Abstract
Variable lymphocyte receptor (VLR) B antibodies of the evolutionary distant sea lamprey are structurally distinct from conventional mammalian antibodies. The different protein architecture and large evolutionary distance of jawless vertebrates suggest that VLR antibodies may represent promising tools for biomarker discovery. Here we report the generation of panels of monoclonal VLR antibodies from lamprey larvae immunized with human T cells and the use of a recombinant monoclonal VLR antibody for antigen purification and mass spectrometric identification. We demonstrate that despite predicted low affinity of individual VLR antigen binding units to the antigen, the high avidity resulting from decameric assembly of secreted VLR antibodies allows for efficient antigen capture and subsequent identification by mass spectometry. We show that VLR antibodies detect their antigens with high specificity and can be used in various standard laboratory application techniques. The lamprey antibodies are novel reagents that can complement conventional monoclonal antibodies in multiple scientific research disciplines.
1. Introduction
The cardinal elements of the adaptive immune system, including the B cell receptor, T cell receptor and MHC molecules are found in all jawed vertebrates, but not in jawless vertebrates (Cooper and Alder, 2006). Although studies suggesting an adaptive immune system in the evolutionary distant jawless vertebrates were conducted almost 50 years ago (Finstad and Good, 1964), the molecular components of the agnathan adaptive immune system were discovered only recently (Pancer et al., 2004). Sequence analyses of transcripts expressed by lymphocyte-like cells of sea lamprey larvae immunized with a cocktail of plant mitogens and particulate antigens led to the discovery of the variable lymphocyte receptor (VLR) B genes, which encode antigen receptors in jawless vertebrates. VLRA and VLRC genes were described in subsequent studies (Rogozin et al., 2007; Guo et al., 2009; Kasamatsu et al., 2010), accentuating the complexity of the adaptive immune system of jawless vertebrates.
Unlike mammalian antibodies which use the immunoglobulin-fold as basic structural unit and are composed of individual heavy and light chains, VLR antibodies are decameric protein complexes generated by iteration of a single polypeptide chain containing beta-sheet forming leucine-rich repeats (LRR) as basic structural units (Pancer et al., 2004). An incomplete VLR gene in germline configuration is flanked by a large number of LRR cassettes, which are copied into the maturing VLR gene by a gene conversion-like process (Alder et al., 2005; Rogozin et al., 2007). The mature VLR gene consists of a signal peptide, a capping N-terminal LRR, followed by a conserved LRR1 unit, 1–9 variable LRRv units, a capping C-terminal LRR unit and a stalk region, the latter being necessary for cell surface expression of the VLR antibody and for multimerization of the secreted gene product (Pancer et al., 2004; Herrin and Cooper, 2010). Our initial studies on monoclonal VLR antibodies demonstrated the high degree of specificity with which VLR antibodies detect their antigen (Herrin et al., 2008). This specificity is in accordance with a combinatorial VLR repertoire predicted to exceed 1014 individual antibody sequences (Rogozin et al., 2007).
Structural analyses of three monoclonal VLR antibodies complexed to their respective antigens revealed a solenoid shape of the individual VLR unit with the antigen interacting region located at the inner concave surface of the protein (Han et al., 2008; Velikovsky et al., 2009; Kirchdoerfer et al., 2012). Importantly, the antigen also makes contact with residues located in a flexible and highly variable loop structure that protrudes from the capping C-terminal LRR unit. In the first solved structure, the VLR antibody forms a pocket for the comparatively small erythrocyte H-trisaccharide antigen between the relatively rigid parallel beta-sheets of the VLR backbone and the flexible C-terminal loop sequences (Han et al., 2008). In a second study, a hen egg lysozyme (HEL)-specific VLR antibody was shown to bind the antigen by inserting the C-terminal VLR loop into the active site of the enzyme in addition to forming interactions with residues located in the LRR backbone of the VLR antibody (Velikovsky et al., 2009). Importantly, these structural analyses indicate that antigen recognition by VLR antibodies is distinct from antigen recognition by conventional immunoglobulin-based antibodies. The unique origins and structural characteristics of VLR antibodies suggest that these proteins have the potential to complement conventional antibodies in biomedical research applications and for biomarker discovery studies. Here we describe the generation of monoclonal VLR antibodies to human T lineage lymphocytes and demonstrate applicability of monoclonal VLR antibodies for affinity purification and mass spectrometric identification of the cell surface antigens.
