Abstract
BACKGROUND & AIMS
An increased number of macrophages in adipose tissue is associated with insulin resistance and metabolic dysfunction in obese people. However, little is known about other immune cells in adipose tissue from obese people, and whether they contribute to insulin resistance. We investigated the characteristics of T cells in adipose tissue from metabolically abnormal insulin-resistant obese (MAO) subjects, metabolically normal insulin-sensitive obese (MNO) subjects, and lean subjects. Insulin sensitivity was determined by using the hyperinsulinemic euglycemic clamp procedure.
METHODS
We assessed plasma cytokine concentrations and subcutaneous adipose tissue CD4+ T-cell populations in 9 lean, 12 MNO, and 13 MAO subjects. Skeletal muscle and liver samples were collected from 19 additional obese patients undergoing bariatric surgery to determine the presence of selected cytokine receptors.
RESULTS
Adipose tissue from MAO subjects had 3- to 10-fold increases in numbers of CD4+ T cells that produce interleukin (IL)-22 and IL-17 (a T-helper [Th] 17 and Th22 phenotype) compared with MNO and lean subjects. MAO subjects also had increased plasma concentrations of IL-22 and IL-6. Receptors for IL-17 and IL-22 were expressed in human liver and skeletal muscle samples. IL-17 and IL-22 inhibited uptake of glucose in skeletal muscle isolated from rats and reduced insulin sensitivity in cultured human hepatocytes.
CONCLUSIONS
Adipose tissue from MAO individuals contains increased numbers of Th17 and Th22 cells, which produce cytokines that cause metabolic dysfunction in liver and muscle in vitro. Additional studies are needed to determine whether these alterations in adipose tissue T cells contribute to the pathogenesis of insulin resistance in obese people.
Keywords: Metabolically Normal Obesity, Metabolically Abnormal Obesity, Lymphocytes
Obesity is associated with insulin resistance, which is directly associated with increased triglyceride accumulation in the liver,1–3 and is an important risk factor for type 2 diabetes, the metabolic syndrome, and coronary heart disease.4,5 However, not all obese individuals develop insulin resistance and metabolic abnormalities. About one third of obese adults are metabolically normal based on insulin sensitivity measured by using the hyperinsulinemic-euglycemic clamp technique,6,7 and about one third have normal intrahepatic triglyceride content assessed by using magnetic resonance spectroscopy.8 It is not known why weight gain and body fat accumulation cause insulin resistance in some people but not in others. Data from a series of studies conducted in the last 15 years have demonstrated that metabolic dysfunction in obese individuals is associated with adipose tissue inflammation, suggesting a possible link between the metabolically abnormal, insulin-resistant obese phenotype and a dysregulation of adipose tissue immune function.9–13 Since the first observations of increased levels of tumor necrosis factor—α that adipose tissue from obese mice and people have compared with lean controls,14,15 data from subsequent studies support the notion that obesity is associated with chronic low-grade inflammation, which leads to development of metabolic dysfunction.16,17
The major research focus in obesity-related inflammation has been on adipose tissue macrophages, which has led to the concept that obesity is associated with increased adipose tissue macrophage infiltration18,19 in conjunction with a switch in macrophage population from an anti-inflammatory to a pro-inflammatory state.16 Recently, data from studies conducted in rodent models have indicated that the distribution of adipose tissue and hepatic T lymphocytes might also have an important role in obesity-related adipose tissue inflammation and metabolic dysfunction, and in the development of steatosis and steatohepatitis.20–24 However, the potential importance of alterations in adipose tissue lymphocytes in the pathogenesis of metabolic dysfunction in obese people is not known.
The purpose of the present study was to determine if metabolically abnormal (insulin-resistant) obesity (MAO) is associated with an altered polarization of adipose tissue CD4 T lymphocytes compared with lean and metabolically normal (insulin-sensitive) obese (MNO) subjects, and if this specific polarization could be mechanistically related with insulin resistance in the liver and skeletal muscle. Our observations identify a characteristic signature of adipose tissue—resident T cells in MAO subjects. In addition, the cytokines produced by these lymphocyte subsets cause metabolic dysfunction in liver and muscle in vitro.
Research Design and Methods
Human Studies
Subjects
A total of 36 obese (mean ± SD, body mass index [BMI] 40.6 ± 9.2 kg/m2) and 9 lean (BMI 22.7 ± 1.9 kg/m2) men and women were recruited for this study. The glucose infusion rate (GIR; in mg/kg fat free mass [FFM]/min) needed to maintain euglycemia (~100 mg/dL) during the hyperinsulinemic-euglycemic clamp procedure was used to divide obese subjects into tertiles according to their whole-body insulin sensitivity. Subjects in the first tertile (lowest GIR) were considered to be MAO (n = 13, GIR ≤ 6.9 mg/kgFFM/min) and subjects in the third tertile (highest GIR) were considered to be MNO (n = 12, GIR ≥ 10.5 mg/kgFFM/min).
A second cohort of obese subjects, scheduled to have a bariatric surgical procedure (n = 19, age 43 ± 11 years, BMI 47.4 ± 9.0 kg/m2), was enrolled to obtain skeletal muscle tissue (during an outpatient visit) and liver tissue (during bariatric surgery).
