SCOPE OF THE PROBLEM
For the estimated 25.6 million Americans more than the age of 20 years who have diabetes, the risk of having a myocardial infarction or congestive heart failure is about twice as great as those without diabetes.1,2 In addition, it is estimated that 20% to 30% of patients admitted to the hospital with acute coronary syndrome, and 20% to 40% of those admitted with congestive heart failure exacerbation, have diabetes.3-5 Diabetes is believed to be an independent risk factor for heart failure.6-8 However, the association between acute and chronic hyperglycemia and outcomes after acute cardiovascular events is less clear.
Published studies have used different diagnostic criteria for identification of diabetes and inpatient (or stress-induced) hyperglycemia.9-11 Current estimates suggest that 37% of persons with diabetes are unaware of their diagnosis.12 Within this context, studies have shown that 10% to 34% of patients with myocardial infarction who had hyperglycemia on admission (defined differently) with no known history of diabetes were subsequently diagnosed with diabetes within 1 week of discharge.13-15 The remainder are assumed to have stress-induced hyperglycemia.
In addition, cardiovascular disease (and possibly inpatient outcomes) in patients with diabetes or prediabetes represents a complex interplay of disease processes that may not be adequately modeled by the current diagnostic criteria, which are based on the correlation of laboratory values with the onset and progression of microvascular disease processes over many years of exposure.9,10,16,17 Cardiac risk increases before a patient becomes hyperglycemic by the traditional definition. Furthermore, as discussed later, short-term hyperglycemia or glycemic fluctuations may also have an important effect on outcomes in acutely ill patients, although this is less well established. This situation should be considered when evaluating studies that dichotomously categorize populations as either having or not having diabetes or hyperglycemia.
MECHANISMS FOR HYPERGLYCEMIA IN HOSPITALIZED PATIENTS
In the acutely ill cardiac patient, many reasons coexist for the development of hyperglycemia, whether or not a patient has known diabetes. Certain therapeutic interventions, such as vasopressor agents, glucocorticoids, and parenteral nutrition can worsen or precipitate hyperglycemia.18 Patient-specific factors also probably contribute, including a patient’s underlying insulin resistance and β-cell function. However, hyperglycemia may be exacerbated by the severity of illness itself, which is marked by a proportionate increase in counterregulatory hormones and cytokines that have an adverse effect on insulin sensitivity. For example, in patients without known diabetes who were admitted to the hospital with acute myocardial infarction, cortisol, epinephrine, and norepinephrine (not hemoglobin A1c [HbA1c] or infarct size) were reported to be the primary determinants of glucose levels.19 In patients presenting with chest pain, plasma cortisol, catecholamines, glucose, and insulin were increased across the spectrum from noncardiac chest pain to unstable angina to myocardial infarction.20 Cortisol was associated with glucose levels. Such neurohormonal abnormalities have been known for decades.21,22
Neurohormonal derangements lead to excessive hepatic glucose production and insulin resistance during critical illness.23,24 Although hepatic glucose production seems to be the major player,25,26 peripheral insulin-mediated glucose uptake and nonoxidative glucose disposal are also reduced, at least in sepsis, which parallels the hormonal response observed after myocardial infarction in some ways.27,28 Acute illness is generally associated with a catabolic metabolism that is marked by hyperglycemia as well as lipolysis. Hyperglycemia, lipolysis, and hyperinsulinemia are known to interact in complex ways, contributing to exaggerated inflammatory and counterregulatory hormone responses.29,30 Resolution of hyperglycemia is associated with normalization of the inflammatory response.31
In heart failure, metabolic abnormalities also reflect the severity of symptoms.32,33 Low cardiac output leads to compensatory increases in hormones that are counterregulatory to insulin in patients with heart failure.34,35 Moreover, inflammatory cytokines such as tumor necrosis factor α (TNF-α) are increased and have been shown to directly inhibit insulin signaling.36,37 These changes mirror those observed in acute illness in general, but it is unclear whether harmful effects would be additive to abnormalities already present during heart failure. The question is whether hyperglycemia induced by such neurohormonal changes is harmful or whether it is simply a marker of severe illness.
MECHANISMS OF ACUTE HYPERGLYCEMIA-MEDIATED HARM
Diabetes
The presence of diabetes is believed to increase the risk of death for patients who are admitted with the diagnosis of myocardial infarction.38-40 This risk is related both to the direct, short-term sequelae of the infarction itself as well as to the heart failure that may subsequently result from it. Microvascular perfusion abnormalities and impaired myocardial energy production are suspected to affect patients with diabetes more prominently than those without diabetes.17 In part, these factors are suspected of predisposing patients with diabetes to larger infarct sizes, as measured by serologic biomarkers and magnetic resonance imaging, as well as rates of postinfarction heart failure that are 2 times greater than for those without diabetes.8,39,41 In the case of heart failure, the correlates of chronic metabolic and neurohormonal derangements are already a feature, including activation of the sympathetic nervous system and renin-angiotensin system,42,43 increased oxidative stress,44-46 endothelial dysfunction,47,48 inefficient myocardial substrate use, and catabolic metabolism.34 Thus, it is less clear whether exacerbation worsens outcomes. The OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment In Hospitalized Patients With Heart Failure) trial found no correlation between in-hospital, 30-day, and 60-day postdischarge mortality for patients who were admitted with both heart failure and diabetes, but this study did not examine the role of glycemic control.5
Hospital Hyperglycemia
The typical chronic complications of diabetes require several years to develop. Outside the hospital, cardiovascular benefits from glycemic control emerge only after long-term follow-up, and any mortality benefit from tight glycemic control seems be limited to patients with recently diagnosed diabetes.49,50 However, it is unclear if these observations can be extended to acute hyperglycemia associated with acute illness. Limited evidence suggests preferential downregulation of glucose transporters under conditions of chronic hyperglycemia as opposed to intermittent hyperglycemia and acute illness, potentially allowing glucose to enter cells unchecked by normal downregulatory responses.51,52 This situation provides a rationale for differential outcomes associated with stress hyperglycemia compared with chronic hyperglycemia. Stress hyperglycemia can also be considered to contribute to glycemic variability (Fig. 1). Outside the inpatient setting, intermittent glycemic excursions are associated with more profound endothelial toxicity than increases in tonic glucose level in vitro,53-55 and glycemic variability is independently associated with higher levels of oxidative stress in patients with type 2 diabetes.56 A study using a euinsulinemic, hyperglycemic clamp in patients with or without type 2 diabetes reported that oscillating glucose levels between 90 and 270 mg/dL resulted in increased endothelial dysfunction and oxidative stress that exceeded the effects of sustained hyperglycemia at 270 mg/dL in both groups.57 This finding was confirmed in another study.58
Fig. 1.
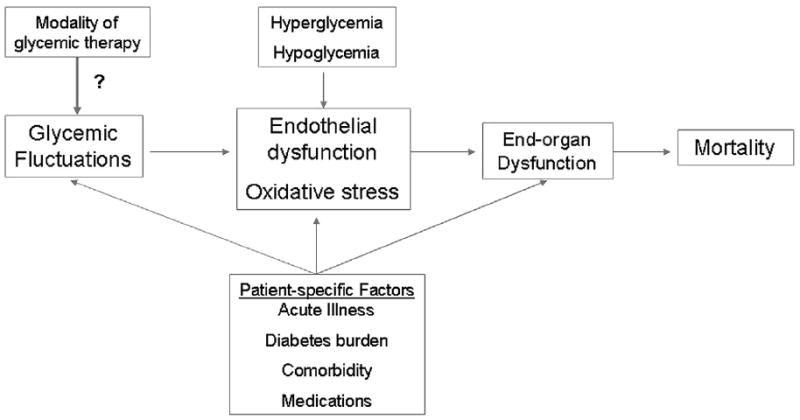
Conceptual framework for a theoretic role of glycemic variability in the acutely ill patient. Glycemic variability may be influenced by patient-specific factors such as acute illness, duration of diabetes, presence of comorbidities, and other medications. Sustained hyperglycemia, hypoglycemia, and glycemic variability may lead to endothelial dysfunction and oxidative stress, which in turn contribute to end-organ dysfunction and death.
