Abstract
Purpose
Retinal ischemia/reperfusion (I/R) injury results in the generation of reactive oxygen species (ROS). The aim of this study was to investigate whether delivery of the manganese superoxide dismutase gene (SOD2) or the catalase gene (CAT) could rescue the retinal vascular damage induced by I/R in mice.
Methods
I/R injury to the retina was induced in mice by elevating intraocular pressure for 2 hours, and reperfusion was established immediately afterward. One eye of each mouse was pretreated with plasmids encoding manganese superoxide dismutase or catalase complexed with cationic liposomes and delivered by intravitreous injection 48 hours before initiation of the procedure. Superoxide ion, hydrogen peroxide, and 4-hydroxynonenal (4-HNE) protein modifications were measured by fluorescence staining, immunohistochemistry, and Western blot analysis 1 day after the I/R injury. At 7 days after injury, retinal vascular cell apoptosis and acellular capillaries were quantitated.
Results
Superoxide ion, hydrogen peroxide, and 4-HNE protein modifications increased at 24 hours after I/R injury. Administration of plasmids encoding SOD2 or CAT significantly reduced levels of superoxide ion, hydrogen peroxide, and 4-HNE. Retinal vascular cell apoptosis and acellular capillary numbers increased greatly by 7 days after the injury. Delivery of SOD2 or CAT inhibited the I/R-induced apoptosis of retinal vascular cell and retinal capillary degeneration.
Conclusions
Delivery of antioxidant genes inhibited I/R-induced retinal capillary degeneration, apoptosis of vascular cells, and ROS production, suggesting that antioxidant gene therapy might be a treatment for I/R-related disease.
Ischemia/reperfusion (I/R)-induced retinal injury is a process whereby hypoxic organ damage occurs after the return of blood flow and oxygen delivery. One key element of the pathologic alteration in retinal I/R injury is the generation of reactive oxygen species (ROS) during the reperfusion, which contributes to visual impairment and blindness in diseases such as diabetic retinopathy, glaucoma, retinal vascular occlusion, and retinopathy of prematurity.1
Superoxide anion (O2−) is one of the major ROS. The release of •O2− in retinal I/R injury was proven either directly by electron paramagnetic resonance or indirectly by showing diminished damage after administration of antioxidant drugs such as EGB 761 extracted from Ginkgo biloba, vitamin E, mannitol, catalase, and several other compounds.2–6 The importance of •O2− is also indicated by the fact that a manganese superoxide dismutase mimetic and transgenic manganese superoxide dismutase inhibited I/R-induced retinal injury and diabetes-induced oxidative stress.6–8 Superoxide dismutase catalyzes the dismutation of •O2− to O2 and the less reactive species, H2O2. Catalase is a potent scavenger of H2O2 and provides another means of inhibiting oxidant stress. It prevents the formation of the more toxic hydroxyl radical (HO•)resulting from the reaction of H2O2 and ferrous ions. Thus, the production of catalase provides additional antioxidative protection during I/R. The delivery of SOD and catalase proteins has successfully prevented retinal I/R injury in rabbits.8
A recent report showed that retinal ischemia and reperfusion cause capillary degeneration similar to diabetes.9 The authors suggest that this I/R model may be used as an acute model in which to test therapies to inhibit capillary degeneration in diabetic retinopathy or other retinopathies. Kowluru et al.6,10 demonstrated that transgenic mice that express elevated levels of manganese superoxide dismutase have higher antioxidant capacity and are protected from damage to the retinal vasculature (formation of acellular blood vessels) as a result of streptozotocin-induced diabetes. Berkowitz et al.11 have recently shown that transgenic expression of SOD1, which encodes the cytoplasmic Cu/Zn superoxide dismutase, also protects retinal vasculature in the same diabetes model. However, overexpression of Cu/Zn superoxide dismutase caused retinal degeneration independent of diabetes.11
The purpose of the present study was to assess the efficacy of manganese superoxide dismutase and catalase gene transfer on I/R-induced retinal capillary injury in mice. We investigated the effect of I/R injury on superoxide and hydrogen peroxide levels, apoptosis of retinal vascular cells, number of acellular capillaries, and levels of 4-hydroxynonenal (4-HNE) protein modifications. We also determined whether increased expression of superoxide dismutase and catalase protected against capillary injury. Our studies show that in this model there is an increase in capillary degeneration, superoxide radical and hydrogen peroxide production and 4-HNE in the retinas. Transfer of superoxide dismutase or catalase genes protected the retinal capillaries from ROS elevation and from I/R-induced injury.
Materials and Methods
Study Animals
Female C57BL/6J mice (10–14 weeks of age) used for this study were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were maintained in cages in temperature-controlled rooms featuring a 12-hour light/12-hour dark cycle (light period from 6 AM to 6 PM). All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Florida, and studies were conducted in accordance with the principles described in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. At study termination, the animals were killed by overdoses of ketamine and xylazine (14 and 30 mg/kg, respectively).
Preparation for Administration of Superoxide Dismutase or Catalase Gene Delivery by Liposomes
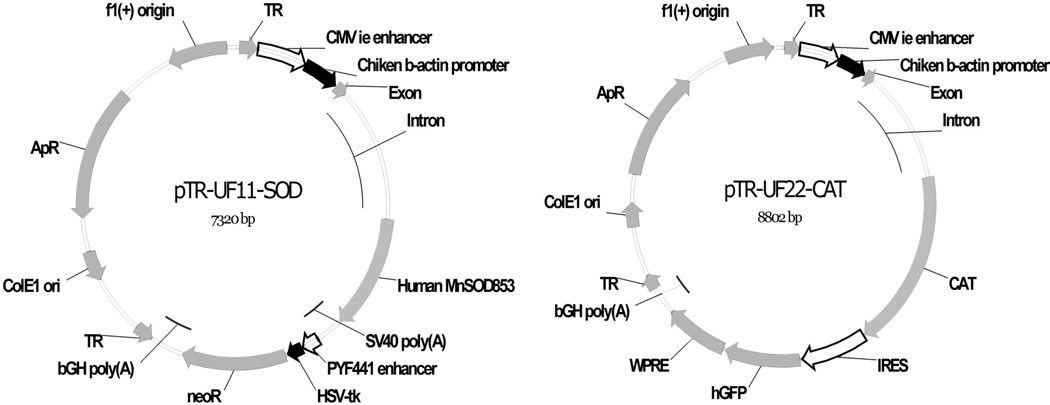
Plasmids pTR-UF11-MnSOD (SOD2) and pTR-UF22-CAT (CAT) were gifts from John Guy (Department of Ophthalmology, University of Florida).12,13 These plasmids express the human SOD2 gene, which codes for Mn-superoxide dismutase, or the human CAT1, which encodes catalase, respectively (Fig. 1). Expression of each gene is driven by the chicken β-actin proximal promoter and the CMV immediate early enhancer (CBA promoter). In addition, pTR-UF22-CAT contains a humanized Gfp gene whose translation is driven by an internal ribosome entry site and the woodchuck hepatitis posttranscriptional regulatory element (WPRE), which increases mRNA processing and transport. Both vectors contain AAV2 inverted terminal repeats for packaging in AAV. The plasmids were purified on cesium chloride density gradients and were combined with cationic liposomes (a gift from Jeffrey Hughes, Department of Pharmaceutics, University of Florida), as described by Shaw et al.14
Figure 1.
