Laboratory mice naturally come in a large variety of colors ranging from black to white, brown to yellow, and spotted.10,11 However, as obvious as this feature is, it refers to the color of the mouse’s hair not of its skin. Investigators are perpetually confused when they shave, clip, or chemically depilate mice to see black mice with pink skin or with patches of pink, grey, or black skin. Knowledge of basic physiological differences between mice and humans provides an obvious answer to this extremely commonly asked question. By examining the skin carefully in several inbred strains or the same inbred strain with various coat color mutations, the answer becomes obvious as well as many other peculiarities of the skin commonly overlooked by most investigators “phenotyping” mutant mice. One must understand normal anatomy and biology in order to correctly interpret both normal and abnormal features (phenotypes).8 We present here evaluation of adults from several different strains with different coat colors to illustrate these important features.
Wild type (normal, +/+) retired female breeders from the following inbred strains were used: BALB/cJ (JR#651, albino, white), C3H/HeJ (JR#659, agouti), C57BL/6J (JR#664, black), B6.Cg-Tyrc-J/J (JR#35, white), and DBA/1J (JR#670, dilute brown). All mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were euthanized by CO2 asphyxiation, shaved with electric clippers (Oster #78005-010, Niles, IL), and photographed on a copy stand with a digital camera.16 This work was done with our Institutional Animal Care and Use Committee’s approval. Skin was removed with a scalpel and cut in a longitudinal head to tail strip, laid flat on aluminum foil, fixed by immersion in Fekete’s acid-alcohol-formalin solution overnight, stored in 70% ethanol, trimmed, processed routinely, embedded in paraffin, sectioned at 6 um, and stained with hematoxylin and eosin.9,17
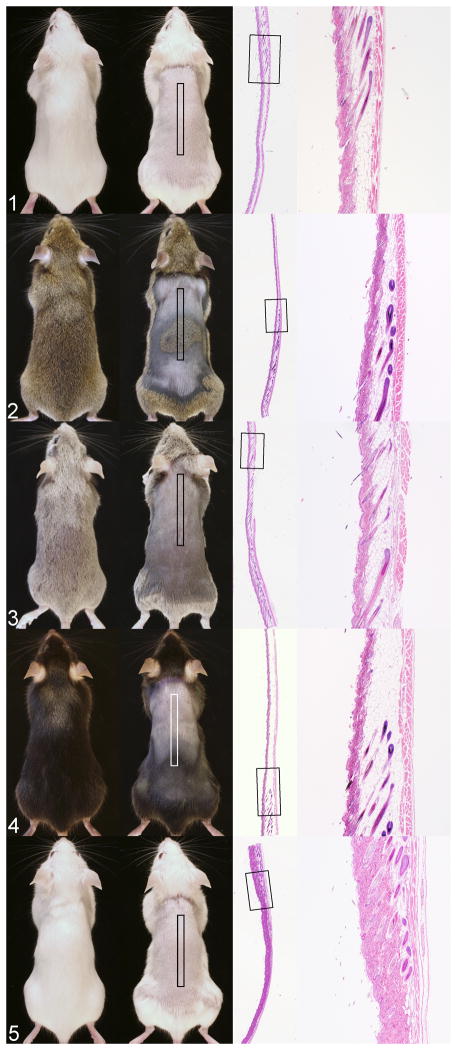
Gross photographs of each of these mice illustrate various shades of pink skin with pigmented areas for the colored mice (Fig. 1–5). Next to each gross image is a scanned image of the skin section to correlate histology with the marked anatomic site. Note that hair follicles vary in size, especially in length, which corresponds to the thickness of the hypodermal fat layer. This is the normal hair cycle in the mouse.6,15 The hair cycle is one of the key points to understanding skin color in the mouse.
Figure 1. Coat and skin colors variations in mice. Each mouse is shown intact (left) and shaved (right) with histology of the skin on the right from the boxed area on the shaved mouse.
Figure 1. BALB/cJ skin and hair are white. Boxed area is uniformly in telogen with a solitary guard hair in anagen. No pigment is evident because this is an albino mouse strain.
Figure 2. C3H/HeJ hair is agouti. The skin has dark and light areas. Light areas are in telogen while dark areas are in late anagen.
Figure 3. DBA/1J mice have a dilute brown coat color. Hair follicles are in catagen and telogen with no evidence of pigmentation within the hair shafts within the follicles. Remnant hair shafts on the surface are pigmented. This strain can have hair shafts with bulbous swellings, a dystrophic hair shaft,12,14 that may result in pigmentary incontenance in the dermis such that the areas in telogen may be more heavily pigmented than similar areas of other inbred mouse strains.
Figure 4. C57BL/6J mice have black hair but their skin has light pink and grey to black areas. The junction of these areas shows the transition from telogen to late anagen. The late anagen hair follicles are heavily pigmented.