Cardiovascular Harm
In the cardiac patient, other lines of evidence exist for a role of glycemic fluctuations in disease onset or modification. In patients admitted with chest pain, glycemic variability was an independent predictor of the severity of coronary artery disease on angiography and was superior to HbA1c.59 Glycemic variability was associated with coronary artery calcium scores in men but not women with type 1 diabetes.60 In a mouse model of diabetes, induced glycemic variability impaired ischemia-induced angiogenesis, independently of mean glucose level, through alteration of the vascular endothelial growth factor pathway.61 A prospective study of patients with type 2 diabetes and ischemic heart disease found that ischemic electrocardiographic changes were more common during rapid glucose changes (>100 mg/dL/h) than during normoglycemia or sustained hyperglycemia.62 Glycemic variability was associated with sympathovagal balance, endothelial function, and left ventricular mass index in patients with type 2 diabetes.63 Thus, it makes sense to consider short-term and long-term glycemic control separately in the hospitalized cardiac patient.
DIABETES, HYPERGLYCEMIA, AND OUTCOMES IN CARDIAC PATIENTS
Chronic Hyperglycemia
The lower limit at which chronic glycemic control (determined by HbA1c) becomes insignificant in acute cardiovascular disease is unknown.10 For example, a recent observational study of patients hospitalized for myocardial infarction found that stepwise increases in HbA1c, even if they were less than 6.5%, were associated with higher 1-year and 3-year mortality.64 On the other hand, a study of 827 patients with diabetes and average HbA1c levels near 8.0% who were admitted with a diagnosis of myocardial infarction found that HbA1c was not associated with in-hospital mortality.65 Therefore, chronic hyperglycemia may be more reflective of long-term outcomes. In heart failure, HbA1c was a progressive risk factor for mortality and hospitalization for heart failure in patients with or without diabetes66 but a U-shaped relationship may exist.67 Heart failure readmission has been associated with increasing HbA1c.68
Acute Hyperglycemia
Increasing admission blood glucose levels have also been shown to have an inverse relationship with in-hospital and long-term postmyocardial infarction survival, independent of a diagnosis of diabetes.64,65,69-71 More specifically, larger deviations of admission glucose level from a patient’s preillness glycemic control, defined by HbA1c (suggesting the presence of stress hyperglycemia), are correlated with 30-day and 1-year mortality.65,72-74 Thus, acute hyperglycemia seems to be a better predictor of mortality than chronic hyperglycemia for myocardial infarction. It is also sometimes difficult to know whether outcomes are observed with improved glycemic control per se or because of secondary effects of insulin itself. In 1 study of 141,680 patients admitted with an acute myocardial infarction,65 three-quarters of the patients with diabetes and admission glucose levels greater than 240 mg/dL received insulin therapy, compared with only 22% of those without diabetes. Thirty-day and 1-year mortality were greater in the patients without diabetes. By comparison, 1 study found no correlation between admission glucose levels and 30-day and 1-year mortality in patients admitted with heart failure.75
Most retrospective studies rely heavily on admission glucose level for analysis, which provides only a snapshot of glycemic control. A recent study of approximately 17,000 patients admitted with myocardial infarction showed that increasing in-hospital mean glucose level was associated with increasing inpatient mortality.76 The effect was observed in patients with and without diabetes, although a higher threshold for harm was identified in those with diabetes. Furthermore, mean hospital glucose level was a better predictor of mortality than admission glucose level. The importance of acute hyperglycemia, relative to chronic hyperglycemia, may be further supported by a study that showed that among intensive care unit (ICU) patients with diabetes who had poor chronic glycemic control (determined by HbA1c), rapid achievement of normoglycemia in the ICU was associated with higher mortality.77 Thus, the unique role of stress hyperglycemia deserves further attention in prospective studies.
Glycemic Variability
One factor that is receiving increasing attention both in the inpatient and outpatient setting is glycemic variability. A large review of more than 7000 medical and surgical ICU patients found that the standard deviation of glucose level was a better predictor of mortality than mean glucose level.78 This finding has been observed in other critical care settings,79,80 but has not been well studied in myocardial infarction. In patients admitted with heart failure exacerbation, glycemic variability, but not mean hospital glucose level, was associated with inpatient mortality.81 There are currently no prospective studies specifically examining outcomes through the pharmacologic manipulation of glycemic variability in the hospital. Moreover, such a study would be technically difficult to perform.
Clinical Trials Data
Over the past decade, awareness has increased about the need to find appropriate management strategies for treating hyperglycemia in hospitalized patients. Recent reports in the medical and surgical ICU have tempered any enthusiasm for strict glycemic control (target of 80–110 mg/dL), because of what has been considered an unacceptable risk of hypoglycemia and possible increase in mortality.82-85 The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial added another layer of complexity when it was ended early because of higher all-cause and cardiovascular mortality in the intensive therapy group compared with the standard therapy group.50 However, in both settings, tight glycemic control was compared with what many consider acceptable glycemic control, not poor control. Therefore, it does not follow that glycemic control should be abandoned in the hospital. For example, in a smaller multicenter randomized controlled trial of 211 surgical patients,86 basal bolus insulin resulted in lower mean glucose level compared with sliding scale insulin (147 vs 172 mg/dL, P < .01), and there was a reduction in the composite outcome of wound infection, pneumonia, bacteremia, and respiratory and acute renal failure (odds ratio [OR] 3.4, 95% confidence interval [CI] 1.5–7.7). In addition, a meta-analysis of clinical trials aiming for at least less than 180 mg/dL also showed benefits in multiple outcomes after cardiac surgery.87
Studies evaluating the management of hyperglycemia in patients with acute nonsurgical cardiovascular disease have been difficult to interpret. DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) 1 and DIGAMI 2 trials attempted to determine if improved inpatient glucose control would decrease mortality after acute myocardial infarction. DIGAMI 1 evaluated the effectiveness of immediate intravenous (IV) insulin therapy followed by insulin-based long-term therapy for patients with diabetes who were admitted with an acute myocardial infarction. Whereas 1-year mortality was reduced, in-hospital and 3-month mortality were not significantly reduced in the group treated with IV insulin.88 DIGAMI 2 was designed to determine whether the mortality benefit was caused by acute IV insulin or long-term subcutaneous insulin.89 Low enrollment and a failure to reach statistically significant differences in plasma glucose and HbA1c levels amongst the 3 groups hampered the study and no difference in short-term and long-term mortality was observed.