Maps of (A) pTR-UF11-MnSOD (SOD2) and (B) pTR-UF22-CAT (CAT). Gene expression was driven by the CBA promoter, a chimera of the CMV immediate early enhancer and the chicken β-actin proximal promoter. pTR-UF22 also contains an IRES-GFP gene and the WPRE posttranscriptional regulatory element. Both plasmids are AAV vectors and contain AAV2-inverted terminal repeats (TR), though gene transfer was mediated by cationic phospholipid vesicles in these experiments.
One eye of each mouse was randomly selected, and 1 µL plasmid (0.5 µg) and liposome mixture was injected in the vitreous cavity 2 days before commencement of the induction of retinal I/R injury. The contralateral eye served as a control and received liposome complexed with a control plasmid (CBA-GFP) and no I/R-induced injury.
Acute I/R-Induced Mice Retinal Microvascular Injury Model
Mice were kept under inhalation anesthesia (isoflurane vapor) during the induction of ischemia. The anterior chamber of the eye was cannulated with a 30-gauge needle attached to a line infusing sterile saline, and the eye was subjected to 2 hours of hydrostatic pressure (80–90 mm Hg; Tono-Pen; Medtronic Solan, Jacksonville, FL) on the anterior chamber. This resulted in retinal ischemia, as confirmed by whitening of the iris and loss of the red reflex. After 2 hours, the needle was withdrawn and the intraocular pressure was normalized, resulting in reperfusion injury.
Detection of ROS
Mice received overdoses of ketamine and xylazine (14 and 30 mg/kg, respectively) 1 day after I/R. Retinas were immediately dissected and then processed for fluorescence staining for comparison with ocular specimens obtained from contralateral control eyes. To detect intracellular ROS, we used two probes (Molecular Probes, Eugene, OR). The probe 2′,7′ dichlorofluorescein diacetate (DCFDA) was used to detect hydrogen peroxide. DCFDA has no fluorescence until it passively diffuses into cells, where an esterase cleaves the acetates, and the oxidation of DCFDA by H2O2 produces a green fluorescent signal. Dihydroethidium (DHE) was used to detect intracellular superoxide (•O2−). Superoxide oxidizes DHE to ethidium, which generates a red fluorescent signal. After a brief rinse in phosphate-buffered saline (PBS), tissues were incubated for 30 minutes at 37°C with a mixture of 10 pM DCFDA or 2.5 µM dihydroethidium. Tissues were washed with PBS, fixed briefly with 4% paraformaldehyde, processed for cryomicroscopy, and observed under a fluorescence microscope (Leitz, Wetzlar, Germany).
Fluorescence Spectrometric Assay of •O2− and H2O2 Production
Fluorescence spectrometry of tissue •O2− and H2O2 production was performed according to a modification of methods described by Benov et al.15 and Mohazzab et al.1 Briefly, retinal homogenates (20 µg) freshly prepared from the mice were incubated with DHE (2.5 µM) and salmon testes DNA (0.05 µg/µL) or DCFDA (10 µM) in a 96-well microplates at 37°C for 30 minutes. The red fluorescence was measured at an excitation of 480 nm and an emission of 610 nm, and the green fluorescence was measured at an excitation of 480 nm and an emission of 520 nm (Safire Microplate Reader; Tecan, Mannedorf, Switzerland). Salmon DNA was added to bind to ethidium and consequently stabilize its fluorescence, thereby increasing the sensitivity of •O2− measurement (>40-fold). Microplate fluorescence spectrometry results were obtained in fluorescence units and were expressed as a percentage of the control. Each retina was tested in six wells (200 µL/well), and each experiment was performed in triplicate.
Preparation of Retinal Vasculature
Retinal vasculature was prepared from freshly isolated eyes that had been fixed with 4% paraformaldehyde for 1 day. Retinas were isolated, washed in water overnight, and incubated with 2.5% trypsin (Invitrogen, Carlsbad, CA) at 37°C for 3 hours and occasionally shaken gently. Nonvascular cells were gently brushed away from the vasculature, and the isolated vasculature was laid out onto slides and used for TUNEL assay and examination of acellular capillaries.
Retinal Vasculature TUNEL Assay
At 7 days after the onset of ischemia, the TUNEL reaction (In Situ Cell Death Detection kit; Roche, Mannheim, Germany) was performed to detect retinal cell death on the isolated vasculature. In each assay, a positive control was set up by treatment with DNase (50 U/100 µL) for 10 minutes to fragment DNA. The retinal vasculature (isolated by the trypsin digest method) was washed extensively in PBS and then permeabilized with 1% Triton ×-100 in PBS. The TUNEL reaction was performed in a humidified atmosphere at 37°C for 1 hour. The number of TUNEL-positive nuclei in different groups was counted throughout the entire retinal vasculature.
Quantitation of Degenerated (Acellular) Capillaries
After quantitation of TUNEL-positive cells, the coverslips were gently soaked away from the slides. Sections were then stained with periodic acid Schiff-hematoxylin. Acellular capillaries were identified as small vessel tubes with no nuclei anywhere along their lengths and are reported per square millimeter of retinal area. They were quantitated by using a modification of methods described by Zheng17 and Tang.18
Immunoblot Analysis
Retinas were dissected, and retinal extracts were prepared by sonication in Laemmli sample loading buffer (without bromphenol blue and without heating). Quantification of total retinal proteins was accomplished by the Lowry method using a RC protein detection kit (Bio-Rad; Hercules, CA). For immunoblot analysis, 20 µg total protein was loaded on 12% SDS polyacrylamide gels run in Tris-glycine buffer. Proteins were electrophoretically transferred to nitrocellulose membranes. To detect manganese superoxide dismutase, the blots were incubated with rabbit anti-MnSOD IgG (Alpha Diagnostic International Inc., San Antonio, TX) at a 1:5000 dilution. To detect catalase, blots were incubated with rabbit anti-catalase (Laboratory Frontiers, Seoul, Korea) at 1/2000. To detect 4-HNE, the blots were treated with anti-4-HNE IgG (Alpha Diagnostic International Inc.) at a dilution of 1:1000. As secondary antibodies, infrared-labeled anti-rabbit IgG (1:10,000) and antimouse IgG (1:10,000) were used. These were obtained from Li-Cor Biosciences (Lincoln, NE), and band intensities were measured on an imaging system (Odyssey Imaging System; Li-Cor Biosciences) using system software. Quantification of MnSOD, catalase, and 4-HNE protein made use of tubulin or β-actin as an internal control. Protein expression levels were then quantitated relative to tubulin or actin in the same sample.