2. Materials and methods
2.1 Animals and immunizations
Lamprey larvae (80–100 mm, Lamprey Services, Ludington, MI) in length were anesthetized (0.1g/l MS222/0.14g/l sodium bicarbonate) and immunized with 2×106 primary lymphocytes enriched for CD4+ T cells in 60μl of 0.66x PBS. The animals were boosted twice at 2 week intervals with an equal number of cells obtained from different donors to avoid the generation of alloantigen-specific VLRs. 10 days after the second boost the animals were sacrificed (1g/l MS222/1.4g/l sodium bicarbonate) followed by exsanguination. Peripheral blood was collected in 0.66xPBS/30mM EDTA, layered on top of 55% percoll and subjected to density centrifugation (400×g, 20 min). Subsequently, the lamprey lymphocytes were collected and the antisera were analyzed for reactivity to primary human PBMC. Out of 3 immunized animals, we chose the animal with the highest polyclonal VLR antibody titer for subsequent expression library generation.
2.2 Human tissues
Peripheral blood was obtained from healthy volunteers of the Vaccine Center of Emory University, Atlanta, GA after informed consent was obtained. Tonsil samples were obtained from Children’s Healthcare of Atlanta and chronic lymphocytic leukemia (CLL) samples from Emory University tissue procurement facility. All studies with human tissues were approved by the Institutional Ethics Review Board and were conducted in accordance with institutional guidelines and the declaration of Helsinki.
2.3 Cells and reagents
Tonsilar single cell suspensions were generated by tissue mincing, filtration through 70 μm wire mesh, and cell centrifugation on a ficoll-hypaque gradient. Blood CD4+ T cells were purified using CD4 microbeads (Miltenyi Biotec, Cambridge, MA) followed by magnetic separation. Hemopoietic cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin/streptomycin, and 50 μM β-mercaptoethanol and HEK293T cells were maintained in DMEM supplemented with 10% fetal calf serum, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin. Antibodies to CD3, CD5 and CD19 were obtained from BD-Biosciences (San Jose, CA). Secondary antibodies were obtained from Southern Biotech (Birmingham, AL) and the VLR-specific monoclonal antibody 4C4 was generated in our laboratory (Alder et al., 2005).
2.4 Generation of VLR expression libraries and recombinant VLR antibodies
RNA was extracted from purified lamprey lymphocytes using Qiagen RNeasy systems (Qiagen, Valencia, CA). Total RNA was used as a template for subsequent random-primed cDNA generation (SuperScript III, Invitrogen, Grand Island, NY), followed by amplification of VLR sequences with gene specific oligonucleotides located in the signal peptide (5′-ATATGCTAGCCACCATGTGGATCAAGTGGATCGCCACGC-3′) and stalk region (5′-ATATACCGGTTCAACGTTTCCTGCAGAGGGCG-3′) of the VLR gene. The amplified gene sequences were digested with the Nhe I and Age I restriction enzymes and cloned into the expression vector pIRESpuro2 (Invitrogen, Grand Island, NY). To generate HA/6xHis-tagged VLR antibodies and monomeric VLR antibodies we used the alternative antisense primer sequences 5′-ATATACCGGTTGGGCATTTCGAGGGGCTAGTGCT-3′ and 5′-TATACCGGTTCAGGGTTTCTGGGTTGTGATCAC-3′, respectively. VLR expression constructs were transfected into 293T cells using polyethylenimine (PEI) at a ratio of 3μg PEI:1μg DNA as described (Reed et al., 2006). 3 days after transfection, the supernatant was harvested and used for staining of primary cells and cell lines. Alternatively, 293T cells transfected with HA/6xHis-tagged VLR clones cells were subjected to treatment with puromycin (1μg/ml) and supernatant from puromycin-resistant cells was used for purification of recombinant VLR proteins using Ni-NTA columns followed by elution with 150mM imidazole.
2.5 Flow cytometric screen of VLR expression library
PBMC were incubated with VLR containing supernatants from transfected 293T cells for 30 min on ice. The cells were washed 2x with PBS/1% BSA followed by incubation with mouse monoclonal antibody (4C4) with VLR specificity at a concentration of 6 μg/ml in PBS/1%BSA for 15 min on ice. Subsequently the cells were washed 2x and incubated with goat anti-mouse PE-labeled secondary antibody. Following this step, the cells were blocked extensively in 5% normal mouse serum, stained with anti-human CD3 and CD19 monoclonal antibodies and analyzed on a FACS CyAN instrument (Dako Cytomation, Carpinteria, CA). FACS data were analyzed using FloJo software. As negative control we used the monoclonal VLR4 antibody that specifically reacts with the BclA antigen of B. anthracis (Herrin et al., 2008).