All subjects completed a comprehensive medical evaluation, which included a history and physical examination, routine blood tests, and a 2-hour oral glucose tolerance test. No subject had any history or evidence of serious disease, took medications that might affect metabolism or the immune system, or had diabetes. Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine in St Louis.
Body composition analyses
Body fat mass and FFM were determined by using dual-energy x-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA). Intra-abdominal adipose tissue volume was quantified by magnetic resonance imaging (Siemens, Iselin, NJ; ANALYZE 7.0 software, Mayo Foundation, MN), and intrahepatic triglyceride content was determined by using proton magnetic resonance spectroscopy (Siemens, Erlanger, Germany), as described previously.25
Hyperinsulinemic-euglycemic clamp procedure to assess insulin sensitivity in vivo
Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine on the evening before the clamp procedure. At 7:00 PM subjects were served a standard meal (12 kcal/kg FFM; 50% carbohydrates, 20% protein, 30% fat) and then fasted until study completion the next day. At 6:00 AM, a primed (22.5 μmol/kg), continuous (0.25μmol/kg/min) infusion of [6,6-2H2] glucose was started and continued for 7.5 hours.26 After infusion of the tracer for 3.5 hours (basal period), a primed, constant infusion of insulin (50 mU·m−2 body-surface-area·min−1) was started and continued for 4 hours (clamp period). At the beginning of insulin infusion, [6,6-2H2]glucose infusion was stopped to account for nearly complete insulin-mediated suppression of endogenous glucose production. Euglycemia (100 mg/dL) was maintained by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose at variable rates. Blood samples were collected before beginning the tracer infusion and every 10 minutes during the final 30 minutes of the clamp procedure to determine glucose and insulin concentrations and glucose kinetics.
Human tissue biopsies
Abdominal subcutaneous adipose tissue biopsies were obtained from lean, MNO, and MAO subjects during the basal stage of the clamp procedure at approximately 7:30 AM, by anesthetizing the biopsy site with an injection of lidocaine and aspirating adipose tissue through a 4-mm liposuction cannula (Tulip Medical, San Diego, CA). Skeletal muscle and liver tissues were obtained from the bariatric surgery patients. Skeletal muscle was obtained during an outpatient visit, by anesthetizing the biopsy site with an injection of lidocaine and using Tilley-Henkel forceps (Sontec Instruments Inc., Centennial, CO) to obtain quadriceps femoris tissue. Liver tissue was obtained from the right lobe by needle biopsy during laparoscopic bariatric surgery. Skeletal muscle, liver, and adipose tissue samples were immediately frozen in liquid nitrogen and stored at −80°C for subsequent analyses. Adipose tissue (~1 g) was also placed in ice-cold saline for immunological analyses.
Analyses of samples
Plasma insulin concentration was measured using a chemiluminescent immunoassay method (Immulite 1000, Diagnostic Products Corporation, Los Angeles, CA). Plasma concentrations of interleukin (IL)-6, CCL5, and IL-7 were measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Plasma concentrations of IL-22 and IL-17 were measured using an enzyme-linked immunosorbent assay (eBioscience, San Diego, CA) as per manufacturer’s protocol, with the following modification: standard curves were extended to allow for better detection sensitivity (IL-22 > 1.95 pg/mL, IL-17 > 0.39 pg/mL). Plasma glucose tracer-to-tracee ratio was determined by using electron impact ionization gas chromatography-mass spectrometry.1
Because of the limited number of CD4+ T cells recovered from the adipose tissue samples, we expanded T cells extracted from adipose tissue in culture by using phytohemagglutinin and IL-2 to obtain a sufficient number of lymphocytes for further analysis. Gene expression of liver and skeletal muscle IL-17 and IL-22 receptors and gene expression of adipose tissue total CD4+ T-cell content and of CCL5 and IL-7 were determined by quantitative polymerase chain reaction. Adipose tissue CD4+ T-lymphocyte polarization was evaluated by using flow cytometry. A detailed description of these analyses is available in the Supplementary Material.
Calculations
Isotopic steady-state conditions were achieved during the final 30 minutes of the basal period and of the clamp procedure and Steele’s equation was used to calculate glucose kinetics.27 Glucose rate of disappearance from plasma was assumed to equal the glucose rate of appearance during basal conditions; during the clamp procedure, glucose rate of disappearance was assumed to equal the sum of endogenous glucose rate of appearance and the rate of infused glucose.
Hepatic insulin sensitivity was determined by calculating the reciprocal of the Hepatic Insulin Resistance Index (the product of basal endogenous glucose production rate and fasting plasma insulin concentration).28 Skeletal muscle insulin sensitivity was assessed as the percent increase in glucose rate of disappearance during insulin infusion.3 The computerized, updated homeostasis model assessment was used to provide an index of whole-body insulin resistance.29
Cell Culture and Rodent Studies
Metabolic effects of IL-17 and IL-22 on human primary hepatocytes
The effect of IL-17 and IL-22 on (1) insulin signaling was determined by measuring phosphorylated Akt in hepatocytes incubated with or without human IL-17 or IL-22, followed by stimulation with human insulin; (2) on glucose release was determined by measuring glucose content in media after incubating hepatocytes with or without human IL-17 or IL-22 followed by stimulation with glucagon with or without human insulin; and (3) rate of glycolysis was determined by measuring the rate of 3H2O formation from of [5-3H]glucose by hepatocytes after a pretreatment period with IL-17 or IL-22, followed by incubation with or without insulin and IL-17 or IL-22. A detailed description of these experiments is available in the Supplementary Material.
