Abstract
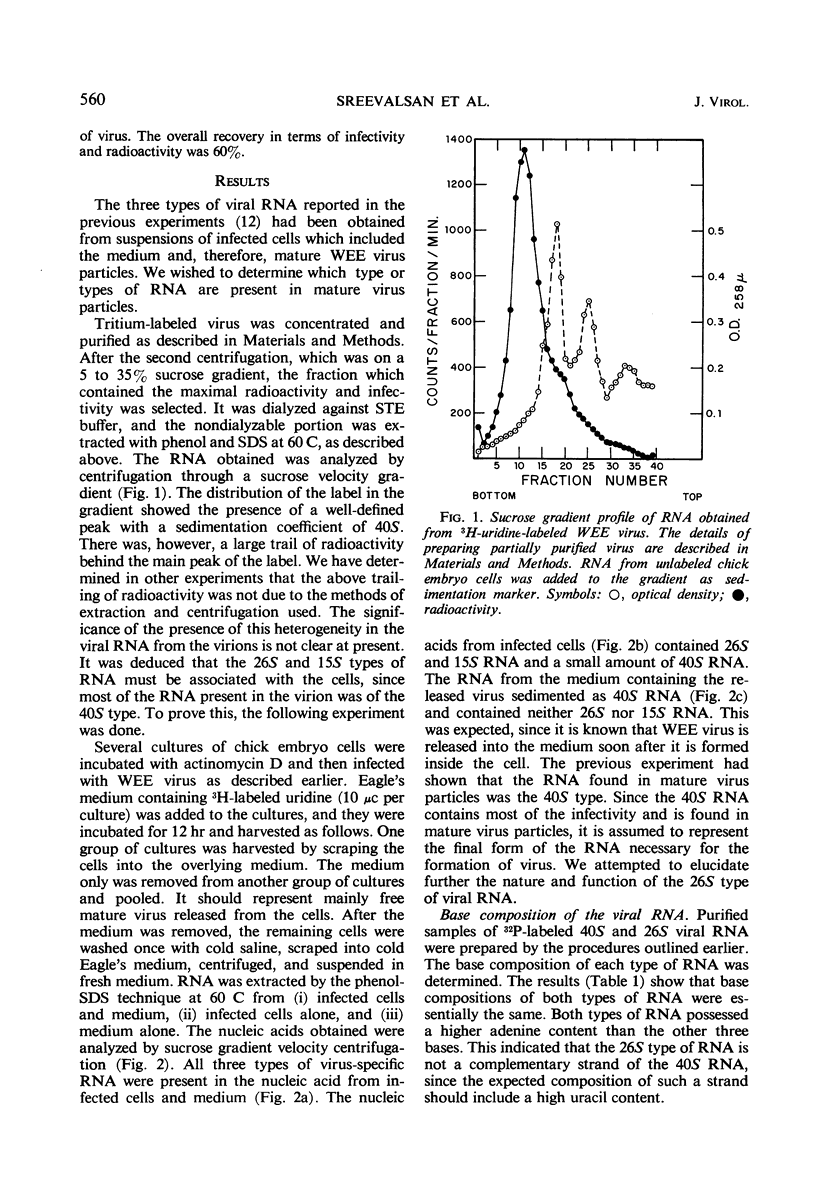
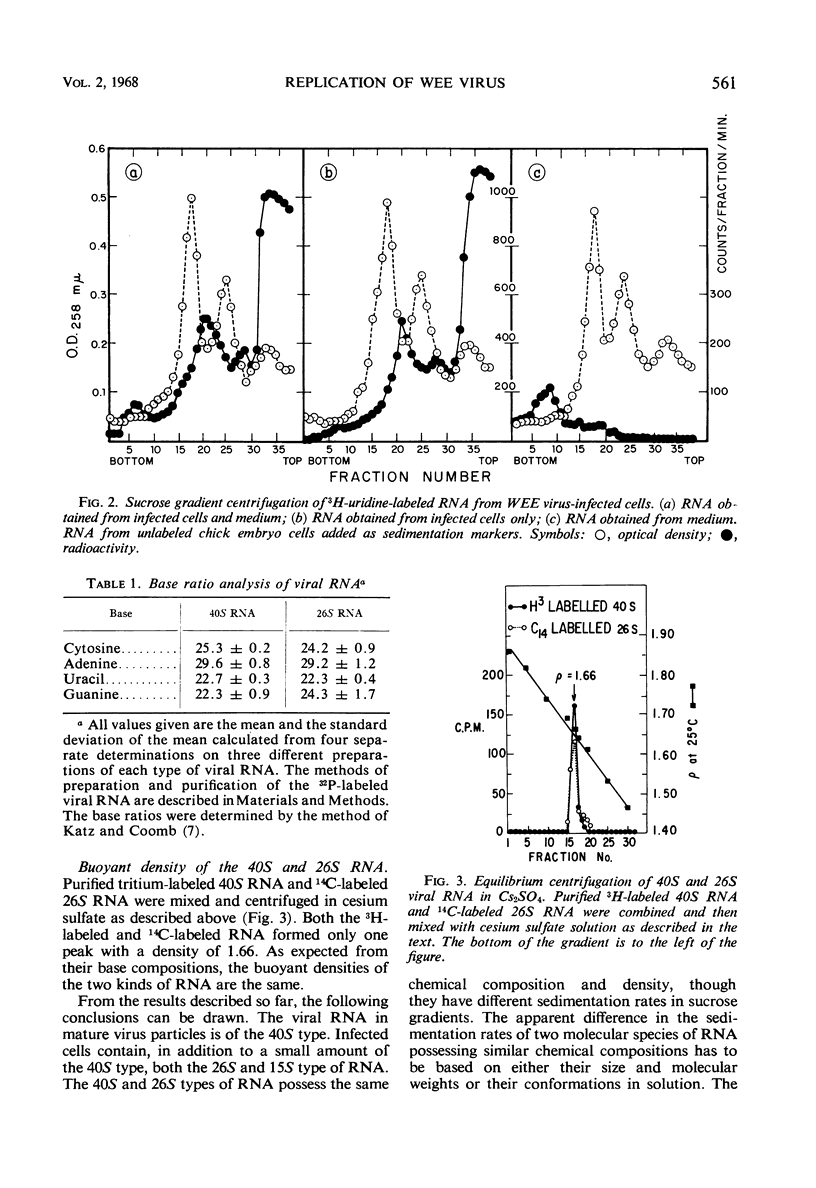
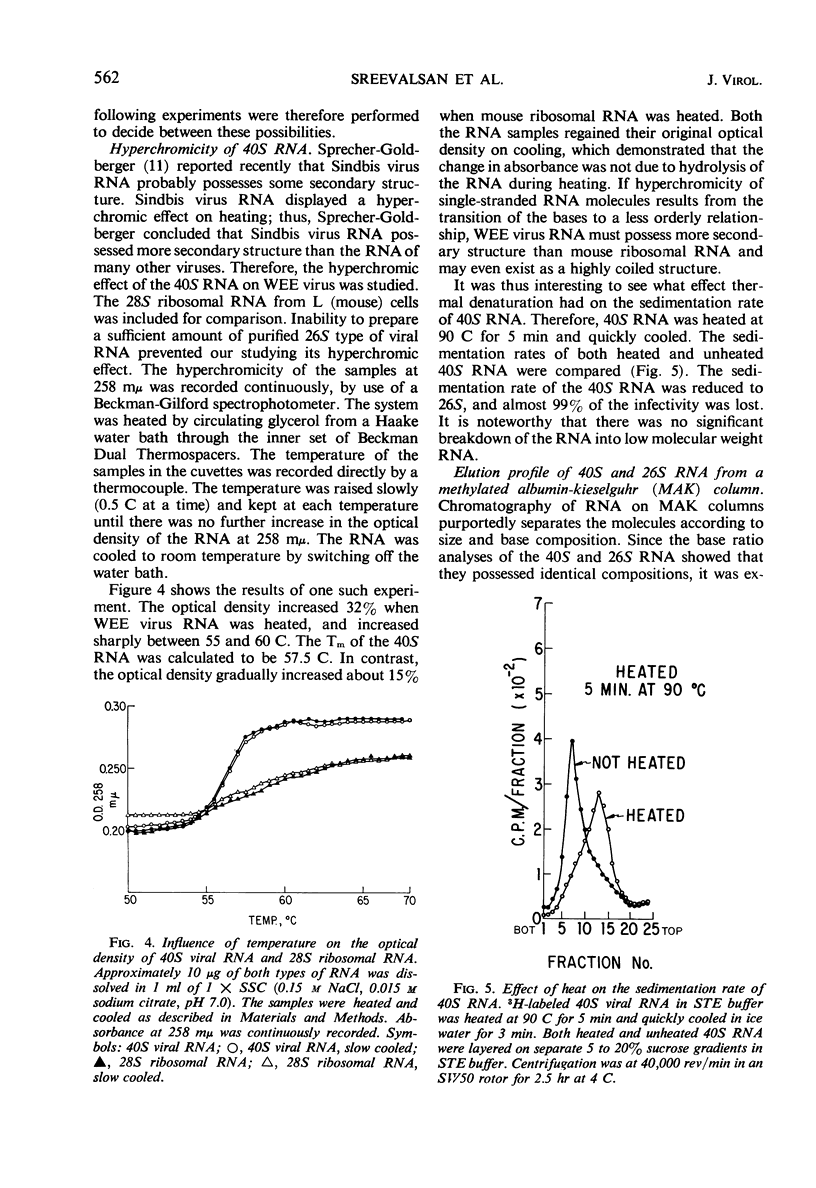
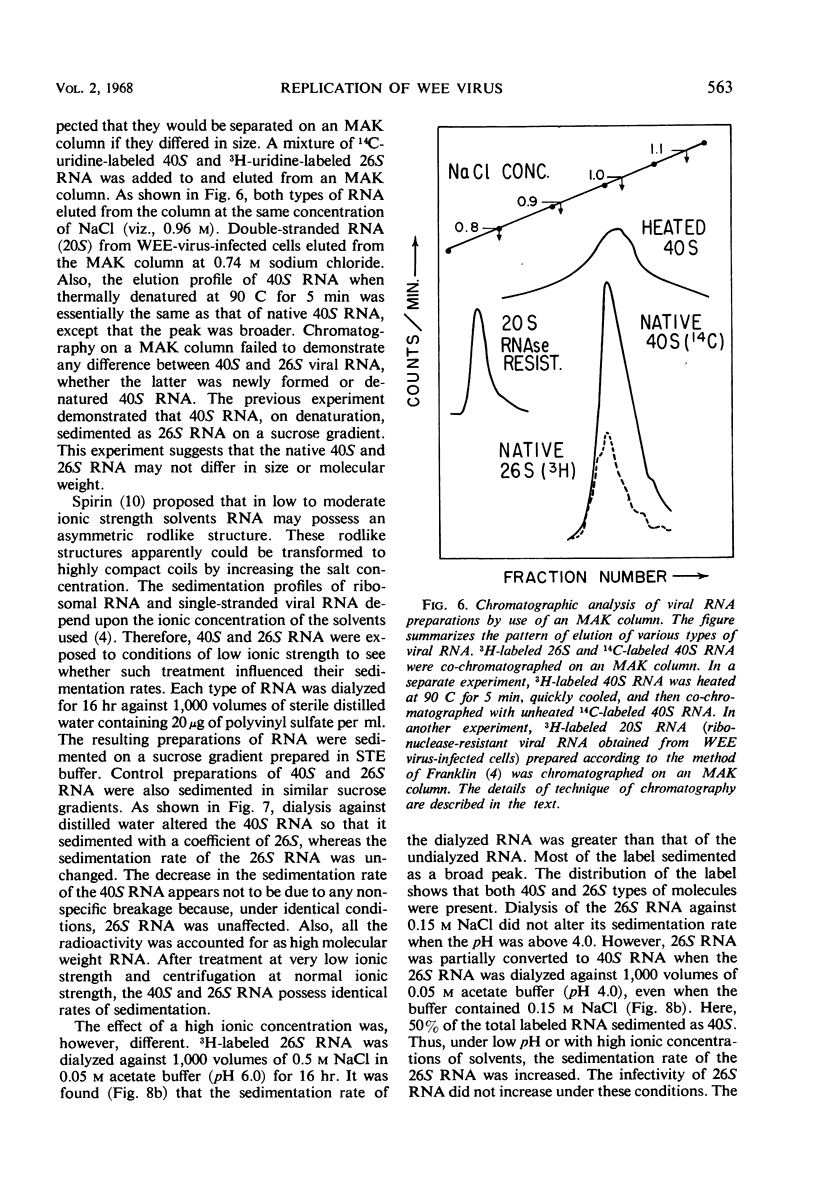
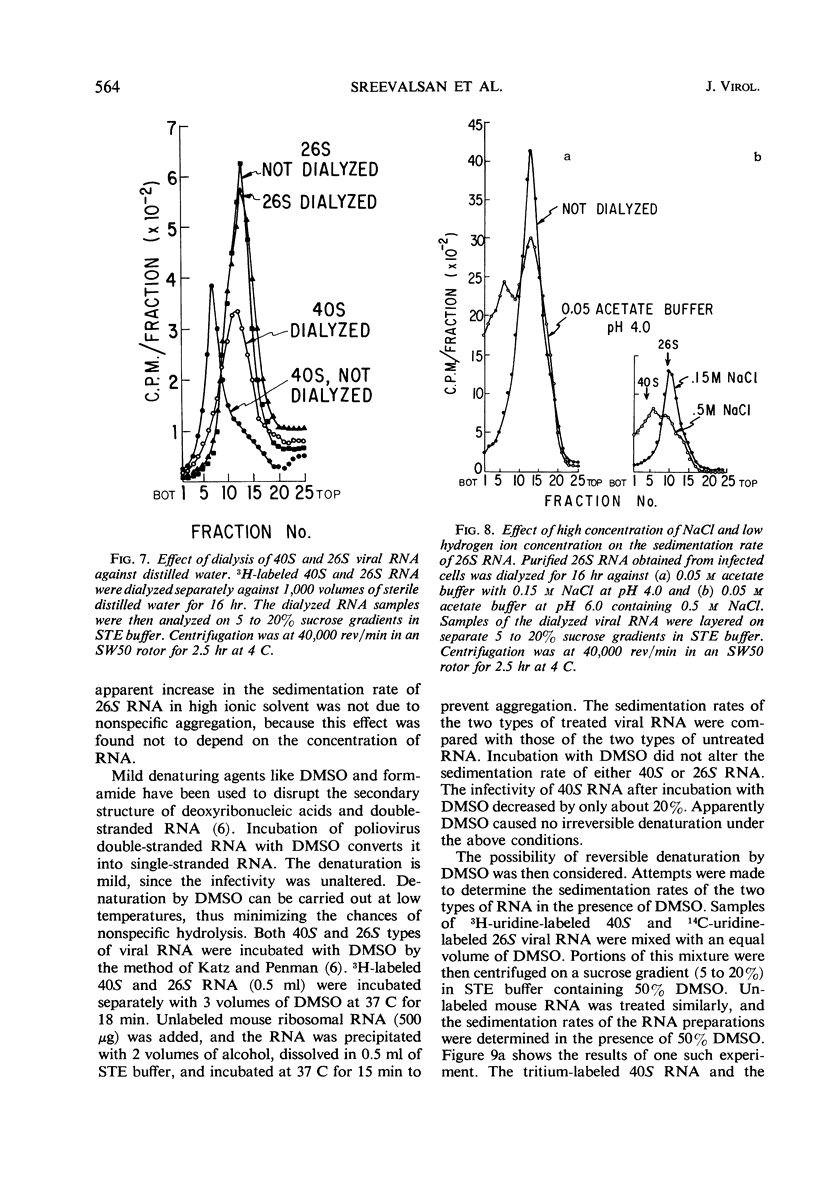
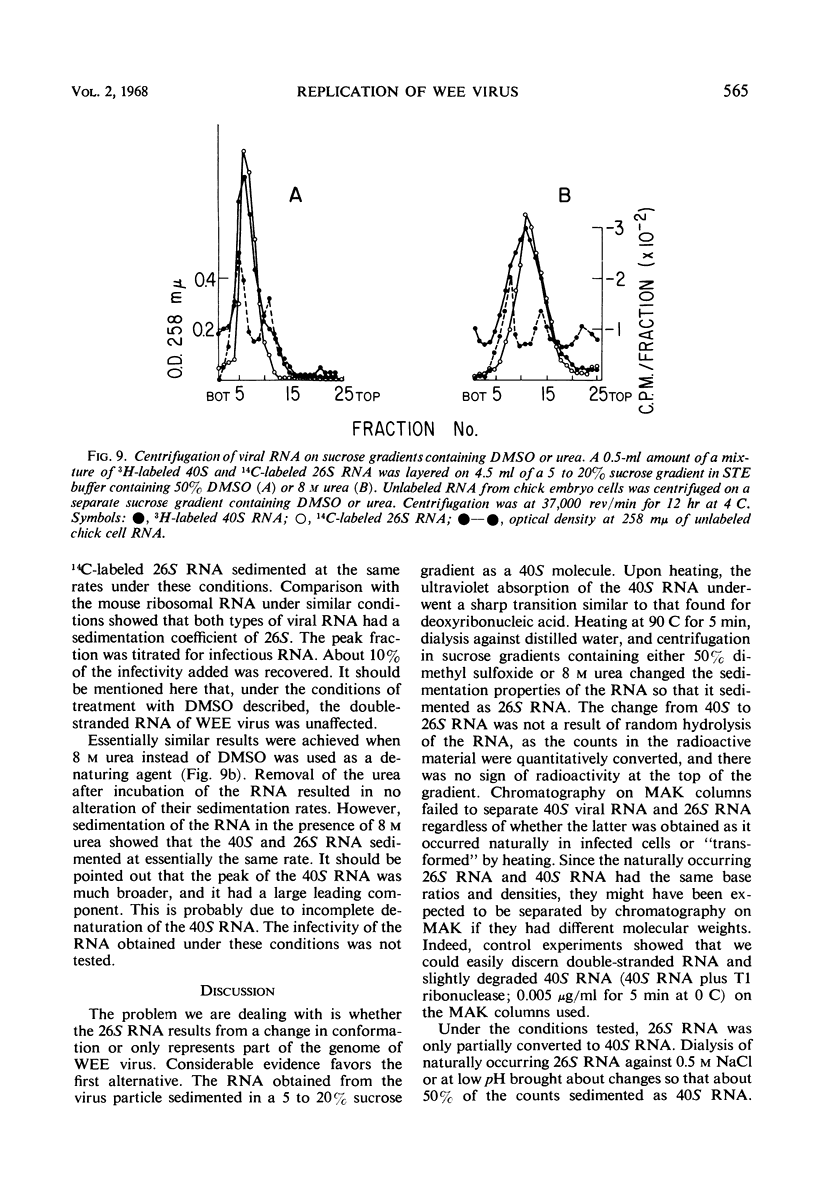
The ribonucleic acid (RNA) from Western equine encephalomyelitis (WEE) virions sedimented through sucrose gradients with a sedimentation coefficient of 40S. Another viral RNA which was always associated with infected cells possessed a sedimentation coefficient of 26S. Both 40S and 26S RNA had identical base compositions and densities. The 40S RNA displayed a hyperchromic effect when heated with a Tm of 57.5 C. When 