62.1 | 61.6 | 61.8 | 61.6 | 61.6 |
| Dietary calcium, mg/d | 463 | 1336 | 397 | 1170 | 782 | 815 | 681 | 719 |
| Supplemental calcium, mg/d | 127 | 163 | 336 | 423 | 0 | 289 | 0 | 554 |
| White, non-Hispanic, % | 90 | 94 | 86 | 93 | 93 | 93 | 88 | 91 |
| College and postcollege, % | 40 | 49 | 26 | 35 | 45 | 48 | 27 | 33 |
| Married, % | 83 | 84 | 46 | 42 | 86 | 84 | 45 | 45 |
| Self-reported health, excellent, % | 19 | 24 | 17 | 22 | 22 | 22 | 19 | 20 |
| BMI, kg2/m | 27.0 | 27.0 | 26.6 | 26.2 | 27.2 | 26.9 | 27.1 | 26.1 |
| Current smoker, % | 16 | 9 | 21 | 11 | 12 | 10 | 18 | 13 |
| Former smoker, % | 54 | 52 | 35 | 37 | 53 | 55 | 35 | 36 |
| Physical activity >= 5 times/wk, % | 17 | 24 | 13 | 20 | 20 | 23 | 14 | 18 |
| History of hypertension, % | 37 | 33 | 35 | 30 | 35 | 35 | 35 | 32 |
| History of high cholesterol, % | 46 | 47 | 50 | 50 | 47 | 46 | 50 | 49 |
| Multivitamin use, % b | 47 | 56 | 55 | 66 | 17 | 90 | 14 | 81 |
| Current MHT use, % | NA | NA | 42 | 47 | NA | NA | 37 | 49 |
| alcohol consumption, g/d | 36.5 | 9.0 | 11.2 | 3.7 | 40.8 | 17.5 | 6.1 | 6.2 |
| Fruits and vegetables, servings/1000 kcal | 3.1 | 3.6 | 3.9 | 4.5 | 3.4 | 3.7 | 4.2 | 4.5 |
| Red meat, g/1000 kcal | 45 | 30 | 36 | 21 | 40 | 37 | 32 | 28 |
| whole grains, servings/1000 kcal | 0.47 | 0.74 | 0.52 | 0.74 | 0.62 | 0.68 | 0.63 | 0.70 |
| total fat, % of energy | 31 | 29 | 33 | 26 | 31 | 30 | 31 | 29 |
| total energy, kcal/d | 2071 | 2058 | 1569 | 1562 | 2037 | 2041 | 1572 | 1563 |
| magnesium intake, mg/d | 191 | 256 | 198 | 286 | 175 | 265 | 188 | 270 |
Abbreviations: BMI, body mass index; MHT, menopausal hormonal therapy; NA, not applicable.
All within-sex group comparisons were significant (p<.05) using the Kruskal Wallis (for continuous variables) and Chi-sq (for categorical variables) test
Mean values otherwise specified
Multivitamins included the “stress-tab type”, “therapeutic or theragran type”, and “one-a-day type”. Only the latter two contained calcium
In both men and women, dietary calcium intakes were inversely associated with both total CVD and heart disease mortality in age-adjusted models (table 2). However after adjusting for potential CVD risk factors, the associations were substantially attenuated and became null in women. Among factors controlled in the multivariate model, variables related to smoking were the strongest confounders. Restricting analyses to supplemental calcium nonusers did not change the associations between dietary calcium intake and CVD mortality (data not shown).
Table 2.