Effect of IL-17 and IL-22 on skeletal muscle glucose uptake in rat soleus and epitrochlearis muscles
This research protocol was approved by the Animals Studies Committee of Washington University. Strips of soleus and epitrochlearis muscles from male Wistar rats were incubated with glucose, with or without human IL-17 or rat IL-22, and with or without the addition of a maximally effective insulin concentration. Glucose transport activity was measured by using 2-deoxygluxose. A detailed description of these experiments is available in the Supplementary Material.
Statistical Analyses
All datasets were tested for normality according to Kolmogorov-Smirnov criteria, and not normally distributed variables were appropriately transformed for analyses (log-transformed or ranked). One-way analysis of variance with planned contrasts was used to evaluate differences between MAO and the other 2 groups (MNO and lean subjects). Trend analysis was performed for selected variables to describe the trend from lean to MNO to MAO groups. Levene’s test was used to assess the homogeneity of group variances on each dependent variable. Student t test for unpaired samples was used to compare differences between vehicle and IL-17 or IL-22 treatment in primary human hepatocytes and rat muscles. Results are presented as mean ± SD or median with quartiles, unless otherwise indicated. A P value ≤.05 was considered statistically significant. Analyses were performed using SPSS software (version 19, SPSS Inc., Chicago, IL).
Results
Metabolic Variables and Body Composition of the Study Subjects
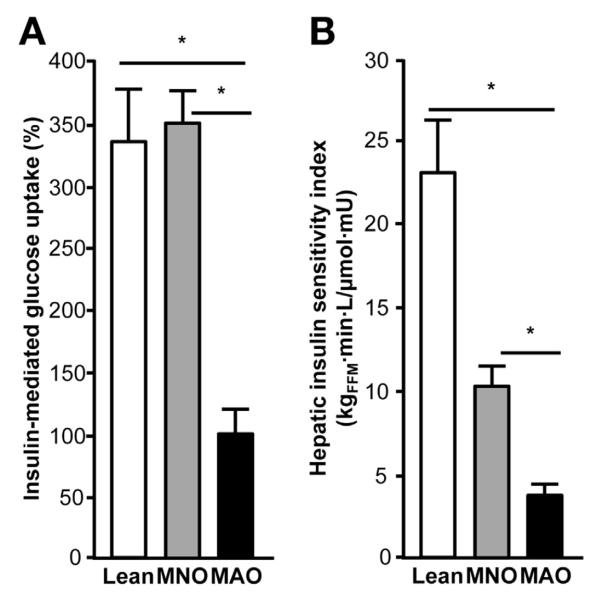
The characteristics of the study subjects are shown in Table 1. Although percent body fat was similar among obese subjects, fat distribution differed between the MNO and MAO groups. MAO subjects had greater intrahepatic triglyceride content than both lean and MNO subjects. Intra-abdominal fat volume was also greater in the MAO than in the lean group, and tended to be greater in MAO than MNO subjects. Fasting plasma glucose concentration was not different among groups, but MAO subjects had greater plasma insulin concentration and homeostasis model of assessment—insulin resistance values than both MNO and lean subjects. Basal hepatic glucose production rate was not different between groups (MAO = 16.3 ± 3.2 μmol/kgFFM/min; MNO = 15.7 ± 2.1 μmol/kgFFM/min; lean = 12.5 ± 2.6 μmol/kgFFM/min; P > .05); however, hepatic insulin sensitivity (assessed by using the Hepatic Insulin Sensitivity Index) and skeletal muscle insulin sensitivity (assessed by insulin-stimulated increase in skeletal muscle glucose uptake) were much lower in the MAO than in the MNO and lean groups (Figure 1A and B).
Table 1.
