Abstract
Background
Mechanisms for increased cardiovascular risk in HIV-1-infected adults are incompletely understood, but platelet activation and immune activation leading to a prothrombotic state have been proposed as significant contributors. Aspirin has antiplatelet and immunomodulatory properties. We explored whether 1 week of low-dose aspirin attenuates platelet activation and immune activation in HIV-1-infected and virologically suppressed adults on antiretroviral therapy.
Methods
Platelet activation and immune activation were measured in HIV-1-infected subjects virologically suppressed on antiretroviral therapy and controls before and after 1 week of low-dose aspirin.
Results
Compared with control subjects, HIV-1-infected subjects had increased platelet activation, as measured by spontaneous platelet aggregation and aggregation in response to adenosine diphosphate, collagen, and arachidonic acid. After aspirin therapy, percent aggregation decreased similarly in both HIV-1-infected and control subjects to all platelet agonists tested except aggregation in response to arachidonic acid, which remained elevated in the HIV-1-infected group. HIV-1-infected subjects exhibited increased markers of T-cell activation (CD38 and HLA-DR) and monocyte activation (sCD14), which decreased after 1 week of aspirin therapy. Moreover, leukocyte responses to Toll-like receptor stimulation were enhanced after 1 week of aspirin therapy. In vitro studies showed that HIV-1 plasma could activate healthy platelets, which in turn activated monocytes, implicating a direct role for activated platelets in immune activation.
Conclusions
Our data demonstrate that heightened platelet activation and immune activation in treated HIV-1 disease are attenuated by 1 week of aspirin therapy. Aspirin should be further studied for its antithrombotic and immunomodulatory benefits in treated HIV-1 disease.
Keywords: platelets, HIV-1, aspirin, immune activation, aggregation
INTRODUCTION
HIV-1–infected patients have an increased risk of ischemic cardiovascular events,1–3 but the pathogenic mechanisms underlying this risk remain elusive. Although antiretroviral therapy (ART) may contribute to cardiovascular risk due to its metabolic adverse effects,2 HIV-1 itself is an independent risk factor for cardiovascular events.4–6 These increased events correlate with increased markers of immune activation and inflammation, suggesting that a heightened inflammatory state in HIV-1 contributes to thrombosis. Heightened immune activation correlates with disease progression in untreated HIV-1 infection7,8 and persists, albeit to a lesser degree, in ART-treated HIV-1-infected patients and correlates with cardiovascular disease.9
Mechanisms that link immune activation, inflammation, and increased risk of thrombotic cardiovascular disease in HIV-1 infection are incompletely understood. Recent studies have implicated platelet activation as a possible link. Circulating platelets in HIV-1-infected subjects have heightened expression of P-selectin, a molecule which binds P-selectin glycoprotein ligand 1 on endothelium and leukocytes and acts to recruit these cells to areas of injury and thrombosis.10 Circulating platelet–monocyte complexes, another marker of platelet activation, are also elevated in HIV-1-infected subjects.11 Notably, ART-treated subjects were not differentiated from viremic untreated subjects in these stud-ies.10,11 It is therefore unknown whether ART-treated and viro-logically suppressed subjects also manifest heightened platelet activation. Activated platelets have been implicated in throm-botic cardiovascular events because of their proinflammatory and thrombogenic effects,12,13 and clinical studies have demonstrated the importance of platelet activity in coronary artery atherosclerosis and thrombosis. Platelet activity, measured by spontaneous platelet aggregation (SPA), provides information about platelet function and is independently associated with long-term mortality and cardiovascular events.14 Antiplatelet therapy is a cornerstone in the prevention of atherothrombotic events and its effects likely involve the modulation of inflammatory and immune pathways.12,13,15,16 Therefore, we conducted an exploratory study to assess whether low-dose aspirin in HIV-1-infected subjects virologically suppressed on ART would result in decreased platelet activity, immune activation, and markers of inflammation.
METHODS
The study was conducted in accordance with policies of the New York University Langone Medical Center Institutional Review Board, Bellevue Hospital Center, and the central office of the New York City Health and Hospital Corporation.
Peripheral blood was drawn, with consent, from HIV-1-infected subjects (n = 25) with HIV-1 RNA viral load <50 copies per milliliter for at least 6 months on ART and from healthy controls (n = 44). Exclusion criteria for this study included age younger than 18 years of age, nonsteroidal anti-inflammatory drug use in the past week (including aspirin), renal failure (creatinine clearance < 30 mL/min or on dialysis), history of myocardial infarction, coronary artery disease, diabetes, presence of coexisting inflammatory disease, coexisting cancer, active bacterial or fungal infection, predisposition to bleeding, or any antithrombotic therapy (eg, coumadin, cilostazol). Subjects were phlebotomized and urine was collected at baseline and after 7 days of aspirin, 325 mg loading dose followed by 81 mg daily. Subjects fasted overnight and refrained from intensive exercise and tobacco use for 4 hours before an early-morning phlebotomy and urine collection to avoid circadian variation in platelet response.17 Blood was drawn using a 19-gauge needle. The first 2 mL was discarded, and the remaining blood was collected into tubes containing 3.2% sodium citrate for platelet activity and leukocyte studies.
Light Transmission Aggregometry
Light transmission aggregometry was performed according to the manufacturer specification using Helena (Beaumont, TX) AggRAM light transmission aggregometer. As previously described,18 functional assays of platelet activity in response to agonists at various concentrations as follows: adenosine diphosphate (0.4, 1, and 2 µM), collagen (0.05, 0.2, and 1 mg/mL), epinephrine (0.1, 0.4, and 2 µM), or arachidonic acid (150 and 1500 µM) and no agonist (SPA) were performed. Percent aggregation at 5 minutes and maximum percent aggregation at 10 minutes was recorded. All aggregation studies were completed within 2 hours of phlebotomy.
Urinary 11-dehydro-TXB2
Urine samples were stored at −80°C until analysis. Urine samples were thawed and assayed for 11-dehydro thromboxane B2 with a commercially available enzyme immunoassay (Corgenix, Inc. Broomfield, CO).
Immune Activation
Fresh peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll– Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ) from whole blood and were stained with antibodies from BD Pharmigen (Franklin Lakes, NJ): CD8 FITC, HLA-DR phycoerythrin (PE), CD8 PerCP, CD38 allophycocyanin (APC), matching isotype controls for PE and APC, and fixed with 4% paraformaldehyde (Electron Microscopy Sciences). About 100,000 events were acquired for each sample using BD FACSCalibur (BD Biosciences, San Jose, CA), and data were obtained using FlowJo Version 8.8.3 software (TreeStar).