Relative Risks and 95% Confidence Intervals for Cardiovascular Disease (CVD) Deaths for Quintiles of Dietary Calcium Intake in Men and Women
| Dietary calcium intake, Quintile
|
P Value for Trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| MEN | ||||||
| Median intake, mg/d | 478 | 616 | 739 | 898 | 1247 | |
| Person-years | 527,379 | 516,858 | 502,994 | 489,449 | 467,990 | |
| All CVD deaths | ||||||
| No. of cases | 1879 | 1550 | 1519 | 1400 | 1556 | |
| Age adjusted | Ref | 0.81 (0.76, 0.86) | 0.79 (0.74, 0.85) | 0.75 (0.70, 0.80) | 0.86 (0.80, 0.92) | 0.004 |
| Multivariate a | Ref | 0.91 (0.85, 0.98) | 0.96 (0.89, 1.03) | 0.92 (0.85, 0.99) | 1.04 (0.97, 1.12) | 0.08 |
| Heart disease deaths | ||||||
| No. of cases | 1496 | 1204 | 1223 | 1110 | 1249 | |
| Age adjusted | Ref | 0.79 (0.73, 0.85) | 0.81 (0.75, 0.87) | 0.75 (0.69, 0.81) | 0.87 (0.81, 0.94) | 0.01 |
| Multivariate a | Ref | 0.89 (0.82, 0.96) | 0.97 (0.90, 1.05) | 0.92 (0.85, 1.00) | 1.06 (0.97, 1.14) | 0.04 |
| Cerebrovascular disease deaths | ||||||
| No. of cases | 268 | 229 | 214 | 205 | 230 | |
| Age adjusted | Ref | 0.83 (0.69, 0.99) | 0.77 (0.64, 0.92) | 0.75 (0.63, 0.90) | 0.87 (0.73, 1.03) | 0.21 |
| Multivariate a | Ref | 0.92 (0.77, 1.10) | 0.90 (0.74, 1.08) | 0.89 (0.73, 1.07) | 1.02 (0.85, 1.23) | 0.63 |
|
| ||||||
| WOMEN | ||||||
| Median intake, mg/d | 408 | 532 | 648 | 798 | 1101 | |
| Person-years | 397,388 | 397,012 | 394,567 | 392,622 | 386,100 | |
| All CVD deaths | ||||||
| No. of cases | 918 | 785 | 700 | 708 | 763 | |
| Age adjusted | Ref | 0.83 (0.75, 0.91) | 0.73 (0.66, 0.80) | 0.72 (0.66, 0.80) | 0.76 (0.69, 0.84) | <.001 |
| Multivariate b | Ref | 0.99 (0.90, 1.09) | 0.94 (0.85, 1.04) | 0.99 (0.89, 1.10) | 1.04 (0.94, 1.15) | 0.37 |
| Heart disease deaths | ||||||
| No. of cases | 692 | 557 | 497 | 495 | 536 | |
| Age adjusted | Ref | 0.78 (0.70, 0.87) | 0.69 (0.61, 0.77) | 0.67 (0.60, 0.76) | 0.71 (0.64, 0.80) | <.001 |
| Multivariate b | Ref | 0.94 (0.84, 1.05) | 0.90 (0.80, 1.01) | 0.94 (0.83, 1.06) | 0.99 (0.87, 1.12) | 0.93 |
| Cerebrovascular disease deaths | ||||||
| No. of cases | 170 | 189 | 149 | 174 | 178 | |
| Age adjusted | Ref | 1.07 (0.87, 1.32) | 0.84 (0.67, 1.04) | 0.96 (0.78, 1.19) | 0.96 (0.78, 1.18) | 0.54 |
| Multivariate b | Ref | 1.23 (1.00, 1.52) | 1.01 (0.81, 1.27) | 1.21 (0.97, 1.51) | 1.20 (0.95, 1.51) | 0.22 |
adjusted for age at baseline (continuous); race/ethnicity (non-Hispanic white; non-Hispanic, black; and others); education (less than high school, high school graduate, some college and college graduate/postgraduate); marital status (married, not married), health status (excellent, very good, good, fair, and poor); BMI (<18.5, 18.5–<25, 25–<30, 30–<35, ≥35 kg2/m) smoking status (never, former, and current), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, and >60 cigarettes per day); time since quitting (never quit, ≥10, 5–9, 1–4, <1 years), vigorous physical activity (never/rarely; ≤3 times/mo; 1–2, 3–4, and ≥5 times/wk), alcohol (0, <5, 5–<15, 15–<30, and ≥30 g/d), supplemental calcium intake (0, <400, 400–<1000, ≥1000 mg/d), fruit and vegetable intake (continuous), red meat intake (continuous), whole grain intake (continuous), total fat intake (continuous) and total caloric intake (continuous).