Immunohistochemistry for 4-HNE in Retinal Vasculature
Retinal vasculature (isolated by the trypsin digest method 1 day after I/R injury) was washed with PBS. After blocking with BSA, slides were stained with a primary first antibody rabbit anti-4-HNE IgG (Alpha Diagnostic International), 1:300 in buffered saline, at room temperature for 2 hours. To control for nonspecific binding of secondary antibody, primary antibody was omitted from the initial incubations before incubation with a secondary antibody. After the primary antibody incubation, antibody-antigen complexes were detected with Cy3-conjugated anti-rabbit IgG antibodies (1:300). The visualization 4-HNE was performed under 32× magnification through fluorescence microscopy (Axiovert inverted microscope; Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
All results were expressed as the mean ± SD. Data were analyzed by the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test except the results of immunohistochemistry, immunoblots, and TUNEL assay on retinal sections. Those tests were analyzed by ANOVA (immunohistochemistry and immunoblot analysis) by nonparametric sign test (TUNEL). Differences were considered statistically significant at P ≤ 0.05.
Results
Increase in Antioxidant Levels after Liposome-Mediated Gene Delivery
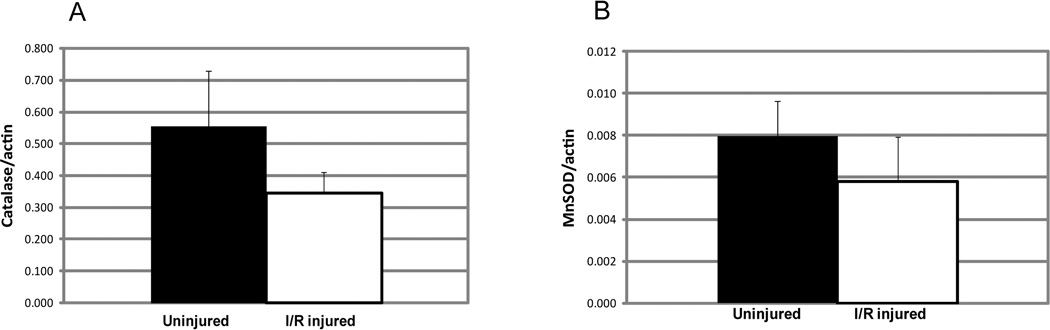
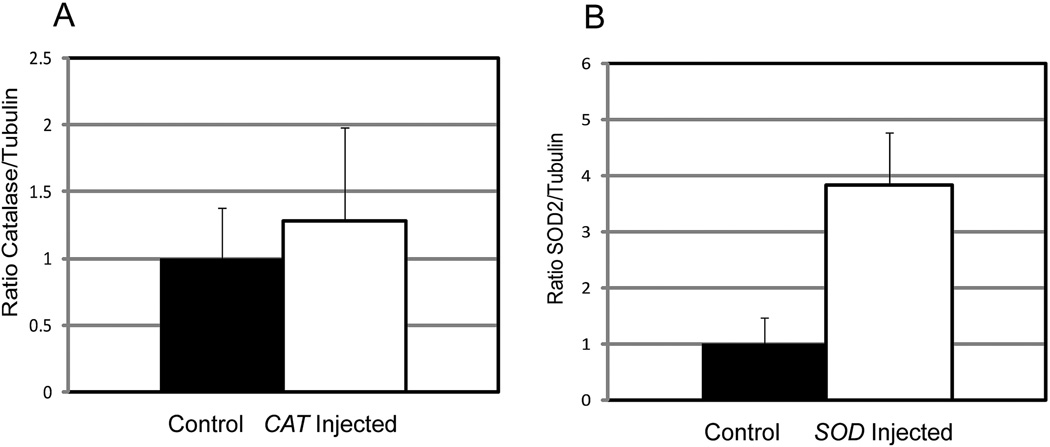
The retinal ischemia/reperfusion technique that we used did not increase levels of MnSOD and catalase (Fig. 2). In fact, there was a trend toward a decrease in both enzymes after I/R injury. A slight decrease in catalase level and a tendency toward a decrease in MnSOD level was also reported by Agardh et al.,19 who used temporary ligation of the blood vessels accompanying the optic nerve to induce ischemia. To determine whether the amount of these antioxidant enzymes could be increased by gene delivery, plasmids encoding human MnSOD and catalase were injected into the vitreous while contralateral eyes were injected with AAV-GFP as a control. As in previous work, we used liposome-conjugated plasmids for gene delivery to the retinal vasculature.14 Two days later, mice were killed and levels of catalase and MnSOD were measured by immunoblot analysis (Fig. 3). Inoculation with the SOD2 plasmid resulted in a nearly fourfold increase in the level of MnSOD (P < 0.007). Levels of catalase were elevated only marginally (27%) by gene delivery, and this increase was not statistically significant. Catalase is present at a high level in the retina, and expression is highest in retinal ganglion cells of rodents.20 Retinal ganglion cells are not efficiently transduced with liposome DNA conjugates after intravitreal injection21; hence, we could not expect a large elevation of catalase in the whole retina.
Figure 2.
Ischemia/reperfusion injury to the mouse retina led to a slight decrease in catalase (A) and MnSOD (B). Mice were euthanatized 24 hours after I/R injury, retinas were dissected, and protein extracts were separated on SDS polyacrylamide gels for immunoblot analysis. The extracts were transferred to nitrocellulose membranes and reacted with antibodies to catalase or manganese superoxide dismutase. Extracts from the same retinas were also probed with antibody to β-actin for normalization. Decreases in catalase and MnSOD did not reach statistical significance (P < 0.18 for catalase; P < 0.23 for MnSOD).
Figure 3.
