Abstract
Compound L-368,899 was successfully alkylated with [11C]iodomethane to generate the oxytocin receptor selective (2R)-2-amino-N-((2S)-7,7-dimethyl-1-(((4-(o-tolyl)piperazin-1-yl)sulfonyl)methyl)bicyclo[2.2.1]heptan-2-yl)-N-[11C]methyl-3-(methylsulfonyl)propanamide ([11C]1) with very high radiochemical purity and high specific activity. PET imaging studies were performed with [11C]1 to investigate brain penetration and oxytocin receptor uptake using rat and cynomolgus monkey models. For rat baseline scans, brain penetration was observed with [11C]1, but no specific uptake could be distinguished in the brain region. By administering a peptide oxytocin receptor selective antagonist for peripheral blocking of oxytocin receptors, the uptake of [11C]1 was amplified in the rat brain temporarily to enable some visual uptake within the rat brain. A baseline scan of [11C]1 in a cynomolgus monkey model resulted in no detectable specific uptake in anticipated regions, but activity did accumulate in the choroid plexus.
Keywords: Oxytocin, Vasopressin, PET imaging, L-368, 899, Pituitary, Receptor Imaging
The oxytocin (OT) system modulates a wide variety of social behaviors, including maternal care, social recognition, and social bonding.1–3 It has been suggested that alterations in the OT system may contribute to the core social deficits in psychiatric disorders such as autism.4–8 Furthermore, several studies suggest that intranasal administration of OT or other drugs that stimulate the OT system may be useful for enhancing social functioning in autism spectrum disorders.9–12 In recent years there has been a significant increase in the number of studies examining the effects of intranasal administration of OT on human behavior.13 The development of a ligand for positron emission tomography (PET) which has a high affinity and selectivity for the oxytocin receptor (OTR) would provide a non-invasive method for localizing and generating quantitative in vivo data of OTR density, data that can only be obtained currently via post mortem techniques. Furthermore, in a clinical setting, an OTR PET ligand may eventually be useful as a biomarker for psychiatric disorders in which social dysfunction may be attributed to altered neural OTR expression. It could also serve as a pharmacodynamic tool to be used for validation of new OT selective, brain penetrant pharmaceuticals. We have recently reported our first preliminary investigations toward the development of a PET imaging agent which is selective for human OTR.14 Although all of our candidates were selective for human OTR over human vasopressin receptor subtypes, the blood-brain barrier and/or p-glycoprotein pump prevented our first set of tracers from successfully imaging the neural OTR in vivo in rat models. While we remain interested in further developing these molecules, we became interested in another class of reported molecules bearing high affinity for OTR (Figure 1), especially since one of the derivatives, L-368,899, was reported to penetrate the blood-brain barrier and used in behavior studies involving the monogamous marmoset model.15–17 The inhibition constants of L-368,899 and its methylamino (1) derivatives for the rodent OTR and vasopressin receptors are included in Table 1 as was reported by Williams et al, Pettibone et al, and with data obtained from NIH’s Psychoactive Drug Screening Program (PDSP) using human cell lines.18 As suggested by the tabulated data, these compounds all have desired selectivity and potency for the OTR in rodents for a PET ligand. It should be noted that the methylamino derivative 1 has nearly identical potency and only slightly less selectivity as the brain penetrating L-368,899. Therefore, carbon-11 methyl alkylation of L-368,899 seemed an obvious route to generate an adequate radiotracer to evaluate this class of molecules as PET imaging radiotracers for neural OTR. Since the selectivity of respective ligands may vary from rodent to human species, 1 was investigated via the PDSP for human OTR and vasopressin receptor affinity. The data suggests increased potency for human receptors while the selectivity remains adequate and with slight improvement across species, which encouraged us to proceed in the development of its positron emitting analogue. We report here the carbon-11 methyl alkylation of L-368,899 and its in vivo evaluation via PET imaging of female rat and male cynomolgus monkey models.
Figure 1.