Characteristics of the Study Subjects
| Lean | Metabolically normal obese | Metabolically abnormal obese | ANOVA | |
|---|---|---|---|---|
| Sex, n (male/female) | 9 (1/8) | 12 (2/10) | 13 (2/11) | |
| Age, y, mean ± SD | 47 ± 15 | 48 ±9 | 38 ± 12 | .092 |
| Body mass index, mean ± SD | 22.7 ± 1.9 | 34.9 ± 4.5a | 43.8 ± 9.0ab | <.001 |
| Body fat mass, %, mean ± SD | 36 ± 4.7 | 44 ± 6a | 48 ± 7a | <.001 |
| IHTG content, %, mean ± SD | 1.8 ± 1.3 | 4.4 ± 3.6 | 11.3 ± 8.7ab | .002 |
| IAAT volume, cm3, mean ± SD | 594 ± 262 | 1386 ± 604 | 1630 ± 450a | <.001 |
| Glucose, mg/dL, mean ± SD | 93 ±7 | 94 ±8 | 99 ±8 | .145 |
| Insulin, mU/L, mean ± SD | 3.1 ± 1.3 | 7.3 ± 2.8 | 21.0 ± 8.5ab | <.001 |
| HOMA-IR, mean ± SD | 0.7 ± 0.3 | 1.7 ± 0.7 | 5.2 ± 2.3ab | <.001 |
| Total-cholesterol, mg/dL, mean ± SD | 192 ± 36 | 174 ± 40 | 164 ± 25 | .173 |
| LDL-cholesterol, mg/dL, mean ± SD | 105 ± 32 | 103 ± 28 | 103 ± 18 | .982 |
| HDL-cholesterol, mg/dL, mean ± SD | 65 ± 18 | 52 ± 12 | 37 ± 6ab | <.001 |
| Triglyceride, mg/dL, mean ± SD | 114 ± 48 | 98 ± 53 | 120 ± 50 | .535 |
ANOVA, analysis of variance; HDL, high-density lipoprotein; IAAT, intra-abdominal adipose tissue; HOMA-IR, homeostasis model assessment of insulin resistance; IHTG, intrahepatic triglyceride; LDL, low-density lipoprotein.
Significantly different from lean group.
Significantly different from metabolically normal group, P < .01.
Figure 1.
Hepatic and skeletal muscle insulin sensitivity in study participants. Hepatic insulin sensitivity (A), assessed by using the Hepatic Insulin Sensitivity Index as a measure of endogenous glucose production in relation to plasma insulin concentration, and skeletal muscle insulin sensitivity (B), assessed as the stimulation of skeletal muscle glucose uptake during insulin infusion, are impaired in MAO subjects compared with MNO and lean subjects. One-way analysis of variance with planned contrasts was used to compare the differences between MAO and the other 2 groups. Values significantly different from the MAO group, *P < .001. Values are mean ± SEM.
MAO Subjects Have a Specific CD4 T-Cell Signature in Adipose Tissue
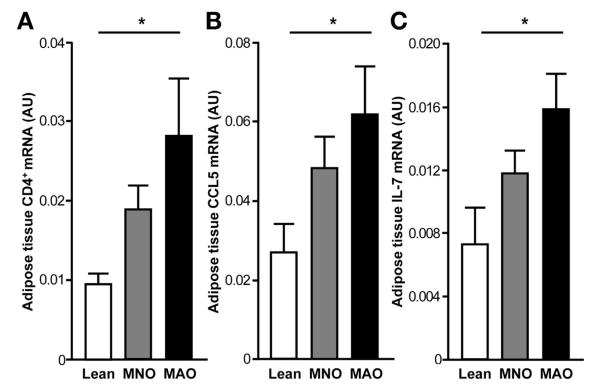
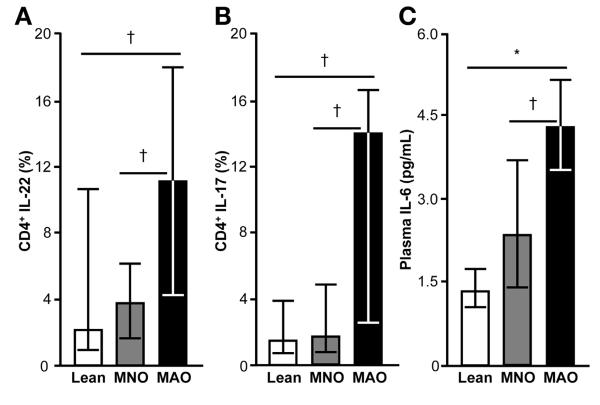
We evaluated adipose tissue CD4+ T-cell content and potential differences in the distribution of CD4+ T-cell populations among MAO, MNO, and lean subjects. Total CD4+ T-cell content, measured by gene expression, increased progressively from lean, to MNO, to MAO subjects (P < .05; Figure 2A). We then analyzed CD4+ T cells for the production of cytokines associated with the T helper (Th) 1 (interferon [IFN] γ), Th2 (IL-13), Th17 (IL-17), and Th22 (IL-22) subsets of Th cells. We found that CD4+ T lymphocytes in adipose tissue of MAO subjects were skewed toward a Th17 and Th22 phenotype; MAO subjects had a greater percent of adipose tissue lymphocytes producing IL-17 and IL-22 cytokines compared with MNO and lean subjects (Figure 3A and B). We did not observe any differences among groups in the percent of lymphocytes polarized toward producing IL-13 (MAO = 13.0% ± 9.7%, MNO = 14.1% ± 10.2%, lean = 13.0% ± 9.7%; P > .05) or IFN-γ (MAO = 68% ± 13%, MNO = 66% ± 22%, lean = 57% ± 24%; P > .05). However, adipose tissue gene expression of CCL5 and IL-7, which are cytokines involved in T-cell proliferation, survival, and recruitment,30–32 increased progressively from lean to MNO to MAO subjects (P < .05; Figure 2B and C).
Figure 2.