Adjusted for variables listed in a and use of menopausal hormone therapy (never, past and current).
Supplemental calcium intake was related to a significantly elevated risk of total CVD and heart disease mortality among men (figure 1). Compared with nonusers, men with >1000mg/d intake of supplemental calcium had significantly higher risk of total CVD death (multivariate RR>1000 vs. 0 mg/day =1.20, [95% CI, 1.05, 1.36]) and heart disease death (multivariate RR>1000 vs. 0 mg/day =1.19, [95% CI, 1.03, 1.37]). Supplemental calcium intake was also related to an increased risk of cerebrovascular disease death in men (p for trend=0.04), but RR for >1000mg/day was not statistically significant with wide 95% CI, probably due to small number of deaths (n=36). No association between supplemental calcium intake and CVD mortality was observed among women. To minimize the impact of other nutrients in multivitamins, we assessed the effect of individual calcium supplement use in those who did not take calcium-containing multivitamins. The highest category of supplemental calcium intake was associated with an increased risk of total CVD death (multivariate RR>1000 vs. 0 mg/day =1.24 [95% CI, 0.97, 1.57]), mainly driven by heart disease death (multivariate RR>1000 vs. 0 mg/day =1.37 [95% CI, 1.06, 1.77]) (supplementary table 1). Consistently null associations were observed in women. Excluding deaths that occurred during the first 2 years of follow-up also did not change the results (data not shown).
Figure 1.
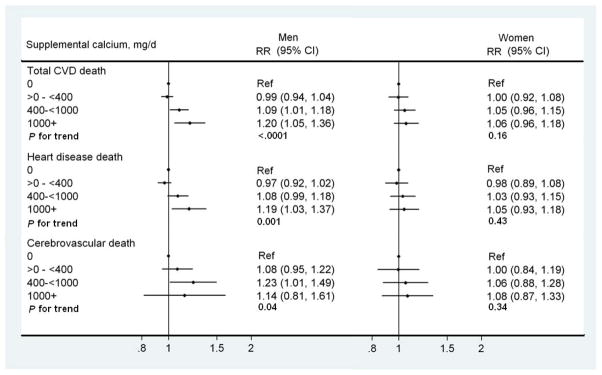
Multivariate relative risks (RRs) and 95% confidence intervals (CIs) for total cardiovascular disease (CVD), heart disease and cerebrovascular disease mortality for categories of supplemental calcium intake. The multivariate RRs were adjusted for age at baseline (continuous); race/ethnicity (non-Hispanic white; non-Hispanic, black; and others); education (less than high school, high school graduate, some college and college graduate/postgraduate); marital status (married, not married), health status (excellent, very good, good, fair, and poor); BMI (<18.5, 18.5–<25, 25–<30, 30–<35, ≥35 kg2/m) smoking status (never, former, and current), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, and >60 cigarettes per day); time since quitting (never quit, ≥10, 5–9, 1–4, <1 years), vigorous physical activity (never/rarely; ≤3 times/mo; 1–2, 3–4, and ≥5 times/wk), alcohol (0, <5, 5–<15, 15–<30, and ≥30 g/d), dietary calcium intake (quintiles), fruit and vegetable intake (continuous), red meat intake (continuous), whole grain intake (continuous), total fat intake (continuous) and total caloric intake (continuous). The use of menopausal hormone therapy (never, past and current) was adjusted in women. Dots indicate the RRs and horizontal lines indicate 95% CIs. The numbers of deaths in category 0 through ≥1000 mg/d were 3947, 2910, 794, and 253 for total CVD deaths, 3171, 2284, 627, and 200 for heart disease death, and 542, 440, 128 and 36 for cerebrovascular disease deaths in men; 1264, 1171, 893, and 576 for total CVD deaths, 931, 839, 607, and 400 for heart disease deaths, and 264, 255, 201, and 140 for cerebrovascular disease deaths in women. Person-years in each category were 1,237,051, 960,869, 234,209 and 725,40 for men, and 581,849, 604,732, 453,105, and 328,002 for women.