GIPS 1 (Glucose-Insulin-Potassium Study-1), GIPS 2 (Glucose-Insulin-Potassium Study-2), and CREATE-ECLA (The Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment and Evaluation-Estudios Clinicos Latino America) are examples of trials that attempted to determine whether insulin itself had a beneficial impact on mortality in patients suffering from acute myocardial infarction.90-92 The impetus for conducting these trials was held in the theory that high-dose glucose-insulin-potassium (GIK) infusion after a myocardial infarction would increase glucose use by myocytes and decrease use of free fatty acids. This situation would result in a decreased production of free oxygen radicals, which may cause further injury to ischemic myocytes. These trials failed to show a consistent benefit, and results were confounded by hyperglycemia in the intervention arms, which exceeded that of control arms. However, post hoc analyses did show that hyperglycemia, defined by a glucose level more than 140 mg/dL at 6 or 24 hours, was associated with increased mortality in patients without known diabetes and in patients who did not receive GIK.93 This finding suggests that a different threshold of harm may exist for patients with or without diabetes, and possibly that GIK may still mitigate the harm. Post-admission hypoglycemia (<70 mg/dL) was not associated with mortality.
Given the limitations of data to guide clinicians in the management of hyperglycemia in acute cardiovascular disease, it is not surprising that from 1997 to 2006, there was no improvement in mortality related to glycemic control in patients hospitalized with acute myocardial infarction.94
MANAGEMENT
Targets
The current American Diabetes Association (ADA)/American Association of Clinical Endocrinology hospital guidelines recommend a target glucose level of 140 to 180 mg/dL based on the control arm of the NICE-SUGAR ICU study in both ICU and non-ICU settings until further data are available (Fig. 2).95 However, NICE-SUGAR did not address whether glycemic control targeting a more modest glucose range (110–140 mg/dL) is better. In particular, hypoglycemia is less common when a more modest target (100–150 mg/dL) is attempted (frequency of blood glucose of <60 mg/dL was 5% in both medical and cardiac care units).96 As a result, an intermediate target glucose range between 110 and 140 mg/dL may be reasonable in certain populations (for example, after cardiac surgery) and institutions and patients in whom it can be performed safely. By comparison, the Endocrine Society recommends meal-specific targets, including a fasting glucose level less than 140 mg/dL and postprandial target of less than 180 mg/dL in noncritical care settings.97 Regardless, a target glucose range attempting to achieve normoglycemia (80–110 mg/dL) is not recommended because of the unacceptable risk of hypoglycemia. Furthermore, this tight glucose range is probably not technically feasible in most circumstances because of limitations of IV infusion algorithms and glucose monitoring.98 Improvements in technology, such as more precise methods of glucose monitoring and computerized (or even closed loop) IV infusion algorithms, are needed to determine whether achievement of normoglycemia is beneficial.99 Until more data are available, separate targets for acute and chronic hyperglycemia are not advocated.
Fig. 2.
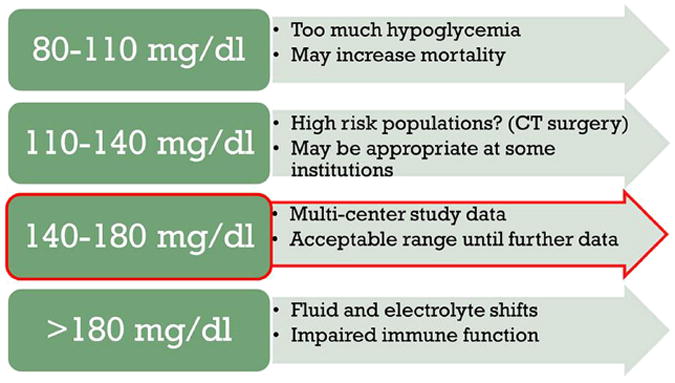
Rationale for current glycemic targets in the hospital.
General Approach
Current hospital and outpatient guidelines as well as large clinical studies focus mainly on the achievement of specific mean glucose targets and not necessarily on how those targets are achieved. The tendency for preoccupation with average glucose level neglects details in overall glucose control that may be linked to outcomes. One cannot just extrapolate successful approaches from the outpatient arena to the inpatient arena, because the dynamic nature of acute illness and the effects of other variables such as nutritional status and renal function necessitate constant vigilance and a flexible management plan. For these reasons, it is of interest to determine whether management of hyperglycemia is better viewed through the lens of minimizing glycemic variability rather than mean glucose level.
It is unknown whether measures that specifically minimize illness-induced glycemic excursions improve outcomes. In addition, some evidence suggests that glycemic variability is a function of patient-specific factors that are difficult to manipulate. Patients with glycemic lability may have long-standing diabetes with both β cell and counterregulatory hormone failure.100 In hospitalized patients, glycemic variability has been associated with age, diabetes, and total insulin requirements.101 However, measures that minimize fluctuations are more likely to achieve overall glycemic control without increasing the risk of hypoglycemia.102,103 Studies in the outpatient setting suggest that physiologic insulin regimens reduce both mean glucose level and glycemic fluctuations.104-106 Physiologic regimens, particularly when used in lieu of traditional sliding scale insulin, could also reduce glycemic variability in the hospital. Dozens of studies have investigated the efficacy of various IV insulin protocols.107,108 However, efficacy is usually not defined in terms of glycemic variability. Computerized IV insulin protocols show reductions in hypoglycemia and hyperglycemia, indicating that they may also reduce glycemic variability.109-111 More studies are needed to recommend for or against specific measures to reduce glycemic variability outside the traditional framework for tight glycemic control in the hospital. The remainder of this section focuses on strategies to provide physiologic glycemic control in hospitalized patients, with particular attention to the needs of the cardiac patient.
Insulin
The current ADA guidelines recommend insulin as the primary modality of therapy in most hospitalized patients with hyperglycemia.96,98 In general, a physiologic regimen containing basal, prandial, and supplemental (correction) insulin components is advised to obtain glycemic control (Table 1).96,98
Table 1.
Approaches to physiologic insulin use in the hospital
| % of Total Daily Insulina | Examples | |
|---|---|---|
| Basal | <50% (if eating) | Long-acting insulin analogue |
| Neutral protamine Hagedorn | ||
| Continuous subcutaneous insulin (pump) | ||
| IV insulin infusion | ||
|
| ||
| Prandial | ≥50% divided evenly over meals (if eating) | Rapid-acting insulin analogue |
| Regular insulin (tube feeds) | ||
|
| ||
| Correction | Varies | See prandial insulin |
| IV insulin infusion | ||
Total estimate insulin per day from all sources. If patient is not eating, then basal insulin may account for most insulin requirements.
IV insulin
IV insulin is advised in patients with severe hyperglycemia and in patients who are critically ill, particularly those with hypotension or who are undergoing major surgery. Issues unique to the acutely ill cardiac patient, such as poor perfusion and edema, may render IV insulin a safer, more effective choice because of slower absorption of subcutaneous insulin and the potential for insulin stacking and delayed hypoglycemia (Table 2).112 IV insulin is often necessary for treatment of hyperglycemia associated with high-dose steroids and total parenteral nutrition. Multiple algorithms have been published,108,109 but few randomized trials comparing algorithms are available.113 In general, a validated protocol should be used, keeping in mind the ease of implementation and use as well as its efficacy and safety.114
Table 2.