Figure 5. B6.Cg-Tyrc-2J/J mice are congenic C57BL/6J mice carrying the albino mutation (in the tyrosinase gene: Tyrc-2J) making them white. Hair follicles in the biopsied area are in telogen to late anagen with no pigmentation.
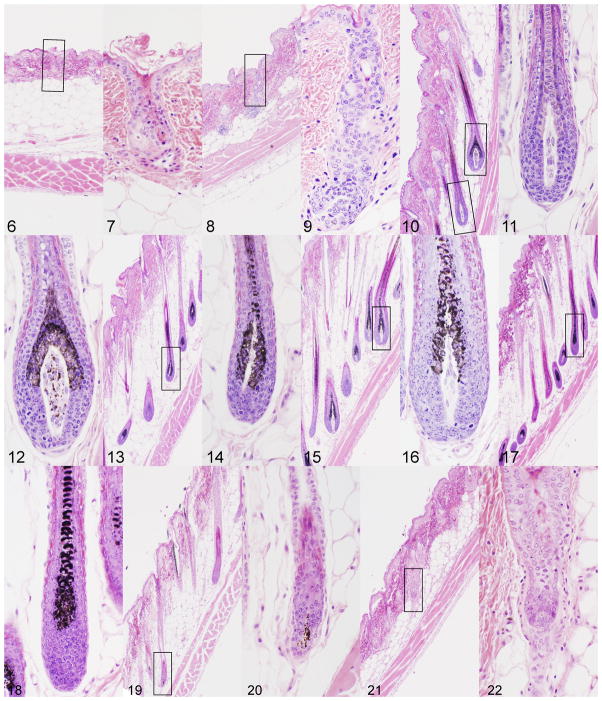
To detail changes in the hair cycle in mice, wax stripping is commonly used to induce the growth phase (anagen) of the hair cycle. While this is a mildly traumatic method that results in mild compensatory acanthosis, the cycle can be followed in a reproducible manner.19 Using this method for an adult, female C3H/HeJ mouse, one can see the progressive changes in hair follicle structure, size, and pigmentation (Fig. 6–22). The layers of the skin change in size, most notably the hypodermal fat layer with the hair cycle. This is commonly misinterpreted to be due mutations in genes that regulate lipid metabolism when it is a normal process.1–4 The interfollicular epidermis of the mouse’s body skin has little to no pigment normally, which is very different from humans. The tail skin is an exception in some strains. Pigment is produced in the actively growing (anagen stage) hair follicle in the bulb where it then is transferred into the hair fiber.5 Because of this wave-like pattern of hair growth, particularly in young mice but less so in adults where the hair cycles in domains rather than waves,7 the hair follicles can be followed down the length of the mouse to observe them change from the resting phase (telogen) through development in the active form that produces the hair fiber (anagen) and then undergoing massive apoptosis and shortening (catagen) as they re-enter telogen. Pigmentation is heaviest from mid to late anagen with little to no pigmentation in telogen. As mice age and damage occurs, pigment escapes the hair follicles to be engulfed by macrophages resulting in what is commonly called pigmentary incontenance. This will give the skin a slight change in color (Fig. 3). The degree of pigmentation can vary in hair follicles based on type of pigment present, as shown in figures 11 and 12 from the agouti C3H/HeJ mouse where some hair follicle bulbs are producing eumelanin while others produce pheomelanin.
Figure 2. Wax stripped adult C3H/HeJ mouse dorsal skin evaluated at 3 day intervals.
Figure 6. Wax stripped skin immediately after the procedure had short hair follicles in telogen.
Figure 7. These telogen follicles (enlarged boxed area in 6) were damaged due to the wax stripping with separation at the level of the companion layer.
Figure 8, 9. Three days later early unpigmented anagen follicles developed.
Figure 10–12. Late stage anagen follicles developed that were pigmented with pheomelanin (Figure 11) or eumelanin (Figure 12).
Figure 13–17. Pigmented late anagen follicles persisted.
Figure 18–22. Massive apoptosis below the level of the sebaceous gland resulted in the onset of catagen. Pigment is evident within the dermal papilla in early (Figure 19) but not late catagen (Figure 21).
These skin color changes have long been known by those of us who study skin and hair biology and these changes provide very useful indicators for staging the mouse hair cycle. There is a great deal of interest in manipulating the hair cycle with drugs to increase hair growth (such as for androgenetic alopecia)13 or to stop hair growth altogether. While performing histopathology is the most definitive way to determine the results of such manipulations,18 quantifying and mapping changes in hair color, using gray scale estimates to pixel counts with digital morphometric analyses, is an inexpensive and efficient way to quantify efficacy.20
This simple analysis illustrates the complexity of the skin as a naturally cycling organ. It is critical to understand these features, especially the dramatic changes in the hair follicles themselves, to understand why there may be no pigmentation (pink skin) to prominent coloration (light to dark grey skin). Likewise when interpreting histologic sections it is important to compare hair cycle stages with changes in the thickness of the fat layer to avoid mistakes in phenotyping new mutants.