We further investigated the relationship between supplemental calcium and total CVD mortality by age, smoking status, BMI, hypertension, hypercholesterolemia (table 3), total magnesium intake, and alcohol consumption (supplementary table 2). The number of deaths and person-years for each subgroup are shown in supplementary table 3. In men, the positive association persisted in most of the subgroups. Smoking status appeared to have a statistically significant interaction with supplemental calcium intake in men, with stronger associations observed in current smokers. In women, the association was null for most subgroups, with the noticeable exceptions of former smokers, women with no history of hypertension, and women who had hypercholesterolemia, among whom supplemental calcium was associated with increased total CVD deaths.
Table 3.
Multivariate Relative Risks and 95% Confidence Intervals for Total Cardiovascular Disease Deaths for Categories of Supplemental Calcium Intake, Stratified by Age, Smoking Status, Body Mass Index and Hypertension
| Supplemental Calcium Intake, mg/d
|
P Value for Trend | ||||
|---|---|---|---|---|---|
| 0 | 0–400 | 400–1000 | >1000 | ||
| MEN | |||||
| Age a | |||||
| <60 | Ref | 0.97 (0.87, 1.09) | 1.15 (0.96, 1.38) | 1.47 (1.09, 2.00) | 0.01 |
| >=60 | Ref | 0.99 (0.94, 1.05) | 1.08 (1.00, 1.18) | 1.15 (1.00, 1.32) | 0.01 |
| p value for interaction | 0.16 | ||||
| Smoking status b | |||||
| Never | Ref | 0.91 (0.82, 1.00) | 1.05 (0.90, 1.23) | 1.04 (0.79, 1.36) | 0.62 |
| Former | Ref | 0.98 (0.92, 1.05) | 1.08 (0.97, 1.20) | 1.17 (0.98, 1.38) | 0.04 |
| Current | Ref | 1.10 (0.99, 1.21) | 1.12 (0.93, 1.34) | 1.33 (0.94, 1.89) | 0.04 |
| p value for interaction | 0.01 | ||||
| Body mass index a | |||||
| < 25 | Ref | 0.93 (0.85, 1.02) | 1.08 (0.94, 1.24) | 1.03 (0.82, 1.31) | 0.45 |
| >=25 and <30 | Ref | 0.97 (0.90, 1.04) | 1.12 (1.00, 1.25) | 1.36 (1.14, 1.63) | <0.001 |
| >= 30 | Ref | 1.10 (1.00, 1.21) | 1.03 (0.87, 1.22) | 1.12 (0.83, 1.50) | 0.36 |
| p value for interaction | 0.19 | ||||
| Hypertension a | |||||
| Yes | Ref | 1.03 (0.94, 1.13) | 1.08 (0.93, 1.25) | 1.44 (1.16, 1.80) | 0.002 |
| No | Ref | 1.02 (0.93, 1.12) | 1.15 (0.98, 1.34) | 1.18 (0.91, 1.52) | 0.06 |
| p value for interaction | 0.80 | ||||
| Hypercholesterolemia a | |||||
| Yes | Ref | 1.04 (0.95, 1.15) | 1.22 (1.05, 1.41) | 1.19 (0.93, 1.51) | 0.01 |
| No | Ref | 0.99 (0.89, 1.10) | 1.05 (0.89, 1.24) | 1.39 (1.08, 1.78) | 0.02 |
| p value for interaction | 0.94 | ||||
|
| |||||
| WOMEN | |||||
| Age a | |||||
| <60 | Ref | 0.99 (0.82, 1.21) | 1.04 (0.83, 1.31) | 0.92 (0.70, 1.22) | 0.68 |
| >=60 | Ref | 1.00 (0.92, 1.09) | 1.05 (0.96, 1.16) | 1.09 (0.97, 1.21) | 0.09 |
| p value for interaction | 0.04 | ||||
| Smoking status b | |||||
| Never | Ref | 0.94 (0.82, 1.09) | 0.99 (0.85, 1.16) | 1.06 (0.89, 1.27) | 0.37 |