SOD2 and CAT gene delivery led to increased retinal levels of MnSOD and catalase. Two days after intravitreal injection of liposomes, conjugates with pTR-UF22-CAT (A), or pTR-UF11-SOD2 (B), retinas were dissected and proteins were prepared for immunoblotting with either catalase-specific (A) or MnSOD-specific (B) antibodies (n= 3). Control eyes were injected with pTR-UF11-GFP. These graphs report the normalized ratio of the detected protein to tubulin in the same extracts. Elevation of MnSOD was statistically significant (P < 0.007). Elevation of catalase level was not (P = 0.21).
Reactive Oxygen Species Increased in I/R-Injured Retinas and Reduced by Delivery of Antioxidant Enzyme Genes
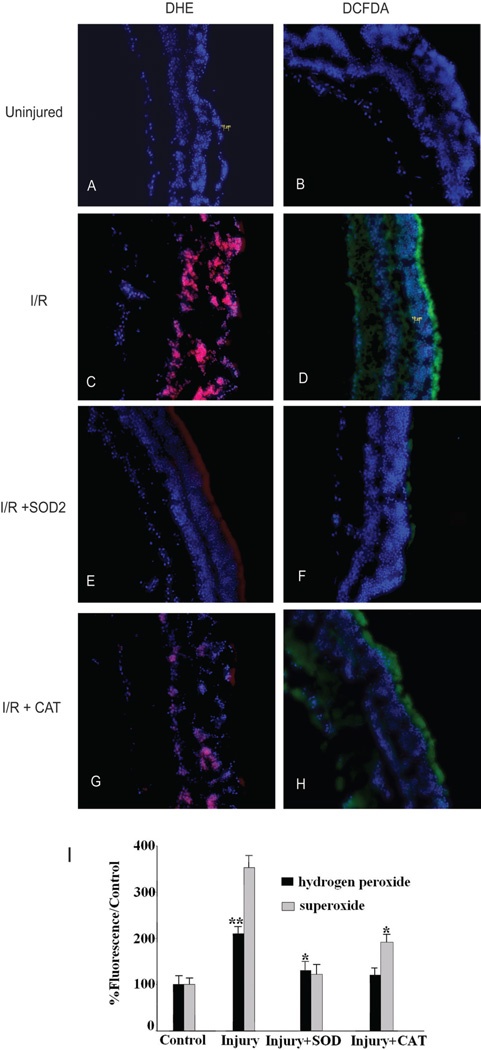
An increase in superoxide levels in retinal mitochondria has been observed in streptozotocin-induced diabetes.6 However, retinal superoxide and hydrogen peroxide levels have not been measured after I/R injury, a model that mimics many of the histologic features of diabetes, but in an accelerated time course. As an initial indicator of ROS activity, we used the fluorescent probes DCFDA and DHE. DCFDA detects H2O2, resulting in a green fluorescence signal, whereas DHE is a probe for •O2− and produces red fluorescence as a result of the complex between ethidium and DNA (Fig. 4). Retinas of mice (n = 18) killed 1 day after the induction of I/R injury were dissected and incubated with DCFDA or DHE. Fluorescence microscopy of the cryosectioned retinal specimens detected superoxide (red labeling with DHE) or hydrogen peroxide (green labeling with DCFDA; Figs. 4C, 4D), relative to control retinas in which superoxide anion and hydrogen peroxide were undetectable (Figs. 4A, 4B). These dyes must be used in fresh, unfixed tissues. Although membrane structures are not evident in these cryosections, DHE or DCFDA staining permeated the entire neural retina, as evident from the DAPI staining of nuclei. To quantitate changes in ROS levels, we measured total fluorescence in retinal homogenates. I/R-induced retinal injury increased DHE staining by 253% and DCFDA staining by 110% compared with that of uninjured control retinas (P < 0.01; Fig. 4I). SOD2 gene delivery significantly reduced the accumulation of superoxide by 91% and hydrogen peroxide by 71% (Figs. 4E, 4F, 4I). SOD2 gene delivery reduced superoxide levels more than hydrogen peroxide levels (Fig. 4I). This result is expected because H2O2 is a product of superoxide dismutase. Similarly, CAT gene delivery reduced the accumulation of superoxide (by 64%) and hydrogen peroxide (by 82%) compared with levels in injured retinas (Figs. 4G, 4H), and, as expected, reduced hydrogen peroxide levels more than superoxide levels (Fig. 4I).
Figure 4.
SOD2 or CAT gene delivery reduced ROS in I/R-injured retinas. (A, B) Superoxide anion and hydrogen peroxide were undetectable in normal retinal sections. (C, D) One day after I/R injury, red labeling with dihydroethidium and green labeling with 2′7′ dichlo-rofluorescein diacetate revealed superoxide anion and hydrogen peroxide in I/R-injured retinas. SOD2 reduced the accumulation of (E) superoxide anion and (F) hydrogen peroxide. Similarly, CAT reduced the levels of (G) superoxide anion and (H) hydrogen peroxide. Sections were costained with DAPI to reveal nuclei. (I) Inhibition of the generation of superoxide and hydrogen peroxide in I/R-induced retinal injury by pretreatment with SOD2 or CAT (n = 18 in each group). Measurements were made by fluorescence spectroscopy in retinal homogenates. **P < 0.01 compared with control group. *P < 0.05 compared with control group.