Adipose tissue gene expression of CD4+ (A), CCL5 (B), and IL-7 (C) in lean, MNO, and MAO participants. Adipose tissue messenger RNA values increased progressively from lean to MNO to MAO subjects (*P value for linear trend <.05, by one-way analysis of variance on log-transformed data). Values are mean ± SEM.
Figure 3.
Adipose tissue T-cell polarization and plasma IL-6 concentrations in lean, MNO, and MAO participants. MAO subjects show a distinctive polarization of CD 4+ T cells expanded from subcutaneous adipose tissue toward IL-22 (A) and IL-17 (B) producing cells. Plasma IL-6 concentration, known to stimulate lymphocyte polarization toward the Th17 and Th22 phenotype, is greater in MAO than both MNO and lean subjects (C). One-way analysis of variance with planned contrasts was used to compare the differences between MAO and the other 2 groups. Values significantly different from the MAO group; *P < .001, †P < .05. Values are median and quartiles.
Plasma IL-22 and IL-6 Concentrations Are Increased in MAO Subjects
Plasma IL-22 concentration was greater in MAO (7.0 ± 4.0 pg/mL) than MNO (2.9 ± 2.2 pg/mL) and lean (3.1 ± 1.9 pg/mL) subjects (P < .05). We were unable to detect IL-17 in plasma, presumably because of inadequate sensitivity of the assay to detect very low plasma concentrations. Circulating IL-6 stimulates lymphocyte polarization toward the Th17 and Th22 phenotype.33 Therefore, we measured IL-6 concentration in plasma and found that MAO subjects had greater plasma IL-6 concentrations than both MNO and lean groups, suggesting that increased circulating IL-6 contributes to the characteristic Th17/Th22 phenotype observed in our MAO subjects (Figure 3C). There were no differences between groups in plasma concentrations of CCL5 (lean: 47 ± 28 ng/mL, MNO: 56 ± 26 ng/mL, MAO: 55 ± 29 ng/mL; P > .05) and IL-7 (lean: 10.8 ± 3.2 ng/mL, MNO: 11.8 ± 3.8 ng/mL, MAO: 11.6 ± 4.1 ng/mL; P > .05).
Human Skeletal Muscle and Liver Express IL-17 and IL-22 Receptors
We evaluated whether the receptors for IL-17 and IL-22 are present in liver and skeletal muscle in human tissue samples because these are the key organs involved in obesity-associated metabolic dysfunction . Skeletal muscle and liver biopsies for this purpose were obtained from a separate cohort of obese subjects undergoing bariatric surgery procedures (Supplementary Table 2). We found that receptors for IL-17 (both IL-17RA and IL-17RC subunits of the heterodimeric IL-17 receptor) and IL-22 (IL-22RA, which is the receptor subunit specific for IL-22) were expressed in both tissues (Supplementary Figure 1).
IL-17 and IL-22 Inhibit Skeletal Muscle Glucose Uptake
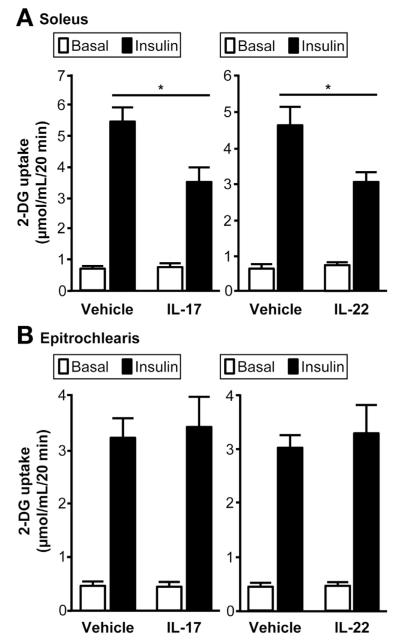
We next sought to determine whether IL-17 and IL-22 could affect skeletal muscle glucose metabolism by using an isolated rat muscle system. We found that both IL-17 and IL-22 receptors were expressed in soleus, but not in epitrochlearis muscle. We then incubated soleus and epitrochlearis muscle strips with either IL-17 or IL-22, and found a marked inhibition of insulin-mediated glucose uptake in soleus (Figure 4A), but no effect on epitrochlearis muscle (Figure 4B), consistent with the presence or absence of IL-17 and IL-22 receptors.
Figure 4.
Effects of IL-17 and IL-22 on rat skeletal muscle 2-deoxyglucose (2-DG) uptake. Incubation of rat muscle strips with IL-17 or IL-22 inhibits insulin-stimulated increase in glucose uptake in soleus muscle (A), but not in epitroclearis muscle (B). Value for muscle incubated with IL-17 and IL-22 is significantly different than value for muscle incubated with vehicle; *P < .01. Values are mean ± SEM.