Activating Agonists
Fresh PBMCs were stimulated in duplicate at 1 million cells per 200 µL media for 18 hours at 37°C, 5% CO2, with type B CPG oligodeoxyribonucleotide (CpGB) (5′-T*C*G*T*C*G* T*T*T*T*G*T*C*G*T*T*T*T*G*T*C*G*T*T*-3′, with asterisks denoting phosphorothioate bonds; 2 µg; Integrated DNA Technologies), lipopolysaccharide (LPS) at 100 ng/mL (Sigma, St. Louis, MO), or 300 ng p24CA equivalents aldrithiol-2 (AT-2) HIV-1MN. AT-2–inactivated HIV-1-1 (AT-2 HIV-1) was prepared as described previously.19
Cytokine Analysis
Plasma samples were stored at −80°C and were diluted 1:20 to 1:80 and were analyzed using cytometric bead array (BD Biosciences—Pharmingen) and multisubtype interferon (IFN) α ELISA kit (PBL Biomedical, Piscataway, NJ).
Inflammatory Markers
Plasma samples were stored at −80°C. A commercial clinical laboratory performed assays for d-dimer (Stago assay), hs C-reactive protein (CRP) [Siemens, Malvern, PA (Dade Behring) Nephelometry], and interleukin (IL) 6 (R&D). Plasma was diluted at 1:10 for soluble P-selectin (sP-selectin) and 1:200 for soluble CD14 (sCD14) and were measured using eBioscience (San Diego, CA) and R&D kits, respectively; all according to the manufacturer specification.
Plasma Platelet Activation and Monocyte Binding Assay
Washed Platelets
Whole blood from healthy donors was collected in 3.2% sodium citrate tubes and within 15 minutes was centrifuged at 200g for 10 minutes. The supernatant, containing platelet rich plasma, was collected, 100 nM prostaglandin E1 (PGE-1) was added, and platelet rich plasma was centri-fuged at 800g for 10 minutes. Supernatant was discarded and Tyrode buffer (10 mM Hepes, 150 mM NaCl, 2.5 mM KCl, 0.3 mM NaH2PO4, 12 mM NaHCO3, 2 mM EDTA, and 0.1% glucose, pH 7.4) was added, containing 100 nM PGE-1 to resuspend the platelet pellet at a concentration of 1.0 × 108 platelets/uL.
Plasma
Blood from all study participants was collected into 3.2% sodium citrate tubes. For healthy donors, within 10 minutes after phlebotomy, 10 uM epinephrine (positive control) or saline was added, incubated for 20 minutes, and then blood was centrifuged for 2500g for 10 minutes. Supernatant plasma from healthy donors treated with or without epinephrine or supernatant plasma from HIV-1+ donors was collected and stored at −20°C.
Plasma Platelet Activation
Fifty microliters of plasma was cocultured with 5 uL of fresh platelets (1.0 × 108 platelets/uL) and incubated for 30 minutes at 37°C. Platelets were washed to remove plasma at 800g for 10 minutes. Platelets were labeled with anti CD61-FITC (Dako) and anti PAC-1 PE antibody or anti P-selectin PE antibody. Samples were analyzed by flow cytometry.
Monocyte Binding Assay
Washed platelets (1.0 × 108/uL) which had been activated with various plasma conditions were cocultured with THP-1 cell line monocytes (1.0 × 106/uL) for 30 minutes at 37°C. Cells were fixed and stained for anti CD61-FITC and anti CD14-PE and analyzed by flow cytometry.
Statistical Analysis
Two-tailed paired Student t tests or Wilcoxon Signed-Rank test were used for paired analyses comparing subjects before and after aspirin. Unpaired 2-tailed Student t tests or Mann–Whitney U tests were used for unpaired analyses. Spearman correlation and χ2 tests were used for analyses of associations.
RESULTS
Subjects demographics
Median age of HIV-infected subjects was 50 (range: 31–71). Nearly 50% of the population was white and 76% were men. About 56% were current smokers. Mean CD4+ T-cell count was 630 (range: 181–1124), mean CD4+ T-cell count nadir was 166 (range: 5–422). Mean years of HIV-1 diagnosis was 9.5 (range: 1–26) and mean years on effective ART therapy with a suppressed HIV-1 RNA viral load was 6 (range: 0.5–21). All subjects with HIV-1 had platelet counts within the normal range [195 × 103/mL (interquartile range: 164–241)]. Current ART regimens are listed in Table 1.
TABLE 1.
Subject Demographics
| HIV-1 Infected (n = 25) |
Uninfected Control (n = 44) |
|
|---|---|---|
| Male sex, n (%) | 19 (76) | 21 (48) |
| Median age, n (range) | 50 (31–71) | 27 (21–60) |
| BMI, n (range) | 23.5 (18.9–43.5) | 23.6 (18–32.9) |
| Race, n (%) | ||
| White | 11 (44) | 25, (57) |
| Black | 13 (52) | 1 (2) |
| Asian | 0 (0) | 15 (34) |
| Other | 1 (4) | 3 (7) |
| Ethnicity, n (%) | ||
| Hispanic | 11 (44) | 3 (7) |
| Non-Hispanic | 14 (56) | 41 (93) |
| Smoking status | ||
| Current smoker | 14 (56) | 2 (5.7) |
| Mean years since HIV-1 diagnosis, n (range) |
9.5 (1–26) | N/A |
| Mean years of effective ART, n (range) |
6 (0.5–21) | N/A |
| Mean CD4 count, n (range) | 630 (181–1124) | N/A |
| Mean CD4 nadir, n (range) | 166 (5–422) | N/A |
| Current antiretroviral usage | 9 NNRTI based | N/A |
| 13 PI based | — | |
| 4 Raltegravir based |
||
| 5 Abacavir containing |
ART-Treated HIV-1-Infected Subjects Have Hyperreactive platelets
Prior data20 demonstrate that the use of submaximal concentrations of agonists identify subjects with a hyperreactive platelet phenotype. HIV-1-infected subjects evidenced significantly higher median SPA [7.9% (4.6–11.8) vs. 4.9% (2.4–8.4), P = 0.045] and higher median aggregation in response to all submaximal agonist concentrations [adenosine diphosphate 0.4 uM, 11.3% (6.6–44.4) vs. 6.4% (2.8–9.7), P = 0.003; collagen 0.05 ug/mL, 5.6% (3.9–12.4) vs. 3.2% (1.8–6.2), P = 0.001; and AA 150 uM, 54.9% (8.2–89.9) vs. 7.4% (3.0–31.0), P = 0.003] except epinephrine 0.1 uM 81.2% (17.0–91.3) vs. 78.8% (13.0–90.7), P = 0.672, than uninfected controls. At higher agonist concentrations, much of the differences observed between HIV-1-infected subjects and healthy controls were attenuated (Table 2).