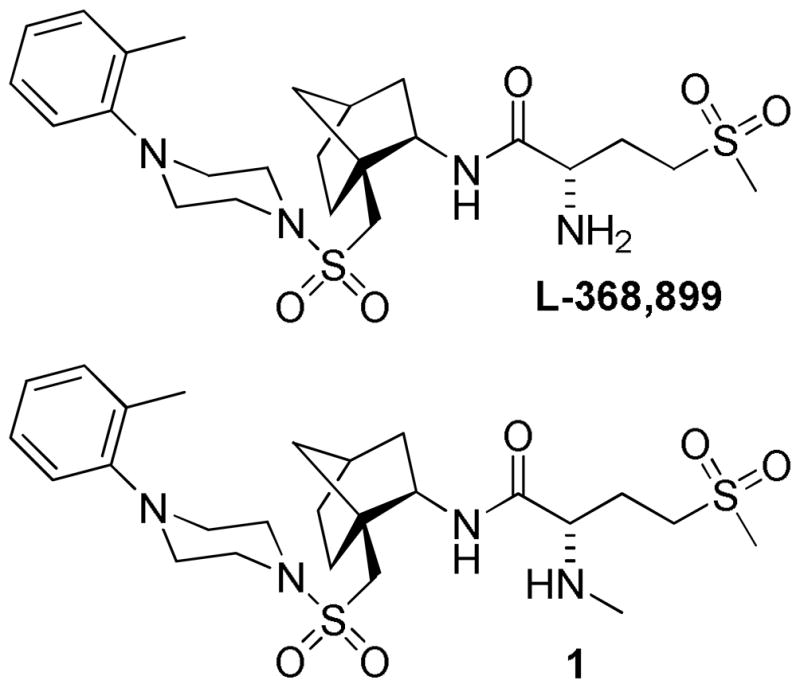
Reported compounds developed through Merck which are selective for OTR.
Table 1.
Inhibition constants of L-368,899 and 1 for OT and vasopressin receptors and their binding affinity (Ki in nM) for the respective human receptors.
| Compound | Rodenta | Humanb | |||||
|---|---|---|---|---|---|---|---|
| IC50 OT | IC50 V1a | IC50 V2 | Ki OTR | Ki V1a | Ki V1b | Ki V2 | |
| L-368,899 | 8.9 | 370 | 570 | 13 | 180 | - | 590 |
| 1 | 9.2 | 320 | 350 | 5.2 | 620 | 1300 | 181 |
The inhibition constants are measured in nanomolar values and were derived from rat uterus (OT), rat liver (V1a), and rat kidney (V2) as previously reported by Williams et al (reference 15).
The Ki values for L-368,899 are as reported by Pettibone et al (reference 17) and measured from the human uterus (OTR), liver (V1a), and kidney (V2). Ki values for 1 were measured in nanomolar values and were derived from human receptor assays generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
The labeling reaction used to generate [11C]1 is outlined in Scheme 1. Both L-368,899 and the cold standard of 1 were synthesized in our laboratories using the previously reported procedures.15 Although L-368,899 is commercially available as a hydrochloride salt, the free base is the desired precursor due to the possible formation of [11C]chloromethane with the presence of chloride ions in the reaction mixture. After multiple trials, our standard dose production of [11C]1 was conducted by preparing a mixture of 2 mg of L-368,899 precursor in 200 μl DMF in a small 1ml V-vial and cooling it to 0 C in an ice bath. A stream of helium and [11C]iodomethane generated from a GE PETtrace MeI Microlab was then bubbled through the mixture. Once activity maxed, the vial was placed in a preheated 110 C oil bath for 10 minutes. The vial was then cooled quickly in its original ice bath, diluted with 500 μl of 50:50:0.1 ethanol: water: triethylamine (HPLC solvent), and injected on an 19 × 100mm Waters XTerra RP18 prep HPLC column with a 5 μm bed. Using the above mentioned HPLC solvent, [11C]1 was eluted with a retention time of 16m 20s at 6ml/min. After being diluted 500% with water, the eluted fractions containing [11C]1 were loaded on a waters tC-18 cartridge, rinsed with 30 ml saline, and product was eluted off with 1.5 ml ethanol into a sterile vial containing 13.5 ml saline. The dose was then passed through 1 μm and 0.2 μm filters using high argon pressure. A dose would normally be prepared in 65 minutes from EOB and result in approximately 5% uncorrected yields of [11C]1 with a specific activity of 1Ci/μmol as calculated from a calibration curve. Radiochemical purity was > 99% and no trace of the starting material (which elutes just before the product) was observed.
Scheme 1.
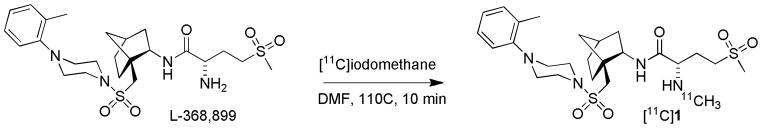
Radiolabeling reaction of L-336,899 with [11C]iodomethane.