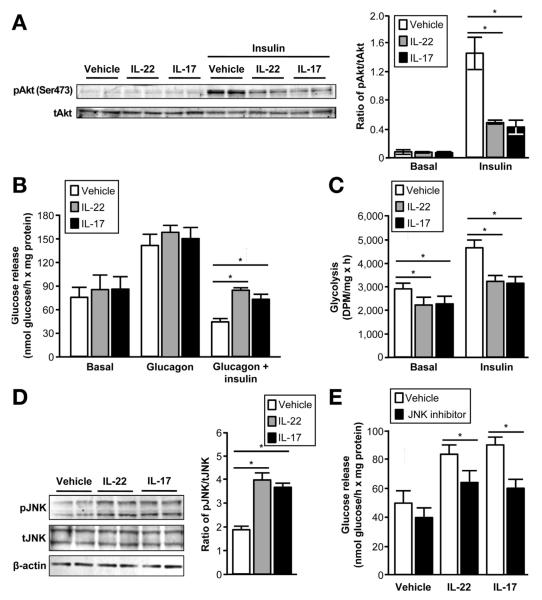
IL-17 and IL-22 Reduce Insulin Sensitivity in Human Hepatocytes
To further determine if IL-17 and IL-22 are involved in the regulation of insulin action in the liver, we evaluated the effects of these cytokines on insulin signaling in primary human hepatocytes. Treatment with either IL-17 or IL-22 led to diminished phosphorylation of Akt Ser473 in response to insulin stimulation (Figure 5A), demonstrating the inhibitory effect of these cytokines on hepatic insulin signaling. We then determined whether IL-17 or IL-22—mediated impairment in insulin action can cause alterations in hepatocyte glucose metabolism. We examined 2 key effects of insulin on hepatic glucose metabolism: insulin-mediated suppression of glucose production and insulin-stimulated increase in anaerobic glucose utilization (glycolysis). Consistent with the observed effects on insulin signaling, the inhibitory effect of insulin on glucose production rate (Figure 5B) and stimulatory effect on glycolytic rate (Figure 5C) were significantly attenuated by pretreatment with either IL-17 or IL-22.
Figure 5.
Effects of IL-17 and IL-22 on insulin-mediated glucose metabolism in primary human hepatocytes. Both IL-17 and IL-22 inhibit insulin-stimulated phosphorylation of Akt (A), insulin-mediated suppression of hepatocyte glucose production (release into the media) (B), and hepatocyte anerobic glucose metabolism (C). Western blotting analyses demonstrate that both IL-22 and IL-17 increase the phosphorylation of JNK (pJNK); total JNK (tJNK) is not affected by treatment with either cytokine (D). Pretreating human hepatocytes with a JNK inhibitor (PD098059) attenuated the increase in hepatocyte glucose production caused by IL-17 or IL-22 treatment in the presence of insulin and glucagon (E). Values for hepatocytes incubated with IL-17, IL-22 or JNK inhibitor are significantly different from vehicle values; *P < .01. Values are mean ± SEM.
We next sought to determine whether activation of c-Jun kinase (JNK), which is known to inhibit insulin action in skeletal muscle and liver,34 might explain the observed effects of IL-17 and IL-22 on glucose production. We found that treatment of human hepatocytes with either IL-17 or IL-22 increased the phosphorylation of JNK without affecting total JNK content (Figure 5D). In addition, pretreating human hepatocytes with a JNK inhibitor (PD098059) attenuated the increase in hepatocyte glucose production caused by IL-17 or IL-22 treatment in the presence of insulin and glucagon (Figure 5E).
Discussion
Chronic low-grade inflammation is associated with obesity-related metabolic complications, including nonalcoholic fatty liver disease (NAFLD), type 2 diabetes, the metabolic syndrome, and coronary heart disease. The discovery that adipose tissue from both obese mice and human subjects is infiltrated with inflammatory macrophages provided a major breakthrough into understanding how obesity and inflammation are inter-related.19,35 However, the focus of research on adipose tissue macrophages largely ignored the potential role of other immune cells in obesity-related inflammation. In the present study, we evaluated subcutaneous adipose tissue T-lymphocyte polarization in lean and obese human subjects who were separated into distinct groups based on skeletal muscle insulin sensitivity determined by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with stable-isotopically labeled glucose tracer infusion, and who had differences in intrahepatic triglyceride content. Our data demonstrate greater total CD4+ T-cell content and polarization of CD4+ T lymphocytes toward IL-17 and IL-22—producing cells in subcutaneous adipose tissue of MAO subjects compared with MNO and lean subjects. This specific adipose tissue polarization was partially mirrored by greater plasma IL-22 concentrations in MAO than MNO and lean subjects. In addition, we found both IL-17 and IL-22 receptors are expressed in human liver and skeletal muscle and that both cytokines inhibit insulin-mediated glucose metabolism in these tissues in vitro, thereby providing a novel putative link between alterations in adipose tissue lymphocyte function and metabolic disease. The observation that lymphocytes producing IL-17 and IL-22 are increased in adipose tissue of MAO, but not MNO and lean subjects, supports the potential importance of this specific lymphocyte signature in obesity-related insulin resistance and could help explain why some obese individuals develop metabolic abnormalities and others do not. However, additional studies are needed to determine whether this is a simple association or a true cause-and-effect relationship.