40S RNA was heated at 90 C and cooled rapidly, it sedimented with a coefficient of 26S. Dialysis of 40S RNA against distilled water changed its sedimentation coefficient to 26S. The presence of 8 m urea or 50% dimethyl sulfoxide in the gradients also altered the sedimentation rate of 40S RNA to 26S. In the latter case, the 26S RNA retained 10% of the infectivity originally added as 40S RNA. Dialysis of 26S RNA against 0.5 m NaCl or 0.05 m acetate buffer at pH 4.0 altered it so that about 50% of the radioactivity sedimented with a coefficient of 40S. Chromatography on methylated albumin-kieselguhr columns failed to separate 40S RNA from 26S RNA. Viral RNA either exists in two conformations which sediment differently in sucrose or contains an extremely labile portion near the center and is easily broken into two equal pieces.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN F., CARTWRIGHT B. VIRUS SPECIFIC RIBONUCLEIC ACIDS IN BABY HAMSTER KIDNEY CELLS INFECTED WITH FOOT-AND-MOUTH DISEASE VIRUS. Nature. 1964 Nov 28;204:855–856. doi: 10.1038/204855a0. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Temperature-sensitive mutants of Sindbis virus: biochemical correlates of complementation. J Virol. 1967 Oct;1(5):956–962. doi: 10.1128/jvi.1.5.956-962.