TABLE 2.
ART-Treated HIV-Infected Subjects Have Hyperreactive Platelets
| Baseline % Platelet Aggregation |
1 Week Aspirin % Platelet Aggregation |
||||||
|---|---|---|---|---|---|---|---|
| HIV-1 | Control | P | HIV-1 | Control | P | ||
| ADP | 0.4 uM 300s | 4.0 (1.0–12.0) | 0.0 (0.0–1.5) | <0.0001 | 3.0 (0.0–9.0) | 0.0 (0.0–2.0) | 0.0171 |
| 0.4 uM MAX | 11.3 (6.6–44.4) | 6.4 (2.6–10.1) | 0.0027 | 9.4 (3.1–13.9) | 6.3 (2.9–10.0) | 0.1753 | |
| 1 uM 300s | 35.0 (10.0–87.0) | 27.5 (3.2–88.3) | 0.5629 | 13.0 (6.0–20.1) | 9.0 (4.8–13.4) | 0.0905 | |
| 1 uM MAX | 59.3 (25.8–88.3) | 43.0 (25.6–91.5) | 0.9481 | 28.4 (17.8–41.1) | 34.7 (25.7–40.1) | 0.1766 | |
| 2 uM 300s | 81.0 (42.0–92.0) | 88.0 (73.0–93.0) | 0.2803 | 36.0 (13.0–51.0) | 32.5 (21.0–47.0) | 0.8726 | |
| 2 uM MAX | 84.3 (52.5–93.5) | 89.3 (77.2–94.5) | 0.3042 | 52.5 (41.4–65.7) | 59.2 (50.9–63.7) | 0.1533 | |
| COL | 0.05 ug/mL 300s | 2.0 (0.0–7.5) | 0.0 (0.0–0.5) | 0.0003 | 3.0 (0.3–6.0) | 1.0 (0.0–4.3) | 0.0765 |
| 0.05 ug/mL MAX | 5.6 (3.9–12.4) | 3.2 (1.8–6.2) | 0.0011 | 8.4 (3.5–11.3) | 4.7 (3.5–6.5) | 0.0790 | |
| 0.2 ug/mL 300s | 8.5 (0.0–83.5) | 6.0 (0–84.0) | 0.9770 | 5.0 (2.0–9.9) | 3.0 (0.5–5.0) | 0.0631 | |
| 0.2 ug/mL MAX | 12.6 (4.3–88.2) | 11.3 (4.0–88.6) | 0.8485 | 8.1 (4.8–13.9) | 6.7 (4.0v8.9) | 0.1245 | |
| 1 ug/mL 300s | 84.0 (80.0–88.0) | 87.0 (83.8–89.0) | 0.1247 | 20.5 (12.50–31.5) | 16.0 (7.0–24.5) | 0.1272 | |
| 1 ug/ml MAX | 87.4 (81.4–90.7) | 90.0 (86.8–91.5) | 0.1009 | 26.1 (16.0–33.3) | 18.9 (10.9–28) | 0.1087 | |
| EPI | 0.1 uM 300s | 22.0 (9.0–80.0) | 16.0 (7.6–46.8) | 0.1508 | 9.5 (6.0–17.0) | 11.0 (7.8–13.6) | 0.7999 |
| 0.1 uM MAX | 81.2 (17.0–91.3) | 78.8 (13.0–90.7) | 0.6715 | 17.1 (11.4–25.8) | 16.9 (13.2–21.8) | 0.8060 | |
| 0.4 uM 300s | 79.5 (36.8–89.0) | 81.0 (68.5–85.5) | 0.7179 | 19.5 (13.3–30.3) | 22 (13.5–26.5) | 0.7446 | |
| 0.4 uM MAX | 89.2 (81.4–95.2) | 90.6 (87.3–93.4) | 0.6971 | 27.9 (19.6–42.7) | 28.9 (20.8–37.6) | 0.9436 | |
| 2 uM 300s | 83.0 (76.3–91.5) | 88.8 (84.0–90.5) | 0.1357 | 32.5 (23.0–53.0) | 36.5 (23.8–44.0) | 0.8058 | |
| 2 uM MAX | 86.6 (78.3–93.4) | 91.7 (88.1–93.8) | 0.0803 | 44.9 (30.4–65.7) | 44.1 (32.3–54.4) | 0.9539 | |
| AA | 150 uM 300s | 5.0 (1.5–13.8) | 0.0 (0.0–4.0) | 0.0033 | 7.0 (4.0–9.0) | 4.0 (1.0–7.0) | 0.0214 |
| 150 uM MAX | 54.9 (8.2–89.9) | 7.4 (2.7–34.6) | 0.0028 | 9.8 (5.8–16.6) | 6.1 (4.4–9.2) | 0.0036 | |
| 1500 uM 300s | 88.5 (83.5–90.0) | 88.0 (85.0–90.0) | 0.8790 | 18.0 (10.5–23.0) | 9.5 (4.8–15.0) | 0.0044 | |
| 1500 uM MAX | 90.4 (86.3–93.0) | 91.5 (89.0–92.9) | 0.4877 | 20.8 (12.6–49.2) | 12.0 (7.2–23.9) | 0.0062 | |
| SPA | 300s | 3.0 (1.0–8.0) | 2.5 (0.4–4.0) | 0.1390 | 2.0 (1.0–6.0) | 3.0 (2.0–5.3) | 0.4681 |
| MAX | 7.9 (4.6–11.8) | 4.9 (2.3–8.5) | 0.0445 | 7.3 (4.8–13.4) | 6.2 (4.2–8.5) | 0.2223 | |
ADP, adenosine diphosphate; COL, collagen; EPI, epinephrine.
Effects of Aspirin on Platelet Activity in ART-Treated HIV-1-Infected Subjects
Platelet aggregation was measured 24 hours after a loading dose of aspirin 325mg in HIV-1-infected subjects and then again after 1 week of aspirin 81 mg daily in both the HIV-1-infected subjects and the healthy controls. As compared with baseline, platelet aggregation was significantly inhibited after a single dose of 325 mg in HIV-1-infected subjects and after 1 week of aspirin in both groups (controls were not available for single-dose measurements) (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A399). As compared with the control group, HIV-1-infected subject platelet aggregation was inhibited similarly after 1 week of aspirin to all platelet agonists tested except aggregation in response to low-dose and high-dose AA, which remained elevated in the HIV-1-infected group [150 uM, 9.7% (5.5– 15.2) vs. 6.1% (4.5–9.1), P = 0.007; and AA 1500 uM, 22.2% (16.4–46.1) vs. 12% (7.6–21.7), P = 0.002] (Table 2).