Effect of SOD2 and CAT on 4-HNE Protein Modification in I/R-Injured Retinas
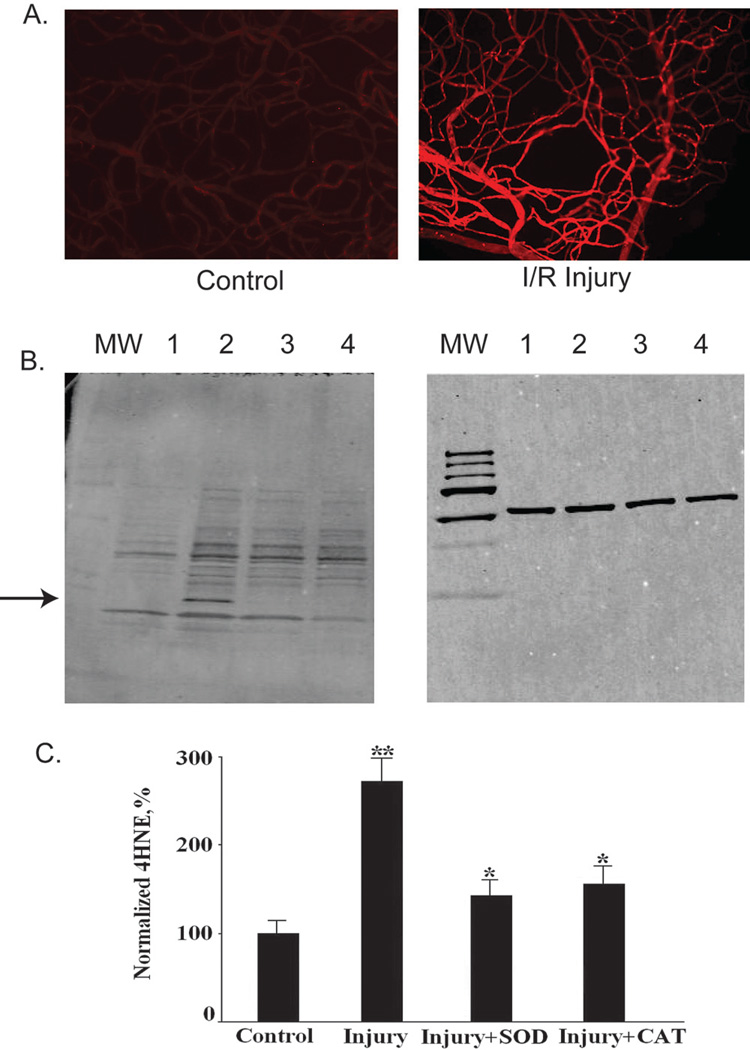
Oxidative damage to lipids results in the production of several reactive aldehydes capable of modifying proteins, including 4-HNE, which is commonly used as a marker of lipid peroxidation. Although DCFDA and DHE indicate the short term elevation of oxidative stress at the time of tissue harvest, measurement of 4-HNE-modified proteins indicates cumulative damage as a result of elevated ROS. We used antibody to 4-HNE to assess the relative levels of aldehyde-modified proteins using both indirect immunofluorescence microscopy of retinal vasculature (Fig. 5A) and Western blot analysis of whole retinal extracts (Figs. 5B, 5C). I/R injury significantly increased the production of 4-HNE protein modifications in retinal capillaries (Fig. 5A). Delivery of the plasmids expressing SOD2 and CAT reduced the production of 4-HNE protein modifications in the retina by 75% and 67.4%, respectively (Figs. 5B, 5C). I/R-induced injury led to the appearance of a 4-HNE-modified protein that migrated with an apparent molecular weight of 17 kDa (Fig. 5B). Gene delivery of SOD2 or CAT inhibited the appearance of this band in Western blots, presumably by blocking modification of the protein (Fig. 5B).
Figure 5.
Administration of SOD2 or CAT inhibited the production of 4-HNE in retinal capillaries after retinal I/R in mice. (A) Examples of 4-HNE immunohistochemistry in the retinal vasculature of I/R-injured retina (right) and control retina (left). (B) Representative Western blots for 4-HNE protein modifications in retina. Left: lane 1: control; lane 2: I/R; lane 3: I/R+CAT; lane 4: I/R+SOD. Right: equal amounts of protein loaded in each gel lane were confirmed by internal reference anti-tubulin antibody. Arrow: 4-HNE-labeled band seen only in the injured, untreated retinas. (C) Densitometric analysis of bands for 4-HNE protein modification. Results for 4-HNE bands were normalized to the level of tubulin probed in the same blots (n= 5 in each group; *P < 0.05 compared with control group; **P < 0.01 compared with injury group).
Effect of I/R on Retinal Capillaries in Mice
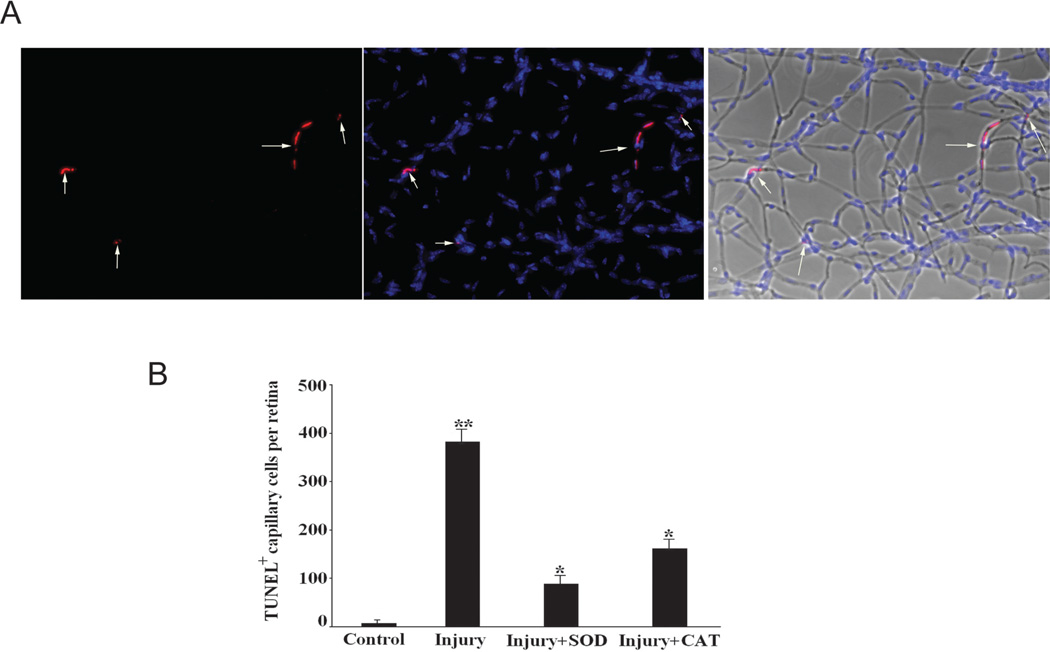
Zheng et al.9 demonstrated an increase in apoptotic cells in the retinal vasculature after I/R injury.9 Examination of retinal vasculature 7 days after I/R injury (Fig. 6A) showed a significant increase in the number of TUNEL-positive capillary cells in the injured retinas compared with the control retinas obtained from the same animal (381 ± 25 vs. 6.4 ± 2.4 TUNEL-positive cells per retina; n = 6 for each group; P < 0.01; Fig. 6B). Delivery of either SOD2 or CAT reduced the number of apoptotic cells in the retinal vasculature: pTRUF11-SOD reduced TUNEL-positive cells by 3.5-fold (87.6 ± 10.6 vs. 381 ± 25), and pTRUF22-CAT reduced the number of positive cells by greater than twofold (161.2 ± 12.3 vs. 381 ± 25; P < 0.05 relative to the injured retinas).