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Martin E. M., Liu S. L., Work T. S. Characterization of the products formed by the RNA polymerases of cells infected with encephalomyocarditis virus. J Mol Biol. 1966 Jan;15(1):77–91. doi: 10.1016/s0022-2836(66)80210-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some physical properties of single-stranded, double-stranded, and branched viral ribonucleic acid. J Virol. 1967 Feb;1(1):64–75. doi: 10.1128/jvi.1.1.64-75.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S., COMB D. G. A NEW METHOD FOR THE DETERMINATION OF THE BASE COMPOSITION OF RIBONUCLEIC ACID. J Biol Chem. 1963 Sep;238:3065–3067. [PubMed] [Google Scholar]
- KELLY R. B., GOULD J. L., SINSHEIMER R. L. THE REPLICATION OF BACTERIOPHAGE MS2. IV. RNA COMPONENTS SPECIFICALLY ASSOCIATED WITH INFECTION. J Mol Biol. 1965 Mar;11:562–575. doi: 10.1016/s0022-2836(65)80011-0. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SPRECHER-GOLDBERGER S. THE EFFECT OF IONIC STRENGTH AND SLOW COOLING ON THERMOLABILITY OF INFECTIOUS RIBONUCLEIC ACIDS. Arch Gesamte Virusforsch. 1964;14:268–271. doi: 10.1007/BF01555098. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr Heterogeneous RNA's occurring during the replication of Western equine encephalomyelitis virus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):974–981. doi: 10.1073/pnas.55.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]