To estimate the potential of [11C]1 for crossing the blood-brain barrier, its log P7.4 was measured and determined to be 2.62 using a previously reported method.19 This value was deemed satisfactory to achieve brain penetration.
To validate the functionality of [11C]1 as a PET tracer for imaging neural OTR, the compound was investigated in vivo via PET imaging using Sprague-Dawley rats (n=4; weighing 180–200 g). The rats were subcutaneously injected with estradiol benzoate (10 – 12 μg in 0.2 ml sesame oil) once a day for 3 days prior to the day of the scan to maximize OTR expression in the hypothalamus. After being anesthetized with a ketamine/xylazine cocktail (100 and 10 mg/kg respectively via ip) the rats were affixed with a tail vein catheter and strapped in the prone position on the microPET bed. A 10 minute transmission scan was performed using a cobalt-57 source to provide both attenuation correction and anatomical details. At exactly 30 seconds after emission scanning began, 500 μCi of [11C]1 was injected into the subjects and a 45 minute emission scan was performed. Animals were euthanized with compressed CO2 following the scan.
The images generated from the sum of the 45 minute baseline scan are shown in Figure 2. After thorough examination of the data at various time points, we concluded there was no specific uptake of [11C]1 within the rat brain during the baseline scan despite some observed penetration into the brain. The similar patterns of the time-activity curves generated for both the brain region and muscle region suggests the possibility of brain penetration through the entirety of the scan (Figure 4a), but there is clearly lower overall uptake within the brain region. Significant uptake was observed in the vicinity of the pituitary gland as indicated by these time-activity curves and the arrows in Figure 2. To confirm if this uptake was specific to oxytocin and in an attempt to amplify the amount of [11C]1 reaching the brain region, periphery blocking studies were performed by injecting 5 mg/kg of desGly–NH2, d(CH2)5[Tyr(Me)2, Thr4] ornithine vasotocin (OVT), an oxytocin selective peptide antagonist, ten minutes prior to the injection of [11C]1.20 There were no visual changes observed in the pituitary uptake after administration of OVT suggesting uptake in the pituitary was not blocked. The time-activity curves shown in Figure 3b clearly confirm that the uptake in the pituitary was still significant. This suggests the observed uptake of [11C]1 within the pituitary is not due to specific OTR binding. This could be attributed to affinity to another receptor as the structure of 1 does contain a known pharmacophore in its structure, 4-(2-methylphenyl)piperazine. These time-activity curves also suggest the overall uptake in the muscle and pituitary was slightly lower when the periphery OTR was blocked with OVT. Interestingly, when OVT was administered, the uptake within the brain increased during the first 10 minutes of the scan. To determine whether there was improved localization of [11C]1 in specific areas during this time frame, the sum of the first 10 minutes of the scan were examined. As can be seen in Figure 4, uptake appears in regions comparably to what was observed during the first five minutes of a previously reported PET image derived from our other investigated 18F-labeled OTR selective ligand.14 Significant uptake of [11C]1 is apparent in the dorsal caudate nucleus, which is known to express OTR, however specific uptake in the hypothalamus and amygdala, which have the highest densities of OTR binding were not apparent. The image resolution, 1.65 mm, is not sufficient to distinguish caudate from the choroid plexus, which we suspect is concentrating the ligand in this in vivo study.21 Although these results suggest in vivo selective binding of [11C]1 to the OTR of the brain is not sufficient in the rat species for localization and quantitative assessments using in vivo PET imaging, blocking the peripheral OTR density clearly appears to improve brain penetration of [11C]1. Given the encouraging results with brain penetration and the higher affinity for primate (e.g. human) OTR, we opted to investigate this compound using a non-human primate model.
Figure 2.
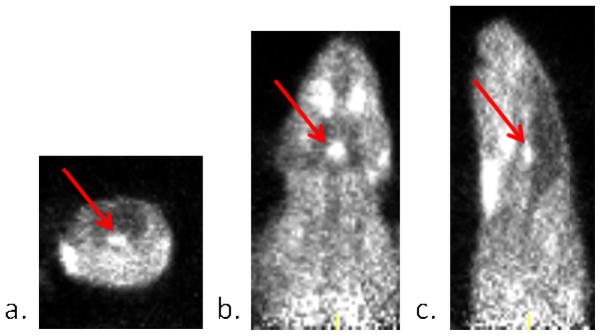
Coronal (a.), transverse (b.), and saggital (c.) views of the sum of a 45 minutes scan of 500 μCi of [11C]1 in a rat model. The arrows point to the observed uptake near the base of the brain in the vicinity of the pituitary gland.