Comparison of IV and SQ insulin in the hospital
| IV | SQ | |
|---|---|---|
| Frequency of titration | Hourly | Daily |
| Time to target glucose | ~12 h | ~3 d |
| Adaptability to clinical status | +++ | ++ |
| Absorption issues | No | Yes |
| Duration of hypoglycemia | <1 h | Hours |
| Labor | +++ | ++ |
Another issue of importance is the patient who is eating while receiving an insulin infusion. Even the most sophisticated insulin infusion algorithms are perturbed in patients who are eating,115,116 likely representing the inability of standardized algorithms to adapt to the rapid glucose level changes induced by eating. In such patients, subcutaneous rapid-acting insulin may be provided to cover meals.117 An alternative is to use an infusion algorithm that provides a programmable temporary step-up in infusion rate after a meal.111,118
Moving patients from IV to subcutaneous insulin should be deferred ideally until a patient is hemodynamically stable, off pressors, extubated, and eating.119 Among patients undergoing postcardiac surgery, those with good glycemic control on stable or minimal insulin requirements meeting the aforementioned criteria are more successful with the transition. In surgical patients, an initial basal insulin dose of 50% to 65% of the daily IV insulin requirements (calculated from the average infusion rate during fasting or adequate subcutaneous prandial insulin coverage) is adequate for many patients 48 hours after surgery.118,120 Inadequate prandial insulin coverage during an infusion may also lead to an overestimation of basal insulin necessary for transitioning a patient off an insulin infusion.118 On the other hand, patients with more chronic hyperglycemia, marked insulin resistance, or shorter duration of infusion after the initial event may require a higher conversion factor or weight-based dosing.121,122 The dosing regimen should be compared with the patient’s home insulin requirements, HbA1c level, and weight (see later discussion).
Subcutaneous insulin
Initiation of insulin
Subcutaneous insulin is the mainstay of treatment of hospitalized patients with diabetes. However, the initiation of insulin can be a challenging proposition for patients who are acutely ill, because a variety of factors, including altered eating habits and a rapidly changing clinical course, may significantly affect insulin requirements over time. It may be helpful to stratify the approach by preadmission glycemic control and therapy (Fig. 3).
Fig. 3.
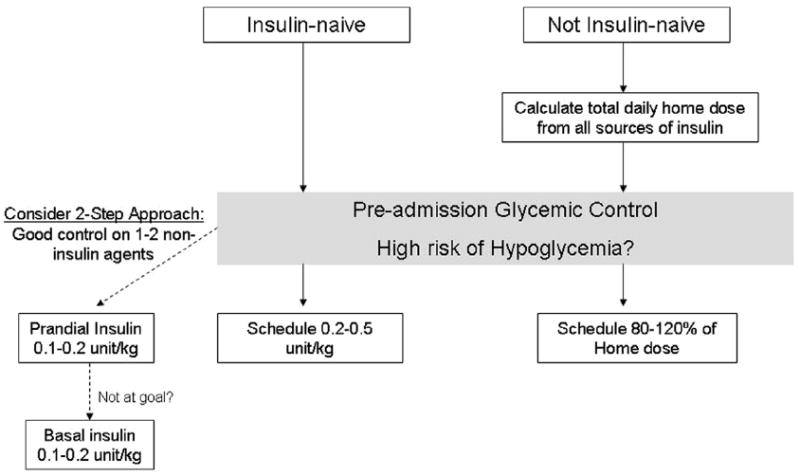
Calculation of the initial total daily dose of insulin in the hospital.
Insulin-naive patients
Poor baseline control: in patients with severe hyperglycemia (>300 mg/dL) refractory to intervention or who have other indications, IV insulin is an efficient means of calculating total insulin requirements, as noted earlier. In patients with moderate hyperglycemia or stable patients with more advanced hyperglycemia, a weight-based algorithm has been used for the initiation of subcutaneous insulin. The total daily dose is calculated as 0.4 to 0.5 units/kg and 50% is administered as basal insulin and 50% as prandial insulin, divided evenly over 3 meals. A lower dose (0.2–0.3 unit/kg) should be considered in patients at risk for hypoglycemia, such as elderly patients or those with renal or liver impairment.98
Mild to moderate baseline control: there are few studies supporting approaches to insulin therapy in insulin-naive patients with more mild to moderate (<200 mg/dL) or intermittent hyperglycemia. These patients may be best treated with a stepwise approach. Outside the hospital, basal insulin is typically initiated before prandial insulin, but in the hospital, prandial insulin may be the preferred first-line therapy in patients who periodically must receive nothing by mouth for tests. If the fasting glucose level remains increased, a low dose of basal insulin can be implemented at 0.1 to 0.2 unit/kg.
Noninsulin-naive patients
In noninsulin-naive patients, continuation of the home insulin regimen requires special consideration. First, it must be emphasized that patients with type 1 diabetes require basal insulin without exception at all times. Otherwise, one must inquire about the patient’s level of adherence, the frequency of glucose self-monitoring, and patterns of hypoglycemia. In all cases, basal insulin is administered at no more than 50% of the total home daily dose (basal plus prandial) and the rest of the total daily requirements are spread out over meals.87,123 In particular, nonphysiologic insulin regimens (particularly in cases in which basal insulin accounts for a large proportion of the total daily dose) need adjustment because patients must often receive nothing by mouth or otherwise may not be eating in the same way with hospital food. In such cases, basal insulin may require reduction in favor of more prandial insulin.
Poor baseline control: the total daily dose may be increased 10% to 20% in patients who are adherent and uncontrolled.
Good baseline control: the total daily dose may be continued without adjustment provided that hypoglycemia is not a significant problem and that a physiologic regimen is used.
Special considerations for prandial and correction insulin
In general, a patient who is eating reasonably well should receive no more than 50% of the total daily insulin dose as basal insulin (Table 3).87,123 Ideally, the prandial insulin dose is tailored to carbohydrate intake (flexible insulin dosing or carbohydrate counting starting at 1 unit per 10 or 15 g of carbohydrates). If fixed meal dosing is used, a consistent carbohydrate diet is necessary. Special precautions should accompany the order (cut dose in half if patient eats 50% of the tray or if glucose level is 70–90 mg/dL, hold dose if <50% of the tray is eaten, and so forth). Correction (supplemental) insulin should also be adjusted based on the patient’s total daily insulin requirements. Coverage for enteral and parenteral feeding requires additional consideration, which is beyond the scope of this article.
Table 3.
Distribution of insulin dosing in hospitalized patients with normal oral intakea based on the total estimated daily dose
| Total Daily Doseb (Units) | Basal Insulin Dose | Fixed Meal Dosec (Per Meal) | Insulin: Carbohydrate (Units: g) | Correction Factor (1 Unit per __ mg/dL More Than 150 mg/dL) |
|---|---|---|---|---|
| <20 | ≤10 | 2–3 | 1:20 | 100 |
| 20–40 | 10–20 | 4–5 | 1:15 | 50 (standard dose) |
| 41–50 | 20–25 | 6–8 | 1:10 (standard dose) | 50 (standard dose) |
| 51–80 | 25–40 | 9–13 | 1:8 | 25 |
| >80 | 40+ | 14+ | 1:5 | 25 |
Assumes patient is eating a typical (carbohydrate-controlled) diet.
Total estimated insulin per day from all sources.
Accompany with appropriate hold parameters (eg, hold if patient eats less than half of tray or if glucose level <80 mg/dL).