Urinary Thromboxane is Higher in HIV-1-Infected Subjects and is Less Responsive to Aspirin Therapy
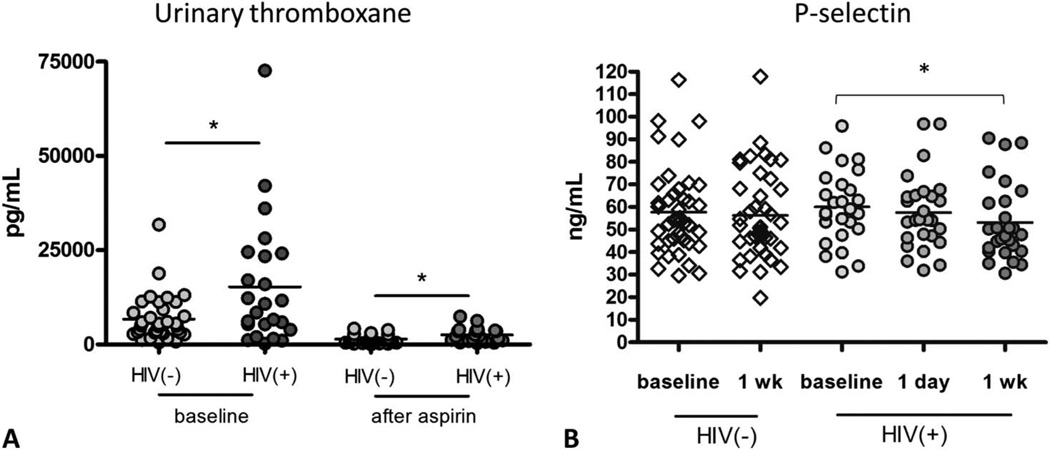
Aspirin irreversibly acetylates platelet cyclooxygenase-1 (COX-1), inhibiting the formation of the potent platelet agonist thromboxane. To further assess platelet activity and the adequacy of COX-1 inactivation by aspirin ex vivo, we measured urinary excretion of the major enzymatic metabolite of thromboxane, 11-dehydro-thromboxane (TX) B2. The basal level of median urinary concentration of 11-dehydro thromboxane B2 was significantly higher in HIV-1-infected subjects compared with controls (9626.8 vs. 7295.2 pg/mL, P = 0.02). After 1 week of aspirin, 11-dehydro thromboxane B2 decreased significantly in both groups but remained significantly higher in the HIV-1-infected group (2255.7 vs. 1422.6 pg/mL, P = 0.04) (Fig. 1A).
FIGURE 1.
Urinary thromboxane is higher in HIV-infected subjects and is less responsive to aspirin therapy. A, Urinary 11-dehydro-TXB2 levels were higher in HIV(+) subjects as compared to HIV(−) control subjects at baseline, (*P = 0.02) and after 1 week of aspirin (*P = 0.04), Mann– Whitney test. B, Plasma P-selectin levels decreased in ART-treated HIV-infected subjects after 1 week of aspirin, paired t test, *P < 0.05. P-selectin levels do not decrease in uninfected control subjects after 1 week of aspirin.
ART-Treated HIV-1-Infected Subjects Have Increased Soluble P-Selectin, Which is Decreased After a Week of Aspirin Therapy
Soluble P-selectin (sP-selectin) is a biomarker for platelet/endothelial activation and is considered a risk factor for vascular disease.21 sP-selectin enhances procoagulant activity by inducing leukocyte-derived microparticle production and promotes activation of leukocyte integrins. There was a trend for higher median sP-selectin in HIV-1-infected as compared with HIV-1-uninfected control subjects at baseline (58.2 ng/mL vs. 54.1 ng/mL) and a significant decrease in median sP-selectin in HIV-1-infected patients after 1 week of aspirin (58.2 ng/mL vs. 47.7 ng/mL) but not after 1 day (58.2 ng/mL vs. 54.3 ng/mL) (Fig. 1B).
T-Cell and Monocyte Immune Activation is Higher in ART-Treated HIV-1-Infected Subjects Than Uninfected Control Subjects and is Decreased After one Week of Aspirin Therapy
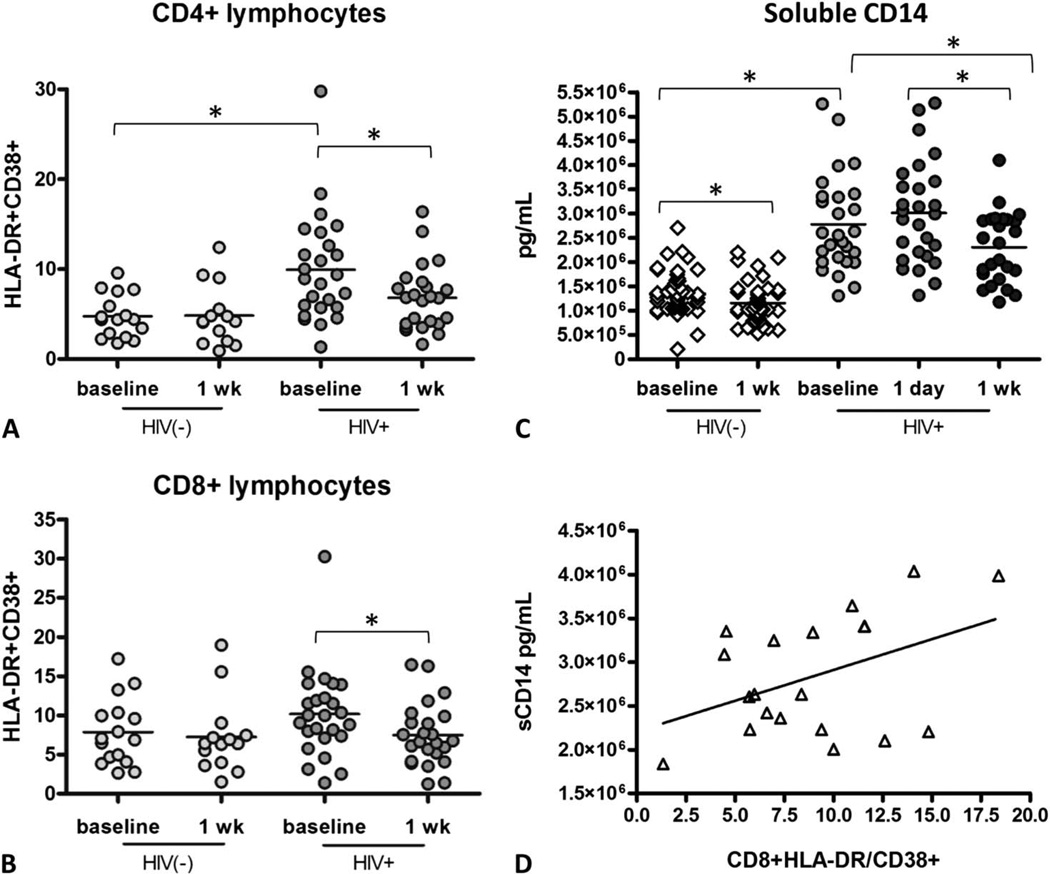
Previous studies have shown that immune activation in ART-treated HIV-1-infected subjects is higher than in uninfected control subjects but lower than in untreated HIV-1-infected subjects.22,23 Because aspirin has been shown to have immunomodulatory properties,24,25 we tested whether aspirin may affect T-cell or monocyte activation markers. T-cell activation was evaluated by measuring activation markers CD38 and HLA-DR on CD4+ and CD8+ lymphocytes. In accordance with prior studies, we found that immune activation is higher in HIV-1-infected subjects on ART than HIV-1-uninfected control subjects. HIV-1-infected subjects had significantly higher %CD4+HLADR+CD38+ lymphocytes than HIV-1-uninfected control subjects (median 8.95% vs. 4.4%, P = 0.001) and there was a trend for higher %CD8 +HLADR+CD38+ lymphocytes in HIV-1-infected than uninfected control subjects (median 9.98% vs. 6.95%, P = 0.13). In response to 1 week of aspirin, there was a significant decrease in HIV-1-infected subjects’ CD4+HLADR+CD38+ lymphocytes (median 8.95% vs. 6.81%, P = 0.005) and CD8 +HLADR+CD38+ lymphocytes (median 9.98% vs. 6.70%, P = 0.015) but not in uninfected control subjects’ CD4 +HLADR+CD38+ lymphocytes (median 4.40% vs. 4.19%) and CD8+HLADR+CD38+ lymphocytes (median 6.95% vs. 6.42%) (Figs. 2A, B).