Figure 4.
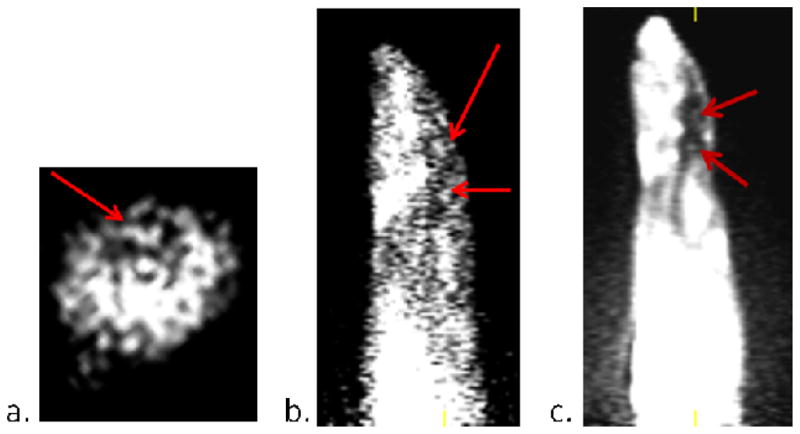
a.) Coronal slice of a PET image generated from 10 minutes of scanning 450 μCi post injection of 5mg/kg of OVT showing the caudate putamen. b.) Saggital slice of a PET image generated from 10 minutes of scanning 300 μCi post injection of 5mg/kg of OVT showing the caudate putamen and ventral tegmental area. c.) Saggital slice of a PET image generated from 5 minutes of scanning 300 μCi of [18F]4 as previously reported (shown for anatomical comparison).
Figure 3.
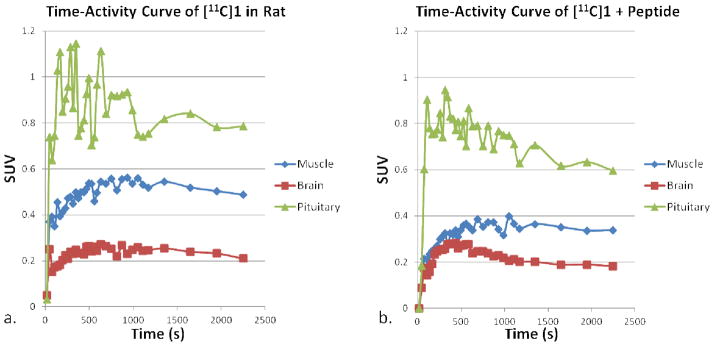
Time-activity curves showing uptake levels in the brain, muscle, and pituitary gland during (a.) 45 minute PET scan of [11C]1 and (b.) a 45 minute scan of [11C]1 10 minutes after an injection of 5mg/kg of OVT.
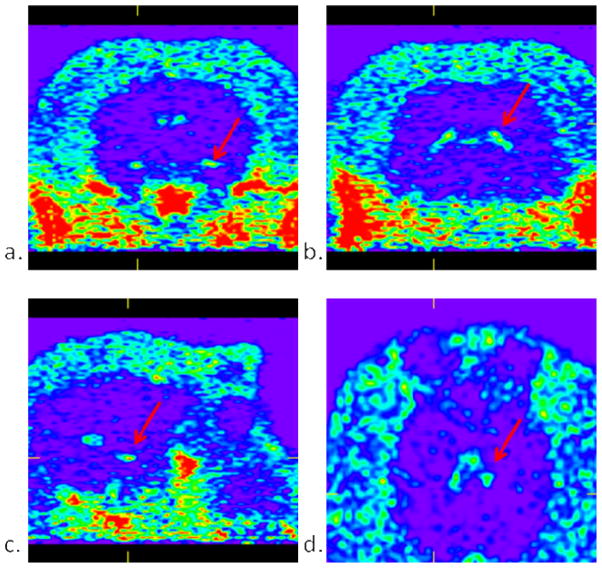
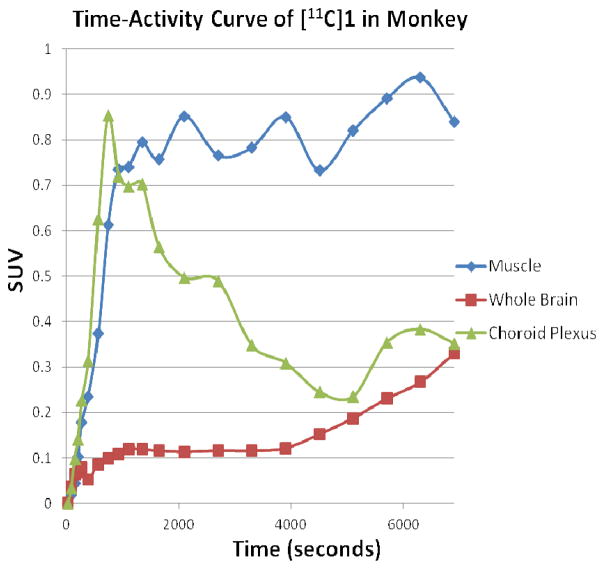
A male cynomolgus monkey weighing 8.91 kg was injected with 8.3 mCi of [11C]1 via bolus injection and scanned for 2 hours from time of injection. The images generated from the sum of the 2 hour scan (Figure 5) show uptake primarily located in the choroid plexus. Time-activity curves generated from uptake in the choroid plexus, muscle tissue distribution and the whole brain are shown in Figure 6. As can be seen, the uptake in the choroid plexus reaches an equilibrium level as seen in the muscle tissue and then gradually washes out completely over the course of the scan. As there is no other area of uptake seen in the brain, and no significant spike of activity in whole brain time-activity curve, it is believed that [11C]1 failed to efficiently penetrate the brain. As there are no reports of OTR in the choroid plexus, we currently do not offer an explanation for the uptake seen in the choroid plexus, although there are many other reports of this phenomenon suggesting it may related to the P-glycoprotein function.22–24 It should also be noted that OTR have not been adequately characterized in the primate brain, so we do not know where the areas of highest OTR densities are located. Indeed, radioligands that efficiently label OTR in rodents using autoradiography fail to generate selective OTR binding in the macaque brain, raising the possibility that the macaque may not be ideally suited for testing OTR PET ligands.25 A better understanding of the localization of OTR in the macaque brain, or a primate with known brain OTR distribution (e.g. the marmoset) is clearly needed to validate OTR PET ligands in this early stage of discovery.26