Adjustment of therapy
Daily adjustment of therapy should be directed at hypoglycemia first. Targeted reduction in insulin doses should be commensurate with the degree and frequency of hypoglycemia and risk of adverse consequences. In patients whose illness is marked by significant stress hyperglycemia (such as after cardiac surgery or large myocardial infarction), one must also allow for continued reduction in insulin requirements as the stress of the illness dissipates.118,122 Continued preemptive dose reduction (10%–20% per day) may be required in patients who are tightly controlled (glucose level consistently <100 mg/dL) with severe illness.98 Patients with chronic hyperglycemia or less acute illness may require less aggressive dose reduction.123 Home insulin requirements may be a helpful guide. In patients with persistent hyperglycemia, randomized controlled trials have indicated that daily dose adjustment of 10% to 20% per day is reasonable in patients receiving basal bolus insulin analogues.87,124
Periprocedural care
The cardiac patient is particularly prone to changes in oral intake because of the need for frequent procedures. In general, it is not necessary to withhold basal insulin entirely. Although limited prospective data are available, 1 retrospective review reported that among patients who were told to take 50% of their home dose of basal insulin before surgery, hypoglycemia was uncommon (2% among patients with a preoperative glucose level <200 mg/dL; 0% among those with preoperative hyperglycemia).124 However, postoperative hyperglycemia was persistent in most patients with preoperative hyperglycemia. By comparison, patients who are undergoing procedures such as cardiac stress testing or catheterization may have a smaller stress response, and a reduction of 50% may still be reasonable. However, the necessity for dose reduction may be negligible and omissions may be minimized by prophylactically adjusting the home regimen to a more physiologic distribution at the time of admission, as discussed earlier. In patients with type 1 diabetes, minimal reductions (<20%), are necessary, and generally only in patients with tight glycemic control.125
Noninsulin Therapy
Oral agents
As stated earlier, for a variety of reasons guidelines advise discontinuation of oral agents in most hospitalized patients with diabetes (Table 4). Oral sulfonylureas may result in prolonged hypoglycemia in patients with even modest renal dysfunction, a common comorbidity in the cardiac patient, or nil-by-mouth status.126,127 Metformin is associated with lactic acidosis in a variety of conditions, such as renal insufficiency (which is especially relevant in patients receiving IV contrast), decompensated heart failure, or hypoxia.128-131 Although the risk is low, the estimated mortality is still high.129,130,132 Thiazolidinediones are well known to be associated with congestive heart failure.132,133 Furthermore, they have a slow onset of action and thus have limited use in the inpatient setting.
Table 4.
Comparison of insulin and noninsulin hypoglycemic agents in the hospital
| Noninsulin Agents | Insulina | |
|---|---|---|
| Frequency of titration | Days to weeks | Daily |
|
| ||
| Adaptability to changes in nutritional status | + | +++ |
|
| ||
| Duration of hypoglycemia | None (M, T, G) | Hours |
| Days (S) | ||
|
| ||
| Other cautions | Renal dysfunction (M, S) | Rare |
| Liver disease (M, S, T) | ||
| Lactic acidosisb (M) | ||
| Nausea/vomiting/pancreatitis (G) | ||
| Heart failure (T, M) | ||
| Elderly (M, S) | ||
DPP-4 inhibitors are generally safe but have limited efficacy and there are limited data for use in the hospital.
Abbreviations: G, GLP-1 (exenatide, liraglutide, pramlintide); M, metformin; S, sulfonylureas; T, thiazolidinediones.
Using a physiologic regimen.
Metformin-associated lactic acidosis is increased in patients with renal dysfunction (and IV contrast administration), acidosis, respiratory failure, acute heart failure, and hemodynamic compromise.
GLP-1 based therapy
Glucagonlike peptide 1 (GLP-1)-based therapies have garnered increasing interest for the treatment of diabetes or hyperglycemia in patients with diabetes. These agents do not generally cause hypoglycemia in the absence of other therapies that cause hypoglycemia.134,135 There are GLP-1 receptors in the myocardium, and preclinical and early clinical studies raise the possibility that there may be beneficial cardiac effects, such as reduction of ischemic preconditioning and improved left ventricular function.136 Furthermore, these therapies counteract the effects of inappropriate glucagon release, a pathologic feature of diabetes and stress hyperglycemia alike.72 There are 2 main approaches to augmenting GLP-1 to treat hyperglycemia: (1) GLP-1 receptor agonists or mimetics and (2) inhibition of dipeptidyl peptidase IV (DPP-IV), the enzyme that degrades GLP-1.
GLP-1 receptor agonists are generally more potent and have the advantage of promoting weight loss long-term.137,138 These agents have been studied intravenously in small studies of hospitalized cardiac patients, but nausea is a major potential side effect and it is too early too tell if cardiac benefits are present.139-143
DPP-IV inhibitors are generally well tolerated but have more limited efficacy.136 However, in some patients with mild hyperglycemia, they may be considered. Meta-analyses from randomized controlled trials suggest cardiovascular benefits from these agents versus active comparators, although most studies were short-term.144,145
The long-term safety of these agents is unknown and the safety and efficacy of GLP-1–based therapies have not been extensively tested in hospitalized patients. Further study therefore is warranted.
Other approaches
Many other noninsulin therapies are under investigation for the treatment of diabetes in the outpatient setting.146 There has been little interest in developing targeted therapies for inpatient use, possibly because of the complexity of the patients and the lack of randomized controlled trials that establish an appropriate glucose target. Noninsulin agents that lower glucose level without causing hypoglycemia are candidates for inpatient therapy. Agents that target major components of stress-induced hyperglycemia, such as hepatic glucose output or insulin resistance in general, are needed. These agents must be safe, effective, and ideally compatible with other IV medications. Until such therapies become available, insulin remains the mainstay of therapy.
Discharge
Sustained glycemic control after discharge may be facilitated with proper planning that begins at the time of hospital admission. Patients should be screened for medication adherence and discharge needs with appropriate social work input early. Diabetes education should be considered early in the hospital course when needed, because education is best received when there is time for continued follow-up and reinforcement.147,148 Likewise, diabetes physician consultation should be considered early in the course when necessary, because the attainment of glycemic control typically requires several days to achieve.87,124 The HbA1c may be used to guide hospital discharge regimens such that those who are well controlled before admission may resume their preadmission therapy, provided there are no contraindications and oral intake has resumed to normal (Table 5). In other patients, 1-step or 2-step intensification of therapy is necessary based on the severity of hyperglycemia, comorbidities, and contraindications.149 Written communication of changes in the diabetes regimen is crucial for patients at the time of discharge, and outpatient follow-up should be ensured.
Table 5.
Home-going strategy based on HbA1ca
| HbA1c (%) | Suggested Regimen |
|---|---|
| >9 | Basal + oral or basal + bolus insulin |
|
| |
| 7–9 | 1-step to 2-step increase in therapy: |
| oral → 2 oral → oral + basal insulin → basal bolus | |
|
| |
| <7 | Resume home regimen |
Strategy assumes that patient is resuming a normal diet and that there are no contraindications.
SUMMARY
More studies are needed to recommend for or against specific measures to reduce glycemic excursions outside the traditional framework for tight glycemic control. However, measures to stabilize glucose through physiologic insulin regimens may have the potential to preserve or enhance the benefits of glycemic control and reduce the risks of hypoglycemia in the hospital. Such efforts also serve to build a united front with outpatient providers in reinforcing the importance of glycemic control for reducing the long-term risk of microvascular complications.
KEY POINTS.
Acute hyperglycemia is associated with worse outcomes in hospitalized cardiac patients.
Hospitalized cardiac patients are at particular risk for developing hyperglycemia and hypoglycemia for a variety of reasons.
Flexible physiologic insulin regimens are appropriate for most hospitalized patients.
Hospitalization provides an opportunity for reinforcement of good diabetes-related self-care behaviors to prevent long-term complications.
Well-designed studies addressing glycemic control in the hospitalized cardiac population are needed.
Acknowledgments
Disclosures: KD discloses receiving research support from Novo Nordisk, as well as consulting fees from Eli Lilly and Pfizer.