FIGURE 2.
T-cell activation is increased in ART-treated HIV-infected subjects and decreases after a week of aspirin therapy. Fresh PBMCs were isolated from blood from HIV(+) subjects and HIV(−) control subjects and were stained for markers of immune activation, fixed, and analyzed by flow cytometry. A, HLADR+CD38+CD4+ and B, HLADR+CD38+CD8+ lymphocytes decrease significantly after 1 week of aspirin therapy, paired t test, *P < 0.05. ART-treated HIV-infected subjects have elevated sCD14, which is decreased after 1 week of aspirin. C, Both HIV(+) and HIV(−) subjects experienced a decrease in sCD14 after 1 week of aspirin therapy, paired t test, *P < 0.05. D, sCD14 correlated with CD8+ lymphocyte immune activation, r = 0.43; P = 0.06.
Monocyte activation was tested by analyzing soluble CD14 (sCD14) levels in plasma. sCD14 levels were higher in treated HIV-1-infected subjects as compared with HIV-1-uninfected control subjects at baseline (median 2.60 × 106 vs. 1.26 × 106 pg/mL; P < 0.001), and sCD14 decreased significantly in treated HIV-1-infected subjects after a week of aspirin (median 2.60 × 106 vs. 2.49 × 106 pg/mL; P < 0.001) and in HIV-1-uninfected control subjects (median 1.26 × 106 vs. 1.04 × 106; P < 0.009) (Fig. 2C). T-cell activation correlated moderately with monocyte activation (r = 0.43; P = 0.06) (Fig. 2D). These data demonstrate that HIV-1-infected subjects have increased levels of T cell and monocyte activation, which subsequently decreases after a week of low-dose aspirin therapy. In this small study, there were no significant associations between aspirin-mediated improvement in T-cell activation (CD4+HLADR+CD38+ or CD8+HLADR +CD38+) or monocyte activation and the following subject characteristics: age, gender, CD4 count, CD4 nadir, smoking status, years since HIV-1 diagnosis, years on effective ART, or ART drug class (data not shown).
One Week of Aspirin Enhances Responsiveness of Leukocytes to TLR Agonist Stimulation
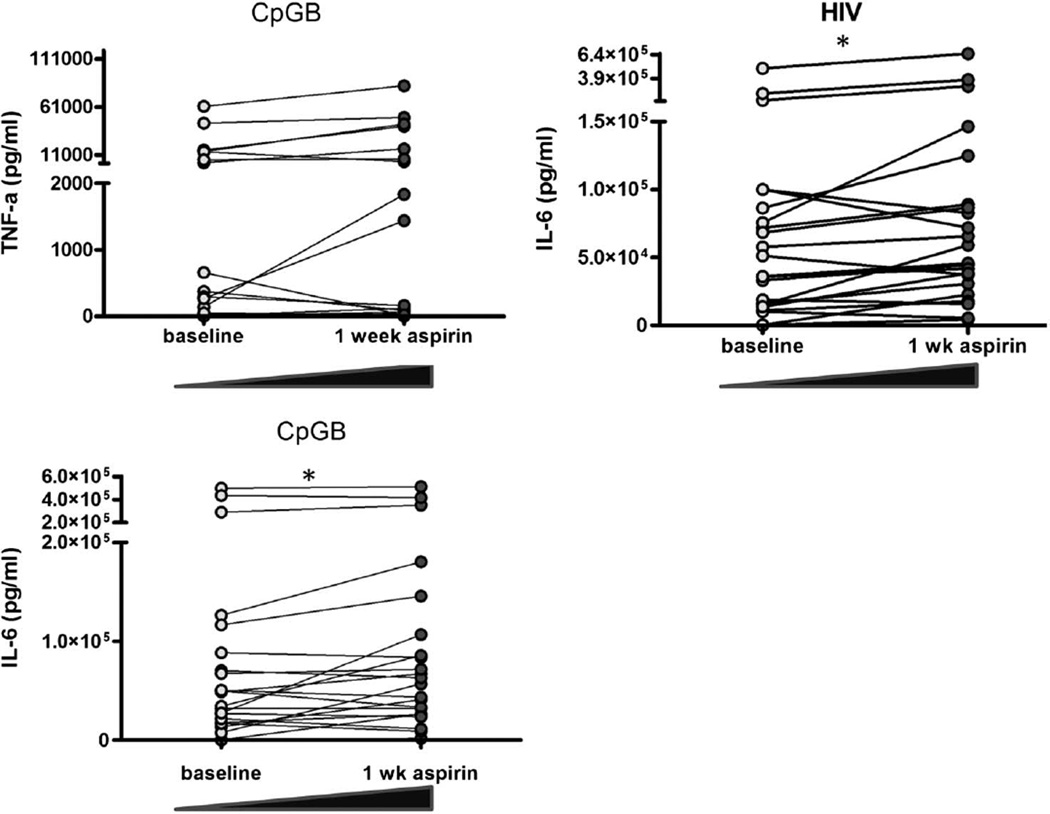
Innate immune cells are less responsive to Toll-like receptor (TLR) stimulation during chronic HIV-1 infection,26,27 possibly as a result of chronic stimulation by inflammatory cytokines.26,28 Because aspirin is anti-inflammatory, we tested whether 1 week of aspirin therapy would improve leukocyte TLR agonist responsiveness in HIV-1 subjects. PBMCs from HIV-1-infected subjects on ART or HIV-1-uninfected control PBMCs were stimulated with relevant TLR 7, 9, and 4 agonists HIV-1, CpGB, or LPS, respectively, for 18 hours and compared with unstimulated PBMCs. After 1 week of aspirin therapy, no differences were observed in mean spontaneous production of cytokines (IL-6, TNFα, IL-1β, and IFNα) by PBMCs from HIV-1-infected subjects. However, in response to CpGB or HIV-1 stimulation, after 1 week of aspirin, PBMCs from HIV-1-infected subjects produced higher levels of IL-6 (mean 108.5 vs. 92.8 ng/mL, P = 0.01 and 107.6 vs. 76.9 ng/mL, P = 0.01, respectively) and a trend toward enhanced production of TNFα in response to CpGB or LPS (mean 11.3 vs. 7.3 ng/mL, P = 0.06 and 38.1 vs. 24.1, P = 0.07, respectively) (Fig. 3). There were no improvements in TLR responsiveness after 1 week of aspirin of PBMCs from HIV-1-infected