Abstract
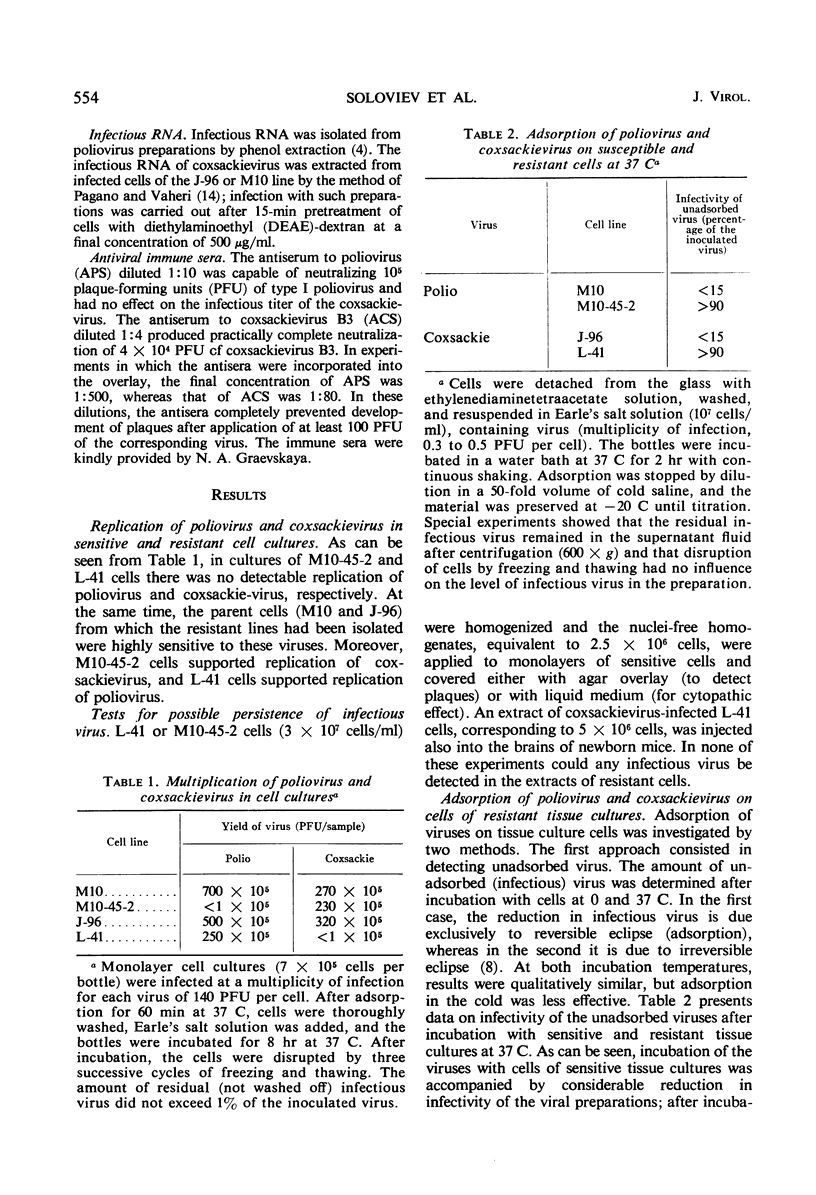
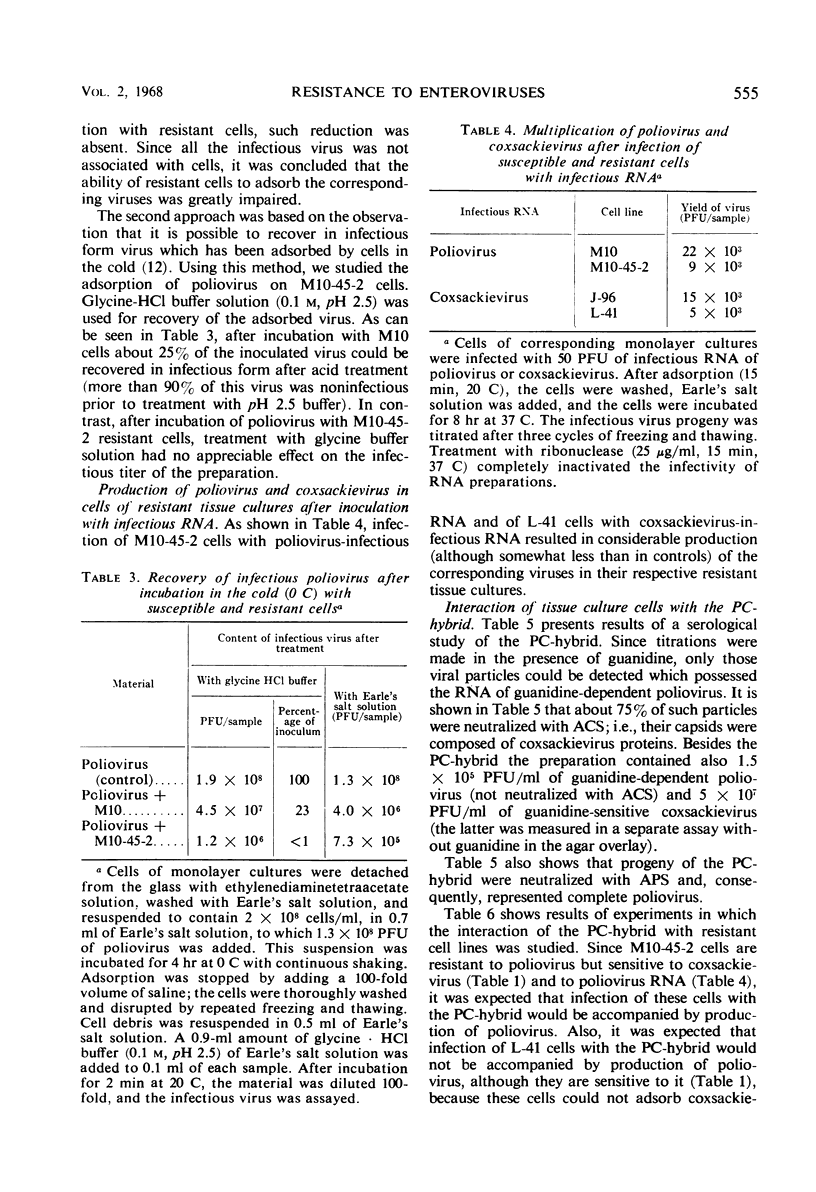
Two cell lines, M10-45-2 and L-41, were studied, each of which possessed specific resistance either to poliovirus or to coxsackievirus. Infection of M10-45-2 cells with poliovirus ribonucleic acid (RNA) and L-41 cells with infectious coxsackievirus RNA was accompanied by production of complete viruses in each of the resistant cell lines. During incubation of the cells with the virus to which they were resistant, the amount of infectious virus did not decrease. Treatment with glycine-HCl buffer solution (pH 2.5) of resistant M10-45-2 cells after incubation with poliovirus at 0 C did not result in recovery of infectious virus, although such release did take place after treatment of sensitive M10 cells. Infection of resistant cells with virus containing poliovirus RNA and coxsackievirus proteins resulted in production of poliovirus in M10-45-2 cells but not in L-41 cells. The resistant cells are apparently unable to adsorb the virus to which they are resistant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOL V. I., MASLOVA S. V., CHUMAKOVA M. Ia. [On the relation between chromatographic behavior and some other properties of poliovirus variants]. Biokhimiia. 1962 Nov-Dec;27:1071–1078. [PubMed] [Google Scholar]
- ALEXANDER H. E., KOCH G., MOUNTAIN I. M., VAN DAMME O. Infectivity of ribonucleic acid from poliovirus in human cell monolayers. J Exp Med. 1958 Oct 1;108(4):493–506. doi: 10.1084/jem.108.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agol V. I., Shirman G. A. Obrazovanie virusnykh chastits za schet fermentnykh sistem i strukturnykh belkov, indutsirovannykh drugim virusom--"pomoshchnikom". Vopr Virusol. 1965 Jan-Feb;10(1):8–11. [PubMed] [Google Scholar]
- CORDS C. E., HOLLAND J. J. ALTERATION OF THE SPECIES AND TISSUE SPECIFICITY OF POLIOVIRUS BY ENCLOSURE OF ITS RNA WITHIN THE PROTEIN CAPSID OF COXSACKIE B1 VIRUS. Virology. 1964 Nov;24:492–495. doi: 10.1016/0042-6822(64)90192-8. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. Variation in plaque-forming ability among parental and clonal strains of HeLa cells. Virology. 