References
- 1.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTER-HEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Nichols GA, Hillier TA, Erbey JR. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 3.Canto JG, Kiefe CI, Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306(19):2120–7. doi: 10.1001/jama.2011.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor JR, Fonarow GC, Zhao X, et al. Diabetes, quality of care, and in-hospital outcomes in patients hospitalized with heart failure. Am Heart J. 2011;162(3):480–486.e3. doi: 10.1016/j.ahj.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) Am Heart J. 2007;154(2):277.e1–8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EF, Velazquez EJ, Solomon SD, et al. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J. 2008;29(6):748–56. doi: 10.1093/eurheartj/ehn062. [DOI] [PubMed] [Google Scholar]
- 7.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 8.Roy B, Pawar PP, Desai RV, et al. A propensity-matched study of the association of diabetes mellitus with incident heart failure and mortality among community-dwelling older adults. Am J Cardiol. 2011;108(12):1747–53. doi: 10.1016/j.amjcard.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–91. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 13.Hashimoto K, Ikewaki K, Yagi H, et al. Glucose intolerance is common in Japanese patients with acute coronary syndrome who were not previously diagnosed with diabetes. Diabetes Care. 2005;28(5):1182–6. doi: 10.2337/diacare.28.5.1182. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara M, Inoue I, Kawagoe T, et al. Is admission hyperglycaemia in non-diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance? Eur Heart J. 2006;27(20):2413–9. doi: 10.1093/eurheartj/ehl271. [DOI] [PubMed] [Google Scholar]
- 15.Wallander M, Malmberg K, Norhammar A, et al. Oral glucose tolerance test: a reliable tool for early detection of glucose abnormalities in patients with acute myocardial infarction in clinical practice: a report on repeated oral glucose tolerance tests from the GAMI study. Diabetes Care. 2008;31(1):36–8. doi: 10.2337/dc07-1552. [DOI] [PubMed] [Google Scholar]
- 16.DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26(3):688–96. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 17.von Bibra H, St John Sutton M. Impact of diabetes on postinfarction heart failure and left ventricular remodeling. Curr Heart Fail Rep. 2011;8(4):242–51. doi: 10.1007/s11897-011-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80:1558–67. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 19.Oswald GA, Smith CC, Betteridge DJ, et al. Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 1986;293(6552):917–22. doi: 10.1136/bmj.293.6552.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stubbs PJ, Laycock J, Alaghband-Zadeh J, et al. Circulating stress hormone and insulin concentrations in acute coronary syndromes: identification of insulin resistance on admission. Clin Sci (Lond) 1999;96:589–95. doi: 10.1042/cs0960589. [DOI] [PubMed] [Google Scholar]
- 21.Kurt TL, Genton E, Chidsey C, 3rd, et al. Carbohydrate metabolism and acute myocardial infarction: circulating glucose, insulin, cortisol and growth hormone responses and excretion of catecholamines. Chest. 1973;64(1):21–5. doi: 10.1378/chest.64.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Vetter NJ, Strange RC, Adams W, et al. Initial metabolic and hormonal response to acute myocardial infarction. Lancet. 1974;1(7852):284–8. doi: 10.1016/s0140-6736(74)92595-1. No abstract available. [DOI] [PubMed] [Google Scholar]
- 23.Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35:S508–18. doi: 10.1097/01.CCM.0000278047.06965.20. [DOI] [PubMed] [Google Scholar]
- 24.Andrewq RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96:513–23. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 25.Jeevanandam M, Young DH, Schiller WR. Glucose turnover, oxidation, and indices of recycling in severely traumatized patients. J Trauma. 1990;30:582–9. doi: 10.1097/00005373-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 26.McGuinness OP, Fugiwara T, Murrell S, et al. Impact of chronic stress hormone infusion on hepatic carbohydrate metabolism in the conscious dog. Am J Physiol. 1993;265:E314–22. doi: 10.1152/ajpendo.1993.265.2.E314. [DOI] [PubMed] [Google Scholar]
- 27.Fan J, Li YH, Wojnar MM, et al. Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock. 1996;6:164–70. [PubMed] [Google Scholar]
- 28.Green CJ, Campbell IT, O’Sullivan E, et al. Septic patients in multiple organ failure can oxidize infused glucose, but non-oxidative disposal (storage) is impaired. Clin Sci (Lond) 1995;89:601–9. doi: 10.1042/cs0890601. [DOI] [PubMed] [Google Scholar]
- 29.Stegenga ME, van der Crabben SN, Blümer RM, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–9. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soop M, Duxbury H, Agwunobi AO, et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxin in humans. Am J Physiol Endocrinol Metab. 2002;282:E1276–85. doi: 10.1152/ajpendo.00535.2001. [DOI] [PubMed] [Google Scholar]
- 31.Stentz FB, Umpierrez GE, Cuervo R, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–86. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 32.Sabelis LW, Senden PJ, Zonderland ML, et al. Determinants of insulin sensitivity in chronic heart failure. Eur Heart J. 2003;5:759–65. doi: 10.1016/s1388-9842(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Motro M, Fisman EZ, et al. Functional class in patients with heart failure is associated with the development of diabetes. Am J Med. 2003;114:271–5. doi: 10.1016/s0002-9343(02)01530-9. [DOI] [PubMed] [Google Scholar]
- 34.Norrelund H, Wiggers H, Halbirk M, et al. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic congestive heart failure. J Intern Med. 2006;260:11–21. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 35.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526–34. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30(4):997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 37.Feinstein R, Kanety H, Papa MZ, et al. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268(35):26055–8. [PubMed] [Google Scholar]
- 38.Stranders I, Diamant M, van Gelder RE, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–8. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 39.Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the prevention of REStenosis with tranilast and its outcomes (PRESTO) trial. Circulation. 2004;109(4):476–80. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 40.Shah AM, Uno H, Kober L, et al. The inter-relationship of diabetes and left ventricular systolic function on outcome after high-risk myocardial infarction. Eur J Heart Fail. 2010;12(11):1229–37. doi: 10.1093/eurjhf/hfq179. [DOI] [PubMed] [Google Scholar]
- 41.Mather AN, Crean A, Abidin N, et al. Relationship of dysglycemia to acute myocardial infarct size and cardiovascular outcome as determined by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:61. doi: 10.1186/1532-429X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pliquett RU, Fasshauer M, Bluher M, et al. Neurohumoral stimulation in type-2-diabetes as an emerging disease concept. Cardiovasc Diabetol. 2004;3:4. doi: 10.1186/1475-2840-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaboury CL, Simonson DC, Seely EW, et al. Relation of pressor responsiveness to angiotensin II and insulin resistance in hypertension. J Clin Invest. 1994;94:2295–300. doi: 10.1172/JCI117593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez Farre A, Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38(6):1400–5. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 45.Polidori MC, Pratico D, Savino K, et al. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. 2004;10(4):334–8. doi: 10.1016/j.cardfail.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39(6):957–62. doi: 10.1016/s0735-1097(02)01721-7. [DOI] [PubMed] [Google Scholar]
- 47.Heitzer T, Baldus S, von Kodolitsch Y, et al. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol. 2005;25(6):1174–9. doi: 10.1161/01.ATV.0000166516.52477.81. [DOI] [PubMed] [Google Scholar]
- 48.Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 49.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 50.ACCORD Study Group. Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanhorebeek I, Van den Berghe G. Diabetes of injury: novel insights. Endocrinol Metab Clin North Am. 2006;35:859–72. doi: 10.1016/j.ecl.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Cohen G, Riahi Y, Alpert E, et al. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113:259–67. doi: 10.1080/13813450701783513. [DOI] [PubMed] [Google Scholar]