| Former | Ref | 1.10 (0.96, 1.27) | 1.19 (1.02, 1.38) | 1.18 (1.00, 1.40) | 0.05 |
| Current | Ref | 0.98 (0.82, 1.17) | 1.13 (0.95, 1.35) | 1.18 (0.98, 1.42) | 0.91 |
| p value for interaction | 0.06 | ||||
| Body mass index a | |||||
| < 25 | Ref | 1.05 (0.92, 1.20) | 1.15 (1.00, 1.32) | 1.13 (0.97, 1.32) | 0.08 |
| >=25 and <30 | Ref | 0.92 (0.81, 1.06) | 0.93 (0.80, 1.08) | 0.94 (0.78, 1.13) | 0.55 |
| >= 30 | Ref | 1.03 (0.88, 1.20) | 1.09 (0.91, 1.30) | 1.18 (0.95, 1.45) | 0.11 |
| p value for interaction | 0.89 | ||||
| Hypertension a | |||||
| Yes | Ref | 0.95 (0.83, 1.09) | 1.05 (0.90, 1.23) | 1.07 (0.90, 1.27) | 0.25 |
| No | Ref | 1.13 (0.96, 1.33) | 1.05 (0.87, 1.26) | 1.36 (1.12, 1.65) | 0.007 |
| p value for interaction | 0.17 | ||||
| Hypercholesterolemia a | |||||
| Yes | Ref | 1.03 (0.88, 1.20) | 1.05 (0.88, 1.24) | 1.21 (1.00, 1.45) | 0.05 |
| No | Ref | 1.05 (0.89, 1.23) | 1.06 (0.89, 1.26) | 1.19 (0.99, 1.44) | 0.08 |
| p value for interaction | 0.69 | ||||
adjusted for age at baseline (continuous); race/ethnicity (non-Hispanic white; non-Hispanic, black; and others); education (less than high school, high school graduate, some college and college graduate/postgraduate); marital status (married, not married), health status (excellent, very good, good, fair, and poor); BMI (<18.5, 18.5–<25, 25–<30, 30–<35, ≥35 kg2/m) smoking status (never, former, and current), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, and >60 cigarettes per day); time since quitting (never quit, ≥10, 5–9, 1–4, <1 years), vigorous physical activity (never/rarely; ≤3 times/mo; 1–2, 3–4, and ≥5 times/wk), alcohol (0, <5, 5–<15, 15–<30, and ≥30 g/d), dietary calcium intake (quintiles), fruit and vegetable intake (continuous), red meat intake (continuous), whole grain intake (continuous), total fat intake (continuous) and total caloric intake (continuous). The use of menopausal hormone therapy (never, past and current) was adjusted in women.
Adjusted for variables listed in a but smoking status.
Total calcium intake had a U-shaped association with total CVD mortality in men (P for nonlinearity, 0.006, figure 2A), with increased total CVD mortality observed at calcium intakes of 1500 mg/d and higher. When we examined the association by quintiles of total calcium intake, compared to the lowest, the highest quintile was significantly associated with elevated total CVD mortality (multivariate RRQ5 vs Q1 =1.12 [95% CI, 1.04–1.20]) and heart disease mortality (multivariate RRQ5 vs Q1=1.12 [95% CI, 1.04–1.21]) (supplementary table 4). A similar positive association was observed between total calcium intake and cerebrovascular mortality, but was not statistically significant. In women, total calcium intake was not associated with deaths from total CVD, heart disease, or cerebrovascular diseases (figure 2B, supplementary table 4).
Figure 2.