Acknowledgments
We thank Jesse Hammer for his skills at assembling these figures.
Funding Sources. This work was supported in part by grants from the National Institutes of Health (RR17436, AR047204, AR49288, AR054407, AR056635, and CA34196 to JPS).
Footnotes
Conflicts of Interest. None declared.
References
- 1.Chase HB. Growth of the hair. Physiol Rev. 1954;34:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- 2.Chase HB. The physiology and histochemistry of hair growth. J Soc Cosmetic Chem. 1955;6:9–14. [Google Scholar]
- 3.Chase HB, Eaton GJ. The growth of hair follicles in waves. Ann NY Acad Sci. 1959;83:365–368. doi: 10.1111/j.1749-6632.1960.tb40912.x. [DOI] [PubMed] [Google Scholar]
- 4.Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116:75–82. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- 5.Chase HB, Rauch H, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiol Zool. 1951;24:1–10. doi: 10.1086/physzool.24.1.30152098. [DOI] [PubMed] [Google Scholar]
- 6.Muller-Rover S, Handjiski B, vanderVeen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 7.Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schofield PN, Gkoutos GV, Gruenberger M, Sundberg JP, Hancock JM. Phenotype ontologies for mouse and man: bridging the semantic gap. Dis Models Mech. 2010;3:281–289. doi: 10.1242/dmm.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour R, Ichiki T, Mikaelian I, Boggess D, Silva KA, Sundberg JP. Necropsy methods. In: Hedrich HJ, editor. Laboratory mouse. Academic Press; London: 2004. pp. 495–516. [Google Scholar]
- 10.Silvers WK. A model for mammalian gene action and interaction. Springer-Verlag; New York: 1979. The coat colors of mice; p. 379. [Google Scholar]
- 11.Steingrímsson E, Copeland NG, Jenkins NA. Mouse coat color mutations: from fancy mice to functional genomics. Dev Dyn. 2006;235:2401–2411. doi: 10.1002/dvdy.20840. [DOI] [PubMed] [Google Scholar]
- 12.Sundberg J, Boggess D, Bascom C, Limberg BJ, Shultz LD, Sundberg BA, King LE, Montagutelli X. Lanceolate hair-J (lahJ): a mouse model for human hair disorders. Exp Dermatol. 2000;9:206–218. doi: 10.1034/j.1600-0625.2000.009003206.x. [DOI] [PubMed] [Google Scholar]
- 13.Sundberg JP, Beamer WG, Uno H, Neste DV, King LE. Androgenetic alopecia: in vivo models. Exp Mol Pathol. 1999;67:118–129. doi: 10.1006/exmp.1999.2276. [DOI] [PubMed] [Google Scholar]
- 14.Sundberg JP, Boggess D, Sundberg BA, Eilersten K, Parimoo S, Filippi M, Stenn K. Asebia-2J (Scdab-2J): A new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundberg JP, King LE. Skin and its appendages: normal anatomy and pathology of spontaneous, transgenic and targeted mouse mutations. In: Ward JM, Mahler JF, Maronpot RR, Sundberg JP, editors. Pathology of genetically engineered mice. Iowa State University Press; Ames: 2000. pp. 181–213. [Google Scholar]
- 16.Sundberg JP, Miller J. Photography of laboratory mice. In: Sundberg JP, Boggess D, editors. Systematic approach to evaluation of mouse mutations. CRC Press; Boca Raton: 2000. pp. 91–100. [Google Scholar]
- 17.Sundberg JP, Peters EM, Paus R. Analysis of hair follicles in mutant laboratory mice. J Investig Dermatol Symp Proc. 2005;10:264–270. doi: 10.1111/j.1087-0024.2005.10126.x. [DOI] [PubMed] [Google Scholar]
- 18.Sundberg JP, Silva KA, McPhee C, King LE. Skin diseases in laboratory mice: approaches to drug target identification and efficacy screening. In: Wiles MV, Proetzel G, editors. Mouse models for drug discovery. Humana Press; Totowa, NJ: 2010. pp. 193–213. [DOI] [PubMed] [Google Scholar]
- 19.Sundberg JP, Taylor DK, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian DC, Sperling LC, Ong D, King LE, Everts H. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol. 2011;42:513–524. doi: 10.1177/0300985810379431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trachy RE, Fors TD, Pickart L, Uno H. The hair follicle-stimulating properties of peptide copper complexes. Results in C3H mice. Ann NY Acad Sci. 1991;642:468–469. doi: 10.1111/j.1749-6632.1991.tb24420.x. [DOI] [PubMed] [Google Scholar]