- 53.Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidatie stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 54.Inhat M, Green D, Ross K, et al. Reduced antioxidant response of retinal and endothelial cells in response to chronic oscillating glucose levels. Diabetes. 2005;54(Suppl 1):2314A. [Google Scholar]
- 55.Risso A, Mrecuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:924–30. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 56.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 57.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 58.Buscemi S, Re A, Batsis JA, et al. Glycaemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabet Med. 2010;27:872–8. doi: 10.1111/j.1464-5491.2010.03059.x. [DOI] [PubMed] [Google Scholar]
- 59.Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snell-Bergeon JK, Roman R, Rodbard D, et al. Glycaemic variability is associated with coronary artery calcium in men with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med. 2010;27:1436–42. doi: 10.1111/j.1464-5491.2010.03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biscetti F, Pitocco D, Straface G, et al. Glycaemic variability affects ischaemia-induced angiogenesis in diabetic mice. Clin Sci (Lond) 2011;121(12):555–64. doi: 10.1042/CS20110043. [DOI] [PubMed] [Google Scholar]
- 62.Desouza C, Salazar H, Cheong B, et al. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26(5):1485–9. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 63.Di Flaviani A, Picconi F, Di Stefano P, et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011;34:1605–9. doi: 10.2337/dc11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timmer JR, van der Horst IC, Ottervanger JP, et al. Prognostic value of admission glucose in nondiabetic patients with myocardial infarction. Am Heart J. 2004;148(3):399–404. doi: 10.1016/j.ahj.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Cao JJ, Hudson M, Jankowski M, et al. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol. 2005;96(2):183–6. doi: 10.1016/j.amjcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 66.Gerstein HC, Swedberg K, Carlsson J, et al. CHARM Program Investigators. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168(15):1699–704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 67.Aguilar D, Bozkurt B, Ramasubbu K, et al. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54(5):422–8. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dungan KM, Osei K, Nagaraja HN, et al. Relationship between glycemic control and readmission rates in patients hospitalized with congestive heart failure during implementation of hospital-wide initiatives. Endocr Pract. 2010;16(6):945–51. doi: 10.4158/EP10093.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 70.Shah B, Amoroso NS, Sedlis SP. Hyperglycemia in nondiabetic patients presenting with acute myocardial infarction. Am J Med Sci. 2012;343(4):321–6. doi: 10.1097/MAJ.0b013e31822fb423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111(23):3078–86. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 72.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petursson P, Herlitz J, Caidahl K, et al. Admission glycaemia and outcome after acute coronary syndrome. Int J Cardiol. 2007;116(3):315–20. doi: 10.1016/j.ijcard.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 74.Ishihara M, Kagawa E, Inoue I, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99(12):1674–9. doi: 10.1016/j.amjcard.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 75.Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119(14):1899–907. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 76.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–27. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 77.Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105–11. doi: 10.1097/CCM.0b013e3181feb5ea. [DOI] [PubMed] [Google Scholar]
- 78.Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244–52. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Wintergerst KA, Buckingham B, Gandrud L, et al. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–9. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 80.Ali NA, O’Brien JM, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36(8):2316–21. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dungan KM, Binkley P, Nagaraja HN, et al. The effect of glycaemic control and glycaemic variability on mortality in patients hospitalized with congestive heart failure. Diabetes Metab Res Rev. 2011;27(1):85–93. doi: 10.1002/dmrr.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 83.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 84.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–48. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 85.Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest. 2010;137(3):544–51. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- 86.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34(2):256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haga KK, McClymont KL, Clarke S, et al. The effect of tight glycaemic control, during and after cardiac surgery, on patient mortality and morbidity: a systematic review and meta-analysis. J Cardiothorac Surg. 2011;6:3. doi: 10.1186/1749-8090-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 89.Malmberg K, Ryden L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26(7):650–61. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 90.van der Horst IC, Zijlstra F, van ’t Hof AW, et al. Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial. J Am Coll Cardiol. 2003;42(5):784–91. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 91.Timmer JR, Svilaas T, Ottervanger JP, et al. Glucose-insulin-potassium infusion in patients with acute myocardial infarction without signs of heart failure: the glucose-insulin-potassium study (GIPS)-II. J Am Coll Cardiol. 2006;47(8):1730–1. doi: 10.1016/j.jacc.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 92.Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293(4):437–46. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 93.Goyal A, Mehta SR, Díaz R, et al. Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation. 2009;120(24):2429–37. doi: 10.1161/CIRCULATIONAHA.108.837765. [DOI] [PubMed] [Google Scholar]
- 94.Holper EM, Abbott JD, Mulukutla S, et al. Temporal changes in the outcomes of patients with diabetes mellitus undergoing percutaneous coronary intervention in the National Heart, Lung, and Blood Institute dynamic registry. Am Heart J. 2011;161(2):397–403.e1. doi: 10.1016/j.ahj.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barth M, Oyen L, Warfield K, et al. Comparison of a nurse initiated insulin infusion protocol for intensive insulin therapy between adult surgical trauma, medical and coronary care intensive care patients. BMC Emerg Med. 2007;7:14. doi: 10.1186/1471-227X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 98.Dungan K, Chapman J, Braithwaite SS, et al. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30:403–9. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 99.Hirsch IB. Intravenous bolus insulin delivery: implications for closed-loop control and hospital care. Diabetes Technol Ther. 2012;14(1):6–7. doi: 10.1089/dia.2011.0222. [DOI] [PubMed] [Google Scholar]
- 100.Murata GH, Duckworth WC, Shah JH, et al. Sources of glucose variability in insulin-treated type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES) Clin Endocrinol (Oxf) 2004;60:451–6. doi: 10.1111/j.1365-2265.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 101.Al-Dorzi HM, Tamim HM, Arabi YM. Glycaemic fluctuation predicts mortality in critically ill patients. Anaesth Intensive Care. 2010;38(4):695–702. doi: 10.1177/0310057X1003800413. [DOI] [PubMed] [Google Scholar]
- 102.Monnier L, Wojtusciszyn A, Colette C, et al. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13(8):813–8. doi: 10.1089/dia.2011.0049. [DOI] [PubMed] [Google Scholar]
- 103.Kauffmann RM, Hayes RM, Buske BD, et al. Increasing blood glucose variability heralds hypoglycemia in the critically ill. J Surg Res. 2011;170(2):257–64. doi: 10.1016/j.jss.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colombel A, Murat A, Krempf M, et al. Improvement of blood glucose control in type 1 diabetic patients treated with lispro and multiple NPH injections. Diabet Med. 1999;16(4):319–24. doi: 10.1046/j.1464-5491.1999.00077.x. [DOI] [PubMed] [Google Scholar]
- 105.Lepore G, Dodesini AR, Nosari I, et al. Effect of continuous subcutaneous insulin infusion vs multiple daily insulin injection with glargine as basal insulin: an open parallel long-term study. Diabetes Nutr Metab. 2004;17(2):84–9. [PubMed] [Google Scholar]