Our data suggest that the mechanism responsible for IL-17 and IL-22—induced insulin resistance is mediated, at least in hepatocytes, by activation of JNK. Data from previous studies have shown that JNK phosphorylates Ser307 on IRS-1, resulting in inhibition and proteolytic degradation of IRS-1, thereby affecting down-stream insulin signaling.34 Our findings are consistent with data from studies conducted in other cell types, which found IL-17 and IL-22 stimulate JNK phosphorylation.36–38 A connection between JNK and insulin resistance has been established by studies showing that JNK knockout mice are protected from obesity-related insulin resistance and JNK inhibitors can act as insulin-sensitizing agents.39 JNK is also a component of the general inflammatory response and is linked to activation of other inflammatory pathways involved in the pathogenesis of insulin resistance and the metabolic dysfunction associated with obesity.39 Therefore, these data collectively suggest that IL-17 and IL-22 in MAO subjects are involved in the pathogenesis of insulin resistance through increased JNK activity in insulin target tissues.
The effect of IL-17 and IL-22 on JNK signaling36–38 suggests the existence of an association between Th17 and Th22 polarization and NAFLD. Although the mechanisms responsible for developing steatosis and chronic liver injury in NAFLD are not clear, data from a series of studies suggest that JNK activation is involved in this process. Hepatic lipid accumulation and development of liver-injury in mouse models of diet-induced NAFLD is mediated by JNK,40 and mice lacking JNK are protected from developing liver steatosis and steatohepatitis.40 In addition, both mice fed a high-fat and high-calorie diet and patients with nonalcoholic steatohepatitis have an increase in hepatic Th17 lymphocytes, and neutralization of IL-17 reduces hepatic inflammation and injury.24 The summation of data from the present study and these previous reports support the notion that an increase in intrahepatic lymphocyte production of IL-17 and IL-22 could be involved in the development of NAFLD.
The results from recent studies conducted in animal models found the distribution of adipose tissue T-lymphocyte subsets is altered by obesity.20–23 In obese mice, CD4+ Th cells in adipose tissue are skewed toward a Th1 phenotype. These cells secrete IFN-γ, which stimulates adipose tissue macrophages to produce inflammatory cytokines (tumor necrosis factor—α and IL-6), which can induce insulin resistance. In lean mice, CD4+ Th cells in adipose tissue are skewed toward IL-4—secreting Th2 cells and regulatory T cells, which counteract inflammation and protect against insulin resistance. In contrast, we did not observe significant differences among our 3 groups of subjects (lean, MNO, and MAO) in the polarization of lymphocytes toward those producing IFN-γ, which would reflect Th1 cells, or those producing IL-13, which would reflect Th2 cells. Similarly, data from a recent study also found no differences in peripheral blood Th1 polarization between obese and lean subjects,41 but found blood lymphocytes were skewed toward a more anti-inflammatory (T regulatory and Th2) phenotype in obese compared with lean people.41 In addition, data from another study, which evaluated gene expression of markers for T cell subsets from visceral and subcutaneous adipose tissue, found both pro-inflammatory (T cytotoxic and Th1) and protective (T regulatory and Th2) lymphocyte populations were greater in adipose tissue from extremely obese than from lean and overweight subjects, and that the ratio of pro-inflammatory to anti-inflammatory T-cell subsets in visceral adipose tissue favored a protective anti-inflammatory T-cell profile.42 These results, in conjunction with the data from our study, suggest that the polarization of CD4+ Th cells observed in obese mice (increase in Th1 and reduction in Th2) is distinct from the polarization seen in obese humans.
The mechanism(s) responsible for the polarization of adipose tissue CD4+ T cells toward Th17/Th22 is not clear, but could be related to an increase in circulating or adipose tissue cytokines, or both. We found plasma IL-6, which promotes the differentiation of CD4+-naïve T cells into Th17 cells,43 was greater in our MAO than MNO and lean subjects. We also found that adipose tissue expression of CCL5 and IL-7, which stimulate T-cell recruitment and proliferation,30–32 was greater in our MAO than MNO and lean subjects. Therefore, the expansion of Th17/Th22 cells in the adipose tissue of our MAO subjects could be a direct consequence of increased circulating IL-6 and adipose tissue cytokine expression.
Our study has several important limitations. First, our data show an association, but do not demonstrate a direct cause-and-effect relationship, between adipose tissue lymphocyte polarization and skeletal muscle or hepatic insulin resistance. Second, our study subjects were primarily comprised of women, so these results might not necessarily apply to men. Third, although we found both liver and skeletal muscle in obese people express IL-17 and IL-22 receptors, we did not determine whether IL-17 and IL-22 receptor expressions differ between lean and obese subjects, and if differences in receptor abundance affect metabolic function. Finally, our study cannot determine if the major ligands for these receptors are derived from circulating IL-17 and IL-22 produced by adipose tissue lymphocytes, or from IL-17 and IL-22 produced locally by specific T-cell subsets that have infiltrated or proliferated in these organs. For example, it has been shown that T regulatory or Th17 cells can infiltrate the liver and either promote44 or prevent45 the development of viral hepatitis—induced fibrosis formation. These limitations need to be addressed in future studies.