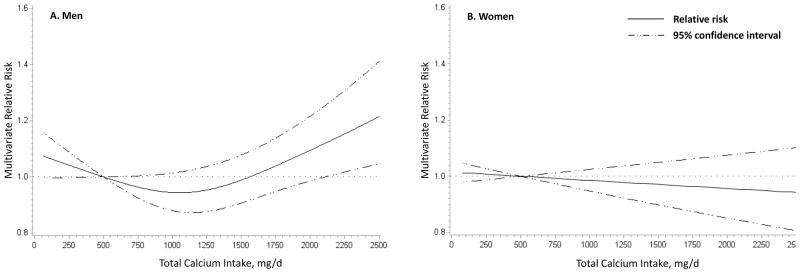
Nonparametric regression curve for the association between total calcium intake and total cardiovascular disease (CVD) mortality for men (A) and women (B). Both models were adjusted for age at baseline (continuous); race/ethnicity (non-Hispanic white; non-Hispanic, black; and others); education (less than high school, high school graduate, some college and college graduate/postgraduate); marital status (married, not married), health status (excellent, very good, good, fair, and poor); BMI (<18.5, 18.5–<25, 25–<30, 30–<35, ≥35 kg2/m) smoking status (never, former, and current), smoking dose (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, and >60 cigarettes per day); time since quitting (never quit, ≥10, 5–9, 1–4, <1 years), vigorous physical activity (never/rarely; ≤3 times/mo; 1–2, 3–4, and ≥5 times/wk), alcohol (0, <5, 5–<15, 15–<30, and ≥30 g/d), fruit and vegetable intake (continuous), red meat intake (continuous), whole grain intake (continuous), total fat intake (continuous) and total caloric intake (continuous). The use of menopausal hormone therapy (never, past and current) was adjusted in women.
Discussion
In this large prospective study we found that supplemental, but not dietary, calcium intake was associated with an increased CVD mortality in men, but not in women. The lack of association between dietary calcium and CVD mortality is generally consistent with previous observational studies. A recent meta-analysis found no effect of dietary calcium on either coronary artery disease or stroke, when comparing the highest intake category to the lowest 23. However the analysis did not examine the dose-response relation of dietary calcium intake to coronary artery disease or stroke. Only a few studies specifically focused on cardiovascular mortality. Dietary calcium was not associated with CVD death in Dutch civil servants 12, US Health Professionals Follow-up Study 13, the Japan Collaborative Cohort Study 14, and the European Prospective Investigation into Cancer and Nutrition study24. However, a study of postmenopausal women in Iowa found a 37% decrease in ischemic heart disease mortality with high dietary calcium intake among those who did not take supplements 10, a finding that we did not observe even after similar restriction was applied. A study of Swedish men also reported with borderline significance that CVD mortality was 23% (RR=0.77, 95% CI: 0.58, 1.01) lower in the highest tertile of dietary calcium intake (>=1599 mg/d) vs. the lowest tertile (<1230 mg/d) 25. The dietary calcium intake in the Swedish cohort was substantially higher than that in the male participants of our study or other studies. It remains to be determined whether very high intake of dietary calcium may offer a protective effect.
Several studies examined the role of supplemental calcium on cardiovascular mortality. The Iowa Women’s Health Study found reduced CVD mortality among users of calcium supplements 10, 26. The Health Professionals Follow-up Study also reported a trend towards decreased fatal ischemic heart disease risk in men with high intakes of supplemental calcium, although the sample sizes were quite small 13. The recent Heidelberg cohort study observed an increased risk of myocardial infarction among calcium supplement users but lacked statistical power to examine CVD mortality 24. To our knowledge, no RCT has tested the effect of calcium supplementation with CVD as a prespecified primary endpoint. Some RCTs did consider cardiovascular disease events as secondary outcomes and most of the earlier studies found no effect of calcium supplementation on CVD 27–28. However, recent secondary analyses of several RCTs have yielded provoking results. Most notably, a reanalysis of the Women’s Health Initiative (WHI) Study observed modestly increased risk of a variety of cardiovascular endpoints, especially myocardial infarction, in the intervention arm 9. The same authors also conducted a meta-analysis of RCTs and showed elevated risk associated with calcium supplementation9. However, the results of the WHI study were heavily weighted in the meta-analysis.
We found a significant interaction by sex. Elevated CVD mortality with increasing supplemental calcium intake was observed only in men; however, we cannot rule out the possibility that supplemental calcium intake may be associated with cardiovascular mortality in women. The sex difference is intriguing. In the reanalysis of the WHI study, adverse effect of calcium supplement intervention was only observed when the analysis was restricted to women who did not take personal supplement at randomization, and personal supplement use by itself was not associated with adverse outcomes regardless of intervention 9. The authors brought up an interesting hypothesis that the abrupt change in calcium intake and subsequent change in serum calcium, instead of overall calcium load, may be responsible for the adverse effects. Dietary supplement use is more prevalent and regular in women than in men and the difference is apparent in populations as young as 20 years old 29. Although no information on duration of supplement use was collected at baseline in our study, it may be reasonable to assume that, on average, male users started taking calcium supplements at an older age. Therefore, women were more likely to have achieved calcium balance and stable calcium levels long before the study, and the effect of calcium supplement became less profound.