Figure 6.
Administration of SOD2 and CAT inhibited capillary cell death after retinal I/R. (A) An example of TUNEL-positive capillary cells (white arrows) in the retinal vasculatures of injured retinas 7 days after I/R by trypsin-digested method (left), DAPI costaining of the same area (middle), and the merged image (right, TUNEL, DAPI, and phase images). (B) Inhibition of retinal I/R-induced capillary cell death by pretreatment with SOD2 and CAT (n= 6 in each group; **P < 0.05 compared with control group; **P < 0.05 compared with injury group).
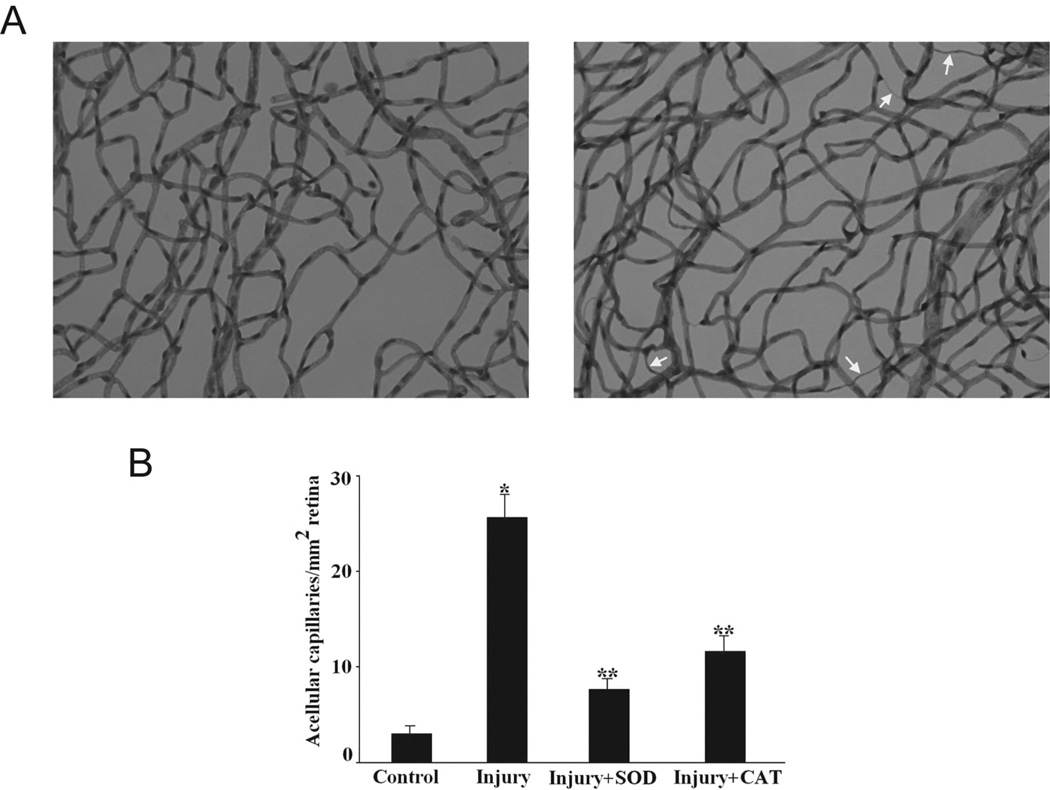
In diabetic retinopathy, the death of retinal endothelial cells and pericytes leads to acellular capillaries.22 In the I/R model, microvessel apoptosis also correlated with degeneration of capillaries. Acellular capillaries appear as thin tubes with no nuclei along their entire length (Fig. 7A). We observed an increase in the number of acellular capillaries in the I/R retina compared with the control retinas (25.7 ± 4.6 vs. 3 ± 1.1 acellular capillaries per square millimeter of retina; n = 6 for each group; P < 0.01; Fig. 7B). Treatment with either the SOD2 or the CAT gene led to a decrease in the number of acellular capillaries that was proportional to the decrease in apoptotic nuclei within the retinal vessels (7.7 ±1.5 acellular capillaries per square millimeter for SOD2 and 11.7 ± 2.9 for CAT).
Figure 7.
SOD2 or CAT gene delivery inhibited capillary degeneration after retinal I/R injury in mice. (A) Examples of acellular capillaries (arrows) in the retinal vasculature of I/R-injured retina (right) and control retina (left). (B) Inhibition of capillary degeneration in injured retinas by pretreatment with SOD2 or CAT (n = 6 in each group; *P < 0.05 compared with control group; **P < 0.05 compared with injury group).
Discussion
Our experiments suggest that delivery of genes for antioxidant enzymes provides protection from retinal capillary degeneration because of increases in oxidative stress, lipid peroxidation, and apoptosis in the I/R model. We found that delivery of SOD2 and CAT genes significantly suppressed the I/R-induced acute retinal injury, as reflected by a marked reduction of retinal capillary degeneration and capillary cell apoptosis. Moreover, liposome-mediated SOD2 or CAT gene delivery suppressed increased •O2− levels and H2O2 production caused by the I/R procedure and significantly attenuated oxidative stress as evidenced by lower 4-HNE protein modifications, indicating that supplementing endogenous MnSOD or CAT has protective effects on retinal capillary cells after retinal I/R injury.
Elevated levels of ROS can be toxic to cells. In particular, the cellular damage that occurs after ischemia may be aggravated by the sudden reintroduction of oxygen into tissues during reperfusion, unleashing a surge in free radical that overwhelms endogenous defenses. As a major cause of tissue damage, ROS consist of superoxide anions produced by the mitochondrion, H2O2 produced by superoxide anions in presence of superoxide dismutase, and peroxynitrite produced by superoxide anions in the presence of nitric oxide.23
Superoxide dismutase is the key enzyme involved in the detoxification of superoxide radicals. There are three forms of the enzyme: the cytosolic copper/zinc superoxide dismutase (Cu/ZnSOD); the extracellular copper/zinc superoxide dismutase (ECSOD); and the mitochondrial MnSOD. Mice lacking Cu/ZnSOD show no overt abnormalities during development and early adulthood but have a shortened lifespan, primarily because of the development of hepatocellular carcinomas.24 Interestingly, late in life, these mice may exhibit some signs of age-related macular degeneration: subretinal deposits and choroidal neovascularization.25 ECSOD knockouts appear healthy and show no obvious spontaneous phenotype, though they die when stressed by high oxygen tension.26 In contrast, MnSOD-deficient mice die of dilated cardiomyopathy within the first 10 days of life,27 and survival is not affected by the overexpression of Cu/ZnSOD.28 These findings indicate that mitochondria are both sources and targets of superoxide radicals. MnSOD is required to maintain tissue function by preserving the activity of mitochondrial enzymes that are susceptible to inactivation by superoxide radicals. The expression of MnSOD and catalase are slightly decreased in the retina after I/R-induced injury (Fig. 2).19 Therefore, maintaining adequate expression and activity of MnSOD and catalase may be critical for retinal protection after I/R-induced injury.