Figure 5.
Images of 8.3 mCi of [11C]1 in a cynomolgus monkey summed up over 2 hours a.) coronal slice showing uptake in the temporal pole area of the choroid plexus. b.) coronal slice showing choroid plexus uptake in the temporal horn of the lateral ventricle. c.) saggital slice showing uptake in the temporal pole area of the choroid plexus. d.) transverse slice showing uptake in the choroid plexus of the third ventricle.
Figure 6.
Time-activity curves generated from a two hour scan of 8.3 mCi of [11C]1 in a cynomolgus monkey showing uptake patterns in the choroid plexus, muscle tissue, and whole brain.
There are additional factors that could have had implications on the results of this study. The possibility of N-methyl demethylation of 1 in vivo could have prevented adequate distribution in time for imaging. The demtheylation was an issue primarily with the N, N-dimethyl analogue as originally reported by Williams et al and it was reported to metabolize only partially to L-368,899 in vivo.15 Nevertheless, enough of the tracer should have remained intact to visualize brain penetration in the first few minutes of the scan. We are currently investigating a more stable N-fluoroethyl analogue of L-368,899 for evaluation to alleviate this issue. Another factor for consideration is the reported oral bioavailability of 17–18% of L-368,899 in the rat and dog, suggesting a relatively rapid metabolism. This was also supported by the necessity of large doses of L-368,899 (20 mg/kg) required to reverse the effects of oxytocin in the marmoset as reported by Smith et al.17 The primary difference between these studies and our study was an iv administration was performed, not an oral administration. Therefore, we still anticipated a rapid influx of activity into the brain at the beginning of the scan if the compound penetrated efficiently. Although our investigated compound was not L-368,899 itself, the results published herein exaggerate the need to further investigate the mechanisms involved in the physiology of the oxytocin system as these results point to the possibility that observed behavioral antagonistic effects of L-368,899 could function through the periphery. To further investigate this possibility and to afford complimentary data to this study, the synthesis of [11C]L-368,899 may be investigated via [11C]methylation at the terminal carbon of the methionine sulfone.