In the subgroup analyses, smoking status was a significant effect modifier, with the adverse effect of supplement calcium only observed among smokers. Smoking can cause a wide range of detrimental effects on the cardiovascular system, and act synergistically with other risk factors to substantially increase the risk for cardiovascular diseases 30. Further study is needed to evaluate the interplay between calcium and smoking. Another potential effect modifier is vitamin D. Several lines of evidence have pointed to a beneficial effect of vitamin D on cardiovascular health 31, suggesting that co-administration of calcium with vitamin D may weaken the adverse impact of calcium. Unfortunately, information on intake of individual vitamin D supplements was not collected in our study and vitamin D in multivitamins is highly correlated with supplemental calcium intake, therefore we were not able to assess the role of vitamin D supplement.
One plausible biological mechanism through which calcium may exert harmful impact on cardiovascular health is vascular calcification, the deposit of calcium phosphate in cardiovascular structures. Emerging evidence has linked calcification of coronary arteries with increased atherosclerotic plaque burden32, risk of coronary heart disease33–34, and mortality35. Vascular calcification is an actively regulated process that not only shares key proteins and pathways, but is also intricately intertwined with bone mineralization 36. It remains unclear whether vascular calcification, like osteogenesis, is also influenced by calcium supplement intake. Among patients with end stage renal disease, daily ingestion of calcium as phosphate-binding agent is positively correlated with coronary artery calcification 37. A report of the WHI study did not find any difference in coronary artery calcification scores between the intervention and placebo groups 38, although personal intake of supplements and poor compliance might mask the real association. In addition, increased blood coagulation and arterial stiffness have also been positively linked with serum calcium and proposed as potential mechanisms by which calcium may affect cardiovascular health39. However, it is worth mentioning that calcium is widely involved in many aspects of human physiology and some of its effects may be beneficial for cardiovascular health, including lower blood pressure 40–41 and improved blood lipid profile 6. To understand the overall effects of calcium, more mechanistic studies are warranted.
Our study has some limitations. First, we did not have information on the duration of supplement use, which might be an important factor mediating the effect of calcium supplement on CVD mortality. Second, although we controlled for multiple CVD risk factors, we could not rule out the possibility that other correlated nutrients also contributed to the observed association, or that the use of calcium supplements is a marker of behavior that is related to the CVD. We also lacked of information on family history of cardiovascular diseases that may also confound our results. Third, with self-reported intake information, we were subject to measurement error. Also, calcium intake was only measured at baseline and, therefore, we were not able to assess change in dietary or supplement intake during follow-up.
Our study has several strengths. Its large size and long follow-up allowed adequate statistical power to test the overall effect of calcium on CVD mortality with, and also assess the associations by age, BMI, smoking status, cardiovascular risk profile and multivitamin intake. We were also able to examine heart disease mortality and cerebrovascular mortality separately. Moreover, we excluded people with chronic diseases at baseline, whose dietary and supplement use pattern might be affected by their prevalent health conditions. We also conducted sensitivity analysis by excluding people who died within the first two years of follow-up, further reducing the likelihood of reverse causality.
In conclusion, our findings suggest that supplemental calcium intake is associated with elevated CVD mortality in men, but not in women. Whether there is a sex difference in the cardiovascular effect of calcium supplement warrants further investigation. Given the extensive use of calcium supplement in the population, it is of great importance to assess the impact of supplemental calcium use beyond bone health.