subjects to produce IL-1β or IFNα. In uninfected controls, there was no significant change either in the spontaneous production of cytokines after overnight incubation or in response to TLR stimulation after 1 week of aspirin in the HIV-1-uninfected control PBMCs. For example, in response to CpGB or HIV-1 stimulation, control PBMCs produced similar levels of IL-6 before and after aspirin (mean: 73.8 vs. 76.7 ng/mL, P = 0.73 and 97.7 vs. 107.3 ng/mL, P = 0.57, respectively), and similar levels of TNFα in response to CpGB or LPS before and after aspirin (mean 8.7 vs. 10.6 ng/mL, P = 0.36 and 13.6 vs. 13.3, P = 0.85, respectively). Our data indicate that 1 week of low-dose aspirin 81 mg daily partly ameliorates the defect in TLR responsiveness in PBMCs from HIV-1-infected subjects.
FIGURE 3.
One week of aspirin therapy improves responsiveness of leukocytes to certain TLR agonists. Fresh PBMCs were incubated overnight with TLR agonists CpG and HIV, and culture supernatants were stored at −20°C. Cytokine Bead Array analyses were performed on culture supernatants by flow cytometry. After 1 week of aspirin, more IL-6 was produced in response to CpGB, P = 0.01, more IL-6 was produced in response to HIV-1, P = 0.01, and more TNFα was produced in response to CpGB, P = 0.06 (all using paired t tests).
One Week of Aspirin Does Not Change Soluble Inflammation Markers
Markers of inflammation IL-6, d-dimer, and CRP are elevated in HIV-1 disease, correlate with morbidity and mortality, and are somewhat reduced with ART but remain elevated when compared with levels in HIV-1-uninfected persons.4,29–31 We investigated whether inflammatory markers decrease after a week of low-dose aspirin therapy in treated HIV-1-infected patients. Median levels of CRP, d-dimer, and IL-6 decreased nonsignificantly after 1 week of aspirin therapy [2.7 mg/L (0.8–3.9) vs. 2.2 mg/L (0.8–3.2)], [0.23 µg/mL (0–0.36) vs. 0.13 µg/mL (0–0.38)], and 2.03 pg/mL (1.18– 2.31) vs. 1.98 pg/mL (1.15–3.68)].
Plasma From HIV-1-Infected Subjects Activates Platelets and HIV-1 Plasma– Activated Platelets Activate Monocytes
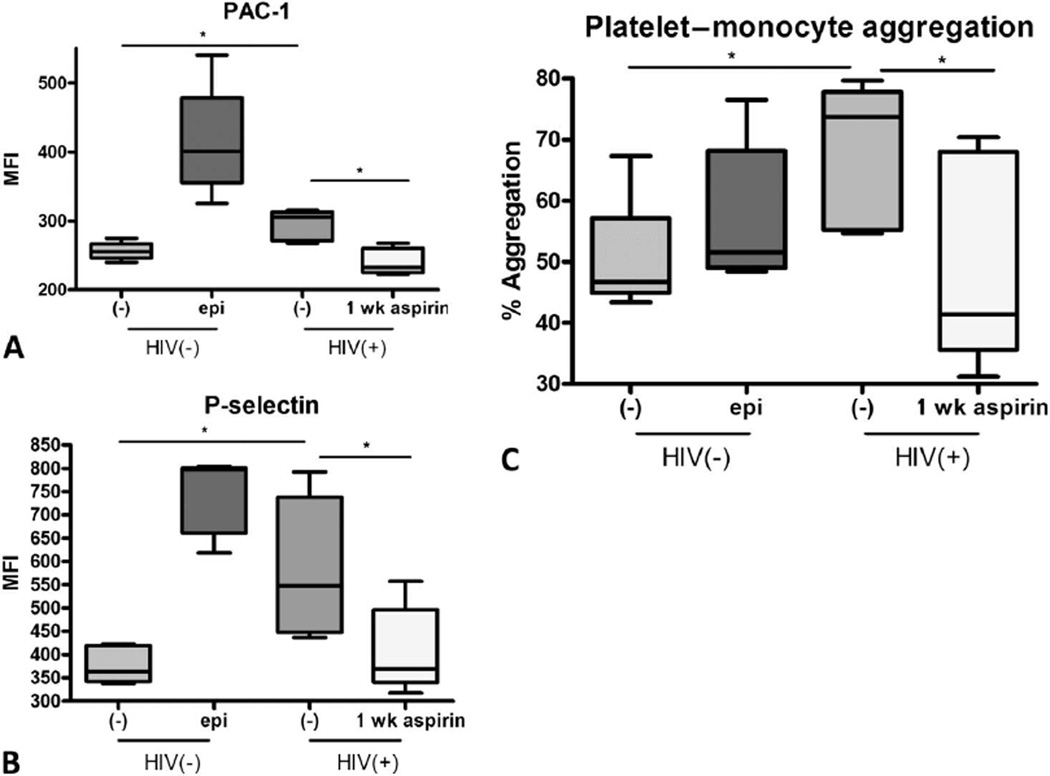
To investigate the mechanism of platelet activation in HIV-1-infected subjects, we exposed platelets from healthy controls to plasma from our HIV-1-infected cohort. Contrary to results with healthy control plasma, plasma from our HIV-1-infected control cohort induced platelet activation, as assessed by increased surface expression of PAC-1 and P-selectin. Platelet activation after incubation with HIV-1-infected plasma was inhibited if the HIV-1 plasma donor had received 1 week of daily aspirin. We next incubated the plasma-activated platelets with THP-1 cell line monocytes. We hypothesized that activated platelets contribute to monocyte activation in HIV-1 disease. Platelets which were cultured with plasma from HIV-1-infected subjects were able to activate THP-1 cell line monocytes, as evidenced by the formation of platelet monocyte aggregates. HIV-1 plasma–activated platelets caused formation of platelet monocyte aggregates significantly more than healthy control-activated platelets. The formation of platelet monocyte aggregates in this assay was significantly abrogated by aspirin therapy, indicating that soluble plasma factors may play a role in platelet hyper reactivity and innate immune activation in HIV-1 infection, which may be reversed by aspirin therapy (Fig. 4).