In conclusion, an OTR selective PET ligand, [11C]1, has been successfully synthesized and investigated within rat and cynomolgus monkey models as a potential candidate for in vivo imaging of neural OTR. PET imaging studies conducted with [11C]1 in the rat suggested [11C]1 may have reached the brain after blocking peripheral OTR with OVT, but the uptake pattern did not recapitulate the distribution of OTR binding in the rat brain as determined by autoradiography, and the uptake that was seen may represent choroid plexus. In a cynomolgus monkey model, uptake was found to temporarily equilibrate within the choroid plexus, an area believed to not contain OTR. The low activity detected in the entire brain throughout the entirety of the scan suggests [11C]1 may not distribute throughout the monkey brain rapidly enough to enable adequate localization and/or quantification of OTR and it may have affinity for the P-glycoprotein pump. However, based on the increase of brain penetration from the rat study observed after blocking the peripheral OTR with OVT, it might be worthwhile to investigate if these effects are observed in a monkey model. Furthermore, studies in primates with known brain OTR receptor distribution are needed to clarify if these results are species specific.
Acknowledgments
We thank Larry Williams, Mel Camp, and Eugene Malveaux for their contributions in the rodent studies. We thank the Yerkes National Primate Center’s imaging suite staff for primate imaging. We thank the NIMH PDSP directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA for their contributions of the human cell line studies. This research was funded by the National Institute of Mental Health through grant 5 R21 MH090776. We also acknowledge NIH MH064692 (LJY) and the National Center for Research Resources P51RR165 (currently P51OD11132) to YNPRC.
Footnotes
Notes:
The animal imaging experiments were performed in compliance with the Emory Institutional Animal Care and Use Committee (IACUC) and radiation safety regulations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donaldson ZR, Young LJ. Science. 2008;322:900. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson JN, Aldag JM, Insel TR, Young LJ. J Neurosci. 2001;21:8278. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross HE, Young LJ. Front Neuroendocrinol. 2009;30:534. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Neurosci Lett. 2007;417:6. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Mol Psychiatry. 2008;13:980. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 7.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Biol Psychiatry. 1998;43:270. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Biol Psychiatry. 2005;58:74. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modi ME, Young LJ. Horm Behav. 2012;61:340. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guastella AJ, MacLeod C. Horm Behav. 2012;61:410. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Young LJ, Flanagan-Cato LM. Horm Behav. 2012;61:227. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AL, Freeman SM, Stehouwer JS, Inoue K, Voll RJ, Young LJ, Goodman MM. Bioorg Med Chem. 20:2721. doi: 10.1016/j.bmc.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PD, Anderson PS, Ball RG, Bock MG, Carroll L, Chiu SH, Clineschmidt BV, Culberson JC, Erb JM, Evans BE, et al. J Med Chem. 1994;37:565. doi: 10.1021/jm00031a004. [DOI] [PubMed] [Google Scholar]
- 16.Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Horm Behav. 2007;52:344. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AS, Agmo A, Birnie AK, French JA. Horm Behav. 57:255. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettibone DJ, Clineschmidt BV, Guidotti MT, Lis EV, Reiss DR, Woyden CJ, Bock MG, Evans BE, Freidinger RM, Hobbs DW, Veber DF, Williams PD, Chiu SHL, Thompson KL, Schorn TW, Siegl PKS, Kaufman MJ, Cukierski MA, Haluska GJ, Cook MJ, Novy MJ. Drug Development Research. 1993;30:129. [Google Scholar]
- 19.Wilson AA, Houle S. Journal of Labelled Compounds & Radiopharmaceuticals. 1999;42:1277. [Google Scholar]
- 20.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Science. 2009;325:862. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ. J Neurosci. 1989;9:1764. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreisl WC, Liow JS, Kimura N, Seneca N, Zoghbi SS, Morse CL, Herscovitch P, Pike VW, Innis RB. J Nucl Med. 51:559. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyal S, Ke B, Muzi M, Link JM, Mankoff DA, Collier AC, Unadkat JD. Clin Pharmacol Ther. 87:579. doi: 10.1038/clpt.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer M, Karch R, Neumann F, Wagner CC, Kletter K, Muller M, Loscher W, Zeitlinger M, Langer O. J Cereb Blood Flow Metab. 30:510. doi: 10.1038/jcbfm.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toloczko DM, Young L, Insel TR. Ann N Y Acad Sci. 1997;807:506. doi: 10.1111/j.1749-6632.1997.tb51953.x. [DOI] [PubMed] [Google Scholar]
- 26.Schorscher-Petcu A, Dupre A, Tribollet E. Neurosci Lett. 2009;461:217. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]