Supplementary Material
Acknowledgments
Funding source: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute and National Institute of Aging, National Institutes of Health, Department of Health and Human Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
References
- 1.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–22. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennekens CH, Barice EJ. Calcium supplements and risk of myocardial infarction: a hypothesis formulated but not yet adequately tested. Am J Med. 2011;124(12):1097–8. doi: 10.1016/j.amjmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Reid IR, Bolland MJ, Grey A. Calcium supplements and risk of myocardial infarction: an hypothesis twice tested. Am J Med. 2012;125(4):e15. doi: 10.1016/j.amjmed.2011.09.006. author reply e17. [DOI] [PubMed] [Google Scholar]
- 4.Bucher HC, Cook RJ, Guyatt GH, et al. Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomized controlled trials. JAMA. 1996;275(13):1016–22. doi: 10.1001/jama.1996.03530370054031. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Elliott P, Allender PS, Pryer J, Follman DA, Cutler JA. Epidemiologic association between dietary calcium intake and blood pressure: a meta-analysis of published data. Am J Epidemiol. 1995;142(9):935–45. doi: 10.1093/oxfordjournals.aje.a117741. [DOI] [PubMed] [Google Scholar]
- 6.Reid IR, Mason B, Horne A, et al. Effects of calcium supplementation on serum lipid concentrations in normal older women: a randomized controlled trial. Am J Med. 2002;112(5):343–7. doi: 10.1016/s0002-9343(01)01138-x. [DOI] [PubMed] [Google Scholar]
- 7.Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–6. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149(2):151–61. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 11.Iso H, Stampfer MJ, Manson JE, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30(9):1772–9. doi: 10.1161/01.str.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 12.Van der Vijver LP, van der Waal MA, Weterings KG, Dekker JM, Schouten EG, Kok FJ. Calcium intake and 28-year cardiovascular and coronary heart disease mortality in Dutch civil servants. Int J Epidemiol. 1992;21(1):36–9. doi: 10.1093/ije/21.1.36. [DOI] [PubMed] [Google Scholar]
- 13.Al-Delaimy WK, Rimm E, Willett WC, Stampfer MJ, Hu FB. A prospective study of calcium intake from diet and supplements and risk of ischemic heart disease among men. Am J Clin Nutr. 2003;77(4):814–8. doi: 10.1093/ajcn/77.4.814. [DOI] [PubMed] [Google Scholar]
- 14.Umesawa M, Iso H, Date C, et al. Dietary intake of calcium in relation to mortality from cardiovascular disease: the JACC Study. Stroke. 2006;37(1):20–6. doi: 10.1161/01.STR.0000195155.21143.38. [DOI] [PubMed] [Google Scholar]
- 15.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 16.Hermansen SW, Leitzmann MF, Schatzkin A. The impact on National Death Index ascertainment of limiting submissions to Social Security Administration Death Master File matches in epidemiologic studies of mortality. Am J Epidemiol. 2009;169(7):901–8. doi: 10.1093/aje/kwn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diet history questionnaire. [Accessed July 17 2012.];National Cancer Institute Web site. Available at: http://riskfactor.cancer.gov/DHQ/
- 18.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152(3):279–86. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 19.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 20.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183–95. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26(20):3735–52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs. 2012;12(2):105–16. doi: 10.2165/11595400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98(12):920–5. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 25.Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol. 2010;171(7):801–7. doi: 10.1093/aje/kwp467. [DOI] [PubMed] [Google Scholar]
- 26.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med. 2011;171(18):1625–33. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869–75. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 28.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 29.Balluz LS, Kieszak SM, Philen RM, Mulinare J. Vitamin and mineral supplement use in the United States. Results from the third National Health and Nutrition Examination Survey. Arch Fam Med. 2000;9(3):258–62. doi: 10.1001/archfami.9.3.258. [DOI] [PubMed] [Google Scholar]
- 30.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83(1):356–62. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152(5):315–23. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 32.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 33.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 34.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 303(16):1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Allison MA, Carr JJ, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 17(4):683–91. doi: 10.1097/gme.0b013e3181d683b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid IR, Bolland MJ, Avenell A, Grey A. Cardiovascular effects of calcium supplementation. Osteoporos Int. 2011;22(6):1649–58. doi: 10.1007/s00198-011-1599-9. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson HO, Nicolson DJ, Cook JV, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;(2):CD004639. doi: 10.1002/14651858.CD004639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Mierlo LA, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20(8):571–80. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