FIGURE 4.
Plasma from HIV-infected subjects activates platelets and HIV-1 plasma–activated platelets activate monocytes. Normal platelets from 5 donors were incubated with control plasma at 37°C for 30 minutes 6 epinephrine or plasma from 5 donor HIV study subjects before and after 1-week aspirin therapy. Normal platelets mixed with plasma from HIV subjects expressed higher platelet activation markers. A, PAC-1; and B, P-selectin at baseline, (*P < 0.05, Mann–Whitney test) but this was abrogated when HIV-1 plasma was used from subjects receiving 1 week of aspirin therapy (paired student t test, *P < 0.05). C, These activated platelets caused THP-1 cell line mon-ocytes to activate more, as measured by CD14+CD61+ double-staining aggregates, Mann–Whitney test, *P ≤ 0.05, but this effect was also abrogated after 1-week aspirin therapy (paired student t test, *P < 0.05). epi, epinephrine.
Altogether, we present new data that platelets from treated and virologically suppressed HIV-1-infected subjects are hyperreactive spontaneously and in response to platelet-activating agonists. We show that 1 week of daily aspirin 81 mg is effective in decreasing platelet activity in HIV-1-infected and HIV-1-uninfected controls. We also present the intriguing findings that aspirin may reverse immune activation in treated HIV-1 infection and enhance leukocyte responses to TLR agonists. We show that platelets from healthy donors are activated by HIV-1 plasma. In turn, HIV-1 plasma–activated platelets contribute to monocyte activation, suggesting that soluble plasma factors in HIV-1-infected subjects play a role in platelet activation and secondarily in leukocyte activation. Together, these data support the conclusion that platelet activity is increased in virologically suppressed HIV-1-infected subjects. Moreover, increased platelet activation may contribute to immune activation, as both platelet and immune activation are ameliorated by aspirin therapy.
DISCUSSION
HIV-1-infected patients have a heightened risk of cardiovascular disease, including myocardial infarction and sudden cardiac death.4,32,33 Although traditional cardiovascular risk factors and antiretroviral medications may contribute to this risk, emerging data suggest a role for immune activation and dysfunction. Multiple studies have shown that low CD4+ T-cell counts are associated with cardiovascular events,6,34 and increased immune activation has been associated with increased carotid artery lesions.35 The link between immune activation and cardiovascular risk in HIV-1 disease remains incompletely understood, but platelet activation may play an important role.
In this study, we found that platelets from HIV-1-infected subjects are significantly more reactive spontaneously and to low-dose platelet-activating agonists than are platelets from HIV-1-uninfected control subjects. At the highest doses of platelet-activating agonists, however, platelets from both HIV-1-infected and HIV-1-uninfected control subjects aggregated maximally and no differences were observed. These data suggest that platelets from HIV-1-infected subjects have a lower threshold to activation, but that this hyperreactivity is dose dependent. When activated platelets are maximally stimulated with platelet agonists ex vivo, they demonstrate a blunted response, presumably related to the platelets having undergone intragranular release in vivo.36 This phenomenon has been termed “exhausted platelets.”37 In addition, as evidenced by ineffective platelet inhibition to AA and elevated urinary thomboxane metabolites after aspirin, COX-1–mediated platelet reactivity remains higher in patients with HIV-1 than HIV-1-uninfected controls, suggesting that longer duration and/or higher dose of aspirin therapy may be needed to completely suppress COX-1–mediated platelet reactivity in ART-treated HIV-1-infected individuals.
One week of low-dose aspirin also decreased markers of T-cell activation and monocyte activation and augmented leukocyte responsiveness to certain TLR agonist stimulants. Healthy platelets incubated with plasma from HIV-1-infected subjects became activated; however, plasma from HIV-1-infected subjects that had been taking aspirin for 1 week attenuated this platelet activation. Furthermore, HIV-1 plasma–activated platelets potently activated monocytes, implicating a direct role for activated platelets in immune activation. Importantly, platelets incubated with HIV-1 plasma from subjects who had taken aspirin for 1 week had a significant reduction in monocyte activation. Therefore, activated platelets may contribute to monocye activaton in HIV-1 infection. The close interaction between platelets and monocytes may help explain the relationship between platelet activity and inflammation, thereby providing a better understanding of the increased cardiovascular risk in HIV-1-infected subjects.
As an antiplatelet and immunomodulating agent, aspirin may diminish immune activation in HIV-1 disease (1) indirectly, through inhibition of platelet activation and (2) directly, through blocking inflammatory pathways in multiple cell types. Platelets express toll-like receptor (TLR) 2, 4, and 938 and can become activated in response to proinflammatory cytokines or infective bacterial agents,39,40 both of which are elevated in HIV-1 infection. Platelets are anucleate; however, they contain a substantial amount of mRNA and all of the translational machinery necessary to rapidly generate their own proteins during hemostatic and inflammatory events. Activated platelets express CD154 (CD40L) and P-selectin which can in turn activate multiple cell types, including endothelial cells, neutrophils, monocytes, and dendritic cells, to produce inflammatory cytokines and chemokines.41,42 The enhanced leukocyte responses we observed after aspirin use are consistent with prior reports. Aspirin has been shown to directly enhance TNFα release from LPS-activated RAW264.7 (macrophage) cells.43 As observed in this study, aspirin therapy may reverse this inhibition of cytokine production by inhibiting platelet activation.