We showed that I/R-induced retinal injury increased the production of •O2− and H2O2 and that delivery of pTR-UF11-SOD2 and pTR-UF22-CAT inhibited this increase. These experiments suggest that •O2− in I/R-induced retinal injury comes from mitochondrial oxidative stress because it is suppressed by mitochondrial superoxide dismutase. We found that the elevation of •O2 − relative to control eyes is greater than that of H2O2 and that the protective effect of MnSOD is greater than that of CAT, suggesting that •O2− is the main cause of retinal injury and that MnSOD is the main antioxidant enzyme that promotes protection in this retinal I/R model. However, despite the fact that the plasmids were administered at the same concentrations, the relative elevation of MnSOD was much greater than that of catalase (Fig. 3), and this may also explain why the delivery of SOD2 was more effective. It is almost certain that the protective effect of both enzymes is exerted in the vascular endothelium, but we measured MnSOD and catalase levels in the entire retina, and the elevations in the relevant cell type are unknown. Vascular endothelial cells, however, are readily transduced by complexes of DNA and cationic liposomes.29
We also observed that the delivery of the SOD2 gene markedly suppressed H2O2 production (Fig. 4I), a finding that is similar to other reports and may be explained through enhanced catalase activity.30,31 A possible explanation is that the higher MnSOD activity resulting from the gene transfer could generate more H2O2 from •O2− and that this high substrate level of H2O2, in turn, would elicit a compensatory response involving the induction of catalase or glutathione peroxidase expression in the retina. In addition, CAT gene delivery reduced retinal •O2− levels (Fig. 4I) possibly by increased MnSOD activity during the I/R procedure. In a study by Dai et al.,32 elevated catalase activity was noted in joint fluid after EC-SOD gene delivery and vice versa; catalase gene delivery also increased MnSOD activity, but neither reached statistical significance.
Free radicals formed during oxidative stress can directly attack polyunsaturated fatty acids and initiate free radical chain reactions that result in lipid peroxidation in cellular membranes. This reaction amplifies the generation of lipid radical species, causing lipid degradation into a variety of oxidized products, including aldehydes, which are extremely reactive and can damage biological macromolecules. Injury can occur distal to the initial site of free radical attack because aldehydes are relatively long-lived compared with free radicals.33,34 4-HNE, the most toxic aldehyde formed during lipid peroxidation,35–38 can covalently modify histidine, cysteine, and lysine residues, leading to stable HNE-protein adducts. The addition of HNE adducts to proteins has been shown to cause functional impairment in cells.33–36 The high level of 4-HNE-modified retinal proteins reported in both aged and light-exposed rats suggests that this aldehyde is abundant in the retina.39,40 The presence of 4-HNE-modified proteins in a porcine model of retinitis pigmentosa suggests that oxidative damage is responsible for damage to cone photoreceptors.41 Measuring protein modifications by 4-HNE has proven useful in assessing the impact of antioxidant therapy for light-induced retinal damage.42–44 Our data reveal that I/R-induced retinal injury increases the production of 4-HNE in retinal capillaries and that MnSOD and CAT significantly reduced 4-HNE protein modification levels of the retinal tissue. This result suggests that MnSOD and CAT can reduce the burden of free radicals and, therefore, inhibit lipid peroxidation.
Elevation of MnSOD and CAT levels rescued I/R-induced damage to retinal capillaries. Zheng et al.9 offer several explanations for the capillary degeneration seen in this model. First, the release of ROS and lipid peroxide from the damaged retina may directly initiate the apoptosis of capillary cells and the subsequent degeneration of capillaries. Second, activated leukocytes or other inflammatory cells such as infiltrating macrophages may stimulate cell death in capillary cells also leading to capillary degeneration. The observation that peripheral leukocytes from the circulation infiltrate to the retina during the inflammatory response after retinal I/R supports this hypothesis.45 Degeneration of retinal vasculature may also be attributable to the failure of endothelial progenitor cells to maintain normal retinal vasculature or to repair damaged vasculature. Endothelial precursor cells can rescue and stabilize degenerating vasculature in mice.46 Finally, thrombosis, pressure-induced damage, and release of toxic factors from the injured retina all could contribute to capillary degeneration in this model. Our results suggest that the production of ROS and lipid peroxides, such as 4-HNE, are proximal causes of retinal capillary degeneration.
Degenerated capillaries in this retinal I/R model are similar in microscopic appearance to acellular capillaries found in diabetic retinopathy. Such degenerated capillaries, which are not perfused, reflect a step in the progression to ischemia, and eventually to aberrant neovascularization.47,48 Relevant to this progression, VEGF expression is suppressed by antioxidants,24 and clinical studies support a strong correlation between increases in intraocular VEGF concentration and the development of proliferative diabetic retinopathy.49 Oxidative stress is thought to be involved in the upregulation of VEGF expression during diabetes.50 Moreover, diabetes-induced retinal vascular dysfunction, including formation of acellular capillaries and increases in retinal VEGF concentrations and lipid peroxidation, were prevented by antioxidant treatment in diabetic rats.6,51 Recently, Aydoğan et al.52 found that VEGF and TNF-α levels were significantly elevated in I/R-injured guinea pig retinas. In our study, acellular capillaries and capillary apoptosis were significantly elevated in I/R-injured retina. Moreover, MnSOD and catalase gene delivery inhibited capillary degeneration and apoptosis, which may implicate VEGF in this process, but further studies are required to determine the relationship between mitochondrial ROS, the production of acellular capillaries, and the release of VEGF.
In conclusion, our findings demonstrate that liposome-mediated SOD2 or CAT gene delivery protect retinal capillaries during retinal I/R through inhibition of oxidative stress, 4-HNE modification protein generation, and retinal cell apoptosis. This approach may point to a potential therapy to inhibit the vasodegenerative phase of diabetic retinopathy. Transfer of antioxidant genes may also be beneficial in the treatment of other ischemic retinal diseases such as glaucoma and retinal vein obstruction. Further study is warranted to determine the clinical use of SOD2 and CAT in the treatment of I/R-related eye disorders.