In conclusion, the accumulation of excessive adipose tissue mass has been considered an important source of pro-inflammatory adipocytokines that contribute to metabolic dysfunction.46,47 However, not all obese people exhibit metabolic abnormalities, demonstrating that increased adipose tissue alone is not adequate to cause adipose tissue inflammation or metabolic dysfunction. The results from our study demonstrate that obese people who are metabolically abnormal (characterized by impaired insulin-mediated glucose metabolism) have a characteristic polarization of CD4+ T cells in adipose tissue, which is different from both lean subjects and obese subjects who are metabolically normal (characterized by greater insulin-mediated glucose metabolism). In addition, the cytokines produced by these lymphocyte subsets cause metabolic dysfunction in vitro in hepatocytes and muscle tissue. Additional studies are needed to determine whether alterations in lymphocyte populations in adipose tissue and possibly other organs are directly involved in the pathogenesis of hepatic and skeletal muscle insulin resistance in obese people.
Supplementary Material
Supplementary Table 1. Primer Pairs Used for Transcript Detection
Supplementary Table 2. Characteristics of Subjects From Whom Skeletal Muscle and Liver Biopsies Were Obtained
Supplementary Figure 1. Gene expression of receptors for IL-17 and IL-22 in liver and skeletal muscle obtained from human subjects. Expression of the interleukin receptors IL-17RA, IL-17RC, and IL-22RA was detected and identified in human liver (black bars) and skeletal muscle (white bars) from obese subjects by using quantitative reverse transcription polymerase chain reaction. Results were analyzed by comparing the threshold crossing of each sample after normalization to the housekeeping 36B4 gene.
Acknowledgments
The authors thank Courtney Tiemann, Martha Hessler, and Emily Smith for assistance in recruiting the study subjects; Janine Kampelman, Melisa Moore, Dr Adewole Okunade, Freida Custodio, and Jennifer Shew for technical assistance, and the staff of the Clinical Research Unit for help in performing the studies. We also are grateful to the study subjects for their participation.
Funding This work was supported by National Institutes of Health grants UL1 RR024992 (Clinical Translational Science Award), DK 56341 (Nutrition and Obesity Research Center), DK 37948, DK60022, DK33301, a grant from Pfizer Inc., and a grant from the Longer Life Foundation.
Abbreviations used in this paper
- FFM
fat free mass
- GIF
glucose infusion rate
- IFN
interferon
- IL
interleukin
- JNK
c-Jun kinase
- MAO
metabolically abnormal insulin-resistant obese
- MNO
metabolically normal insulin-sensitive obese
- NAFLD
nonalcoholic fatty liver disease
Footnotes
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.04.010.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 3.Korenblat KM, Fabbrini E, Mohammed BS, et al. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 9.Farb MG, Bigornia S, Mott M, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 11.Koska J, Stefan N, Dubois S, et al. mRNA concentrations of MIF in subcutaneous abdominal adipose cells are associated with adipocyte size and insulin action. Int J Obes (Lond) 2009;33:842–850. doi: 10.1038/ijo.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears DD, Hsiao G, Hsiao A, et al. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci U S A. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerbacka J, Corner A, Kolak M, et al. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am J Physiol Endocrinol Metab. 2008;294:E841–E845. doi: 10.1152/ajpendo.00653.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 17.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega Martinez de Victoria E, Xu X, Koska J, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 22.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilan Y, Maron R, Tukpah AM, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Bian Z, Zhao L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frimel TN, Deivanayagam S, Bashir A, et al. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 26.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 27.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 29.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 30.Overwijk WW, Schluns KS. Functions of gammaC cytokines in immune homeostasis: current and potential clinical applications. Clin Immunol. 2009;132:153–165. doi: 10.1016/j.clim.2009.03.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 32.Bacon KB, Premack BA, Gardner P, et al. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 33.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 34.Aguirre V, Uchida T, Yenush L, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvester J, Liacini A, Li WQ, et al. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16:469–476. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Iyoda M, Shibata T, Kawaguchi M, et al. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298:F779–F787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 38.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 39.Vallerie SN, Hotamisligil GS. The role of JNK proteins in metabolism. Sci Transl Med. 2010;2:60rv5. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 40.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Weerd K, Dik WA, Schrijver B, et al. Morbid obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a treg- and th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeyda M, Huber J, Prager G, et al. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 2011;19:743–748. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 43.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Chen S, Xu K. IL-17 expression is correlated with hepatitis Brelated liver diseases and fibrosis. Int J Mol Med. 2011;27:385–392. doi: 10.3892/ijmm.2011.594. [DOI] [PubMed] [Google Scholar]
- 45.Claassen MA, de Knegt RJ, Tilanus HW, et al. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315–321. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Primer Pairs Used for Transcript Detection
Supplementary Table 2. Characteristics of Subjects From Whom Skeletal Muscle and Liver Biopsies Were Obtained
Supplementary Figure 1. Gene expression of receptors for IL-17 and IL-22 in liver and skeletal muscle obtained from human subjects. Expression of the interleukin receptors IL-17RA, IL-17RC, and IL-22RA was detected and identified in human liver (black bars) and skeletal muscle (white bars) from obese subjects by using quantitative reverse transcription polymerase chain reaction. Results were analyzed by comparing the threshold crossing of each sample after normalization to the housekeeping 36B4 gene.