This small pilot study provides evidence that activated platelets may contribute to immune activation and inflammation in HIV-1 infection. Importantly, this study also suggests that low-dose aspirin may be a potential intervention for HIV-1-infected subjects on ART to blunt platelet and immune activation and inflammation. As this study was a small nonrandomized open-label pilot study, future studies are needed to confirm and expand upon our findings.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the tireless efforts of the late Luis Vargas for recruitment of study participants. The authors would like to thank the NYU/Bellevue ACTU study nurses Janet Forcht RN, Caroline Sturm-Reganato RN, and Richard Hutt RN for clinical trial services; and student volunteers Stuart Sacks, Lindsay Elbaum, and Christopher Lopez for data collection and study sample transport.
Supported by the Center for AIDS Research pilot project grant P30 AI027742, in part by the National Center for the Advancement of Translational Science (NCATS) Grant No. UL1 TR000038 (M.O., J.S.B.), the the National Institutes of Health National Institute of Allergy and Infectious Diseases Grant No. 5 U01 AI069532 (J.A.A., K.C.), the Grunebaum AIDS research scholarship (M.O.), and by an American Heart Association Fellow to Faculty Award (0775074N) and a Doris Duke Clinical Scientist Development Award (2010055) (J.S.B.).
Footnotes
The authors have no conflicts of interest to disclose.
M.O. designed the study and performed experiments, analyzed and interpreted data, and drafted and revised the article. G.G., L.H., M.M., E.M., V.V. and M.A.N. collected data and performed selected experiments. K.C. contributed to experimental design and collected data. J.A.A. and N.B. contributed to experimental design and revised the article. J.S.B. designed the study, analyzed and interpreted data, and drafted and revised the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
Presented, in part, at the XIX International AIDS Conference, July 2012, Washington, DC.
REFERENCES
- 1.Triant VA. HIV-1 infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205(suppl 3):S355–S361. doi: 10.1093/infdis/jis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-1-infected patients: the data collection on adverse effects of anti-HIV-1 drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 3.Aberg JA. Cardiovascular complications in HIV-1 management: past, present, and future. J Acquir Immune Defic Syndr. 2009;50:54–64. doi: 10.1097/QAI.0b013e31818ceaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Sadr WM, Lundgren JD, Neaton JD et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 5.Lang S, Mary-Krause M, Simon A, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55:600–607. doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV-1 outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 8.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 9.Neuhaus J, Angus B, Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV-1. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayne E, Funderburg NT, Sieg SF, et al. Increased platelet and microparticle activation in HIV-1 infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh MV, Davidson DC, Kiebala M, et al. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J Virol Methods. 2012;181:170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lievens D, Zernecke A, Seijkens T, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arte-rioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 14.Trip MD, Cats VM, van Capelle FJ, et al. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 15.Duffau P, Seneschal J, Nicco C, et al. Platelet CD154 potentiates inter-feron-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001001. 47ra63. [DOI] [PubMed] [Google Scholar]
- 16.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- 17.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 18.Merolla M, Nardi MA, Berger JS. Centrifugation speed affects light transmission aggregometry. Int J Lab Hematol. 2012;34:81–85. doi: 10.1111/j.1751-553X.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossio JL, Esser MT, Suryanarayana K, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger JS, Becker RC, Kuhn C, et al. Hyperreactive platelet phenotypes: Relationship to altered serotonin transporter number, transport kinetics and intrinsic response to adrenergic co stimulation. Thromb Haemost. 2013;109:85–92. doi: 10.1160/TH12-03-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–495. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 22.Ronsholt FF, Ullum H, Katzenstein TL, et al. T cell subset distribution in HIV-1-1 infected patients after 12 years of treatment induced viraemic suppression. J Acquir Immune Defic Syndr. 2012;61:270–278. doi: 10.1097/QAI.0b013e31825e7ac1. [DOI] [PubMed] [Google Scholar]
- 23.Rueda CM, Velilla PA, Chougnet CA, et al. HIV-1-induced T-cell activation/exhaustion in rectal mucosa is controlled only partially by antire-troviral treatment. PLoS One. 2012;7:e30307. doi: 10.1371/journal.pone.0030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroecksnadel K, Winkler C, Wirleitner B, et al. Aspirin down-regulates tryptophan degradation in stimulated human peripheral blood mono-nuclear cells in vitro. Clin Exp Immunol. 2005;140:41–45. doi: 10.1111/j.1365-2249.2005.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 26.Kamga I, Kahi S, Develioglu L, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 27.Donaghy H, Gazzard B, Gotch F, et al. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 28.Finke JS, Shodell M, Shah K, et al. Dendritic cell numbers in the blood of HIV-1-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 29.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV-1. Curr Opin HIV-1 AIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eastburn A, Scherzer R, Zolopa AR, et al. Association of low level viremia with inflammation and mortality in HIV-1-infected adults. PLoS One. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triant VA, Regan S, Lee H, et al. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Lelyveld SF, Gras L, Kesselring A et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS. 2012;26:465–474. doi: 10.1097/QAD.0b013e32834f32f8. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-1-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBane RD, II, Karnicki K, Tahirkheli N, et al. Platelet characteristics associated with coronary artery disease. J Thromb Haemost. 2003;1:1296–1303. doi: 10.1046/j.1538-7836.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien JR. "Exhausted" platelets continue to circulate. Lancet. 1978;2:1316–1317. doi: 10.1016/s0140-6736(78)92087-1. [DOI] [PubMed] [Google Scholar]
- 38.Shiraki R, Inoue N, Kawasaki S, et al. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Jayachandran M, Brunn GJ, Karnicki K, et al. In vivo effects of lipo-polysaccharide and TLR4 on platelet production and activity: implications for thrombotic risk. J Appl Physiol. 2007;102:429–433. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 40.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 41.Elzey BD, Schmidt NW, Crist SA, et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111:3684–3691. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weyrich AS, McIntyre TM, McEver RP, et al. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho JY. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch Pharm Res. 2007;30:64–74. doi: 10.1007/BF02977780. [DOI] [PubMed] [Google Scholar]