1959 Jun;8(2):223–229. doi: 10.1016/0042-6822(59)90006-6. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., CORDS C. E. MATURATION OF POLIOVIRUS RNA WITH CAPSID PROTEIN CODED BY HETEROLOGOUS ENTEROVIRUSES. Proc Natl Acad Sci U S A. 1964 Jun;51:1082–1085. doi: 10.1073/pnas.51.6.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIDY G., SPRUNT K., REDMAN W., ALEXANDER H. E. Sensitivity of populations of clonal lines of HeLa cells to polioviruses. Proc Soc Exp Biol Med. 1959 Oct;102:81–85. doi: 10.3181/00379727-102-25150. [DOI] [PubMed] [Google Scholar]
- OSGOOD E. E., BROOKE J. H. Continuous tissue culture of leukocytes from human leukemic bloods by application of gradient principles. Blood. 1955 Oct;10(10):1010–1022. [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- SOLOVEV V. D., GULEVICH N. E., VARSHAVER N. B. VIRUSOLOGICHESKOE I KARIOLOGICHESKOE IZUCHENIE REZISTENTNO I K VIRUSU POLIOMIELITA KLETOCHNO I LINII. Vopr Virusol. 1963 Sep-Oct;67:580–583. [PubMed] [Google Scholar]
- SOLOVYOV V. D., GULEVICH N. E. Studies on antiviral immunity using tissue culture methods. II. Obtaining cells resistant to poliomyelitis virus. Acta Virol. 1960 Jul;4:220–226. [PubMed] [Google Scholar]
- Solov'ev V. D., Goergadze I. I., Varshaver N. B. Izuchenie estestvennykh izmenenii vospriimchivosti k virusam v perevivaemoi kul'ture kletok J-96 i v klonirovannykh subkul'turakh. Vopr Virusol. 1965 Nov-Dec;10(6):699–703. [PubMed] [Google Scholar]
- VARSHAVER N. B., GULEVICH N. E. IZUCHENIE GENETICHESKIKH OSNOV IMMUNITETA KELTOK. II. KARIOLOGICHESKOE ISSLEDOVANIE REZISTENTNYKH KLETOK LE IKEMII. Vopr Virusol. 1964 Jul-Aug;30:482–489. [PubMed] [Google Scholar]
- VOGT M. A genetic change in a tissue culture line of neoplastic cells. J Cell Physiol Suppl. 1958 Dec;52(Suppl 1):271–285. doi: 10.1002/jcp.1030520413. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- WECKER E., LEDERHILGER G. GENOMIC MASKING PRODUCED BY DOUBLE-INFECTION OF HELA CELLS WITH HETEROTYPIC POLIOVIRUSES. Proc Natl Acad Sci U S A. 1964 Sep;52:705–709. doi: 10.1073/pnas.52.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