Acknowledgments
The authors thank Qiuhong Li, Xiaoping Qi, Jijing Pang, and Marina Gorbatyuk for their expertise and assistance; and John Guy for the gift of pTR-UF11-MnSOD and pTR-UF22-CAT.
Supported by the Shaler-Richardson Professorship Fund and by a grant from the Juvenile Diabetes Research Foundation.
Footnotes
Disclosure: B. Chen, None; S. Caballero, None; S. Seo, None; M.B. Grant, None; A.S. Lewin, None
References
- 1.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina: demonstration of a free radical-mediated mechanism. Invest Ophthalmol Vis Sci. 1991;32:1471–1478. [PubMed] [Google Scholar]
- 3.Szabo ME, Droy-Lefaix MT, Doly M. Direct measurement of free radicals in ischemic/reperfused diabetic rat retina. Clin Neurosci. 1997;4:240–245. [PubMed] [Google Scholar]
- 4.Kim SY, Kwak JS, Shin JP, Lee SH. The protection of the retina from ischemic injury by the free radical scavenger EGb 761 and zinc in the cat retina. Ophthalmologica. 1998;212:268–274. doi: 10.1159/000027305. [DOI] [PubMed] [Google Scholar]
- 5.Hirose F, Kiryu J, Miyamoto K, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004;43:1098–1102. doi: 10.1161/01.HYP.0000123069.02156.8a. [DOI] [PubMed] [Google Scholar]
- 6.Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Dong A, Shen J, Krause M, et al. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- 8.Nayak MS, Kita M, Marmor MF. Protection of rabbit retina from ischemic injury by superoxide dismutase and catalase. Invest Ophthalmol Vis Sci. 1993;34:2018–2022. [PubMed] [Google Scholar]
- 9.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reper-fusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]
- 10.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009;50:2351–2358. doi: 10.1167/iovs.08-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy J, Qi X, Hauswirth WW. Adeno-associated viral-mediated catalase expression suppresses optic neuritis in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1998;95:13847–13852. doi: 10.1073/pnas.95.23.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. SOD2 gene transfer protects against optic neuropathy induced by deficiency of complex I. Ann Neurol. 2004;56:182–191. doi: 10.1002/ana.20175. [DOI] [PubMed] [Google Scholar]
- 14.Shaw LC, Pan H, Afzal A, et al. Proliferating endothelial cell-specific expression of IGF-I receptor ribozyme inhibits retinal neovascularization. Gene Ther. 2006;13:752–760. doi: 10.1038/sj.gt.3302718. [DOI] [PubMed] [Google Scholar]
- 15.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohazzab H, Kaminski PM, Fayngersh RP, Wolin MS. Oxygenelicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol. 1996;270:H1044–H1053. doi: 10.1152/ajpheart.1996.270.3.H1044. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Mohr S, Du YD, Kern TS. Non-uniform distribution of lesions and biochemical abnormalities within the retina of diabetic humans. Curr Eye Res. 2003;27:7–13. doi: 10.1076/ceyr.27.2.7.15455. [DOI] [PubMed] [Google Scholar]
- 19.Agardh CD, Gustavsson C, Hagert P, Nilsson M, Agardh E. Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion. Metabolism. 2006;55:892–898. doi: 10.1016/j.metabol.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Hunt DM, Lu H, Hunt RC. Expression of antioxidant protective proteins in the rat retina during prenatal and postnatal development. Invest Ophthalmol Vis Sci. 1999;40:744–751. [PubMed] [Google Scholar]
- 21.Masuda I, Matsuo T, Yasuda T, Matsuo N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci. 1996;37:1914–1920. [PubMed] [Google Scholar]
- 22.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz T, Kukner AS, Aydemir O, Ozercan HI, Naziroglu M. Aprotinin reduces ischemia-reperfusion injury in the retina of guinea pigs. Eur J Ophthalmol. 2003;13:642–647. doi: 10.1177/112067210301300708. [DOI] [PubMed] [Google Scholar]
- 24.Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 25.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 28.Copin JC, Gasche Y, Chan PH. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic Biol Med. 2000;28:1571–1576. doi: 10.1016/s0891-5849(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]
- 30.He SQ, Zhang YH, Venugopal SK, et al. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869–1879. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 31.Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:681–691. doi: 10.1167/iovs.06-0553. [DOI] [PubMed] [Google Scholar]
- 32.Dai L, Claxson A, Marklund SL, et al. Amelioration of antigeninduced arthritis in rats by transfer of extracellular superoxide dismutase and catalase genes. Gene Ther. 2003;10:550–558. doi: 10.1038/sj.gt.3301916. [DOI] [PubMed] [Google Scholar]
- 33.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 34.Awasthi YC, Yang Y, Tiwari NK, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Crabb JW, O’Neil J, Miyagi M, West K, Hoff HF. Hydroxynonenal inactivates cathepsin B by forming Michael adducts with active site residues. Protein Sci. 2002;11:831–840. doi: 10.1110/ps.4400102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol Aspects Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 38.Szweda LI, Uchida K, Tsai L, Stadtman ER. Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal: selective modification of an active-site lysine. J Biol Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- 39.Kapphahn RJ, Giwa BM, Berg KM, et al. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Yang X, Dong A, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 42.Rex TS, Tsui I, Hahn P, et al. Adenovirus-mediated delivery of catalase to retinal pigment epithelial cells protects neighboring photoreceptors from photo-oxidative stress. Hum Gene Ther. 2004;15:960–967. doi: 10.1089/hum.2004.15.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanito M, Nishiyama A, Tanaka T, et al. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- 44.Tanito M, Kwon YW, Kondo N, et al. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J Neurosci. 2005;25:2396–2404. doi: 10.1523/JNEUROSCI.4866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsujikawa A, Ogura Y, Hiroshiba N, Miyamoto K, Kiryu J, Honda Y. In vivo evaluation of leukocyte dynamics in retinal ischemia reperfusion injury. Invest Ophthalmol Vis Sci. 1998;39:793–800. [PubMed] [Google Scholar]
- 46.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns, IV: diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 48.Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 49.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- 50.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 51.Obrosova IG, Minchenko AG, Marinescu V, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 52.Aydogan S, Celiker U, Turkcuoglu P, Ilhan N, Akpolat N. The effect of thalidomide on vascular endothelial growth factor and tumor necrosis factor-alpha levels in retinal ischemia/reperfusion injury. Graefes Arch Clin Exp Ophthalmol. 2008;246:363–368. doi: 10.1007/s00417-007-0663-9. [DOI] [PubMed] [Google Scholar]