- 106.Saudek CD, Duckworth WC, Giobbie-Hurder A, et al. Implantable insulin pump vs multiple-dose insulin for non-insulin-dependent diabetes mellitus: a randomized clinical trial. Department of Veterans Affairs Implantable Insulin Pump Study Group. JAMA. 1996;276(16):1322–7. [PubMed] [Google Scholar]
- 107.Meijering S, Corstjens AM, Tulleken JE, et al. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systematic review of the literature. Crit Care. 2006;10(1):R19. doi: 10.1186/cc3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson M, Weinreb J, Hoo GW. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care. 2007;30(4):1005–11. doi: 10.2337/dc06-1964. [DOI] [PubMed] [Google Scholar]
- 109.Vogelzang M, Loef BG, Regtien JG, et al. Computer-assisted glucose control in critically ill patients. Intensive Care Med. 2008;34(8):1421–7. doi: 10.1007/s00134-008-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Plank J, Blaha J, Cordingley J, et al. Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients. Diabetes Care. 2006;29:271–6. doi: 10.2337/diacare.29.02.06.dc05-1689. [DOI] [PubMed] [Google Scholar]
- 111.Cavalcanti AB, Silva E, Pereira AJ, et al. A randomized controlled trial comparing a computer-assisted insulin infusion protocol with a strict and a conventional protocol for glucose control in critically ill patients. J Crit Care. 2009;24(3):371–8. doi: 10.1016/j.jcrc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 112.Ariza-Andraca CR, Altamirano-Bustamante E, Frati-Munari AC, et al. Delayed insulin absorption due to subcutaneous edema. Arch Invest Med. 1991;22(2):229–33. [PubMed] [Google Scholar]
- 113.Blaha J, Kopecky P, Matias M, et al. Comparison of three protocols for tight glycemic control in cardiac surgery patients. Diabetes Care. 2009;32(5):757–61. doi: 10.2337/dc08-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Curr Opin Clin Nutr Metab Care. 2010;13(2):198–204. doi: 10.1097/MCO.0b013e32833571db. [DOI] [PubMed] [Google Scholar]
- 115.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28:2418–23. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 116.Smiley D, Rhee M, Peng L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5:212–7. doi: 10.1002/jhm.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dungan K, Hall C, Schuster D, et al. Comparison of 3 algorithms for basal insulin in transitioning from intravenous to subcutaneous insulin in stable patients after cardiothoracic surgery. Endocr Pract. 2011;17(5):753–8. doi: 10.4158/EP11027.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Juneja R, Roudebush C, Kumar N, et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9:232–40. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 119.Furnary AP, Braithwaite SS. Effects of outcome on in-hospital transition from intravenous insulin infusion to subcutaneous therapy. Am J Cardiol. 2006;98(4):557–64. doi: 10.1016/j.amjcard.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 120.Schmeltz LR, DeSantis AJ, Schmidt K, et al. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia. Endocr Pract. 2006;12:641–50. doi: 10.4158/EP.12.6.641. [DOI] [PubMed] [Google Scholar]
- 121.Dungan K, Hall C, Schuster D, et al. Differential response between diabetes and stress-induced hyperglycaemia to algorithmic use of detemir and flexible mealtime aspart among stable postcardiac surgery patients requiring intravenous insulin. Diabetes Obes Metab. 2011;13(12):1130–5. doi: 10.1111/j.1463-1326.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shomali ME, Herr DL, Hill PC, et al. Conversion from intravenous insulin to subcutaneous insulin after cardiovascular surgery: transition to target study. Diabetes Technol Ther. 2011;13(2):121–6. doi: 10.1089/dia.2010.0124. [DOI] [PubMed] [Google Scholar]
- 123.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus as-part versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):564–9. doi: 10.1210/jc.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DiNardo M, Donihi AC, Forte P, et al. Standardized glycemic management and perioperative glycemic outcomes in patients with diabetes mellitus who undergo same-day surgery. Endocr Pract. 2011;17:404–11. doi: 10.4158/EP10316.OR. [DOI] [PubMed] [Google Scholar]
- 125.Mucha GT, Merkel S, Thomas W, et al. Fasting and insulin glargine in individuals with type 1 diabetes. Diabetes Care. 2004;27:1209–10. doi: 10.2337/diacare.27.5.1209. [DOI] [PubMed] [Google Scholar]
- 126.Holstein A, Plaschke A, Hammer C, et al. Characteristics and time course of severe glimepiride-versus glibenclamide-induced hypoglycaemia. Eur J Clin Pharmacol. 2003;59(2):91–7. doi: 10.1007/s00228-003-0592-4. [DOI] [PubMed] [Google Scholar]
- 127.Holstein A, Hammer C, Hahn M, et al. Severe sulfonylurea-induced hypoglycemia: a problem of uncritical prescription and deficiencies of diabetes care in geriatric patients. Expert Opin Drug Saf. 2010;9(5):675–81. doi: 10.1517/14740338.2010.492777. [DOI] [PubMed] [Google Scholar]
- 128.Yeung CW, Chung HY, Fong BM, et al. Metformin-associated lactic acidosis in Chinese patients with type II diabetes. Pharmacology. 2011;88(5–6):260–5. doi: 10.1159/000331867. [DOI] [PubMed] [Google Scholar]
- 129.van Berlo-van de Laar IR, Vermeij CG, Doorenbos CJ. Metformin associated lactic acidosis: incidence and clinical correlation with metformin serum concentration measurements. J Clin Pharm Ther. 2011;36(3):376–82. doi: 10.1111/j.1365-2710.2010.01192.x. [DOI] [PubMed] [Google Scholar]
- 130.Biradar V, Moran JL, Peake SL, et al. Metformin-associated lactic acidosis (MALA): clinical profile and outcomes in patients admitted to the intensive care unit. Crit Care Resusc. 2010;12(3):191–5. [PubMed] [Google Scholar]
- 131.Seidowsky A, Nseir S, Houdret N, et al. Metformin-associated lactic acidosis: a prognostic and therapeutic study. Crit Care Med. 2009;37(7):2191–6. doi: 10.1097/CCM.0b013e3181a02490. [DOI] [PubMed] [Google Scholar]
- 132.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 133.Kaul S, Bolger AF, Herrington D, et al. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(17):1885–94. doi: 10.1016/j.jacc.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 134.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34:S279–84. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs. 2011;71:1441–67. doi: 10.2165/11591400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 136.Davidson MH. Cardiovascular effects of glucagon-like peptide-1 agonists. Am J Cardiol. 2011;108(Suppl 3):33B–41B. doi: 10.1016/j.amjcard.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 137.Vilsbøll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(10) doi: 10.1002/14651858.CD006423.pub2. CD006423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halbirk M, Nørrelund H, Møller N, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298(3):H1096–102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 140.Deane AM, Chapman MJ, Fraser RJ, et al. The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomised double-blind placebo-controlled cross over study. Crit Care. 2009;13(3):R67. doi: 10.1186/cc7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sourij H, Schmölzer I, Kettler-Schmut E, et al. Efficacy of a continuous GLP-1 infusion compared with a structured insulin infusion protocol to reach normoglycemia in nonfasted type 2 diabetic patients: a clinical pilot trial. Diabetes Care. 2009;32(9):1669–71. doi: 10.2337/dc09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Read PA, Hoole SP, White PA, et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4(3):266–72. doi: 10.1161/CIRCINTERVENTIONS.110.960476. [DOI] [PubMed] [Google Scholar]
- 143.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100(5):824–9. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 144.Johansen OE, Neubacher D, von Eynatten M, et al. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11(1):3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122(3):16–27. doi: 10.3810/pgm.2010.05.2138. [DOI] [PubMed] [Google Scholar]
- 146.Tahrani AA, Bailey CJ, Del Prato S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378(9786):182–97. doi: 10.1016/S0140-6736(11)60207-9. [DOI] [PubMed] [Google Scholar]
- 147.Cook CB, Seifert KM, Hull BP, et al. Inpatient to outpatient transfer of diabetes care: planning for an effective hospital discharge. Endocr Pract. 2009;15:263–9. doi: 10.4158/EP.15.3.263. [DOI] [PubMed] [Google Scholar]
- 148.Kimmel B, Sullivan MM, Rushakoff RJ. Survey on transition from inpatient to outpatient for patients on insulin: what really goes on at home? Endocr Pract. 2010;16(5):785–91. doi: 10.4158/EP10013.OR. [DOI] [PubMed] [Google Scholar]
- 149.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–59. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]