2.6 Western blotting and immunoprecipitation
Western blotting and immunoprecipitation experiments with Jurkat cells and transfected 293T cells were performed as described previously with minor modifications (Ehrhardt et al., 2005). Briefly, cells were pelleted and resuspended in lysis buffer containing 1% Nonidet P-40, 50 mM Tris·HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, and the protease inhibitors leupeptin (5 μg/ml), pepstatin (1 μg/ml), aprotinin (5 μg/ml), PMSF (40 μg/ml). The whole cell lysates were incubated with 20μl of a 50% slurry of protein G beads (GE Biosciences) which were pre-coated with anti-HA antibody 12CA5 and the indicated monoclonal VLR antibodies. After over-night incubation at 4°C the beads were washed 3–5 times with lysis buffer and either further processed for mass spectrometric analysis or boiled in SDS-sample loading buffer. Immunoadsorbed proteins were resolved by SDS/PAGE before transfer to nitrocellulose membranes (PALL BioSciences, Ville St. Laurent, Quebec, Canada), which were probed with the indicated antibodies and visualized by using the ECL reagent (Millipore, Billerica, MA).
2.7 LC-MS/MS sample preparation and analysis
VLR32 immunoprecipitates and control precipitates consisting of Jurkat cell lysates incubated with anti-HA antibodies and protein G beads were eluted in 8 M urea/100 mM ammonium bicarbonate at 95 °C. Eluates were reduced with 10 mM DTT for 20 minutes at 60 °C, allowed to cool at room temperature, and alkylated with 10 mM iodoacetamide for 15 minutes at room temperature in the dark. Samples were diluted 4-fold in 100 mM ammonium bicarbonate to reach a concentration of ≤2 mM urea prior to overnight proteolytic digest with 10 mg/ml trypsin at room temperature. The resulting tryptic peptide samples were acidified with trifluoroacetic acid at a final concentration of 1 % prior to desalting and purification using offline C18 reverse-phase chromatography. Samples were then dried in a vacuum centrifuge and re-dissolved in 0.1 % formic acid for LC-MS/MS analysis. Inline C18 reverse-phase chromatography was performed over a 120-minute gradient using an integrated nano-LC system (Easy-nLC, Proxeon Biosystems A/S, Odense, Denmark), coupled to a linear ion trap-Orbitrap hybrid mass spectrometer instrument (LTQ-Orbitrap, Thermo, San Jose, CA). Profile mode MS spectra were acquired at a 60,000 full-width half-maximum (FWHM) resolution in the Orbitrap whereas MS/MS spectra were acquired in the linear ion trap.
2.8 Data base search and protein identification
Tandem mass spectra were extracted from the raw data files (.RAW) using Mascot (Matrix Science, London, UK; version Mascot) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1)) engines to search the ipi.HUMAN.v3.87 database (91464 entries) assuming trypsin digest and allowing a maximum of 1 miss cleavage. Search was performed with a fragment (MS/MS) ion mass tolerance of 0.50 Da and a parent (MS) ion tolerance of 10.0 PPM. Carbamidomethylation of cysteine was specified as a fixed modification and oxidation of methionine was specified as a variable modification. Scaffold (version Scaffold_3.3.1, Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they exceeded specific database search engine thresholds. Mascot identifications required at least ion scores must be greater than both the associated identity scores and 20. X! Tandem identifications required at least -Log(Expect Scores) scores of greater than 2.0. Protein identifications were accepted if they contained at least 2 identified peptides. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
3. Results
In an effort to generate monoclonal VLR antibodies against human T lineage cells we immunized lamprey larvae with CD4+ T cells purified from peripheral blood samples. We screened 151 monoclonal VLR antibodies for binding to peripheral blood mononuclear cells (PBMC). Twelve clones recognized various populations of PBMCs in flow cytometry analyses (Table 1). Three of these (VLR6, VLR32 and VLR97) displayed a T cell-specific binding pattern, whereas the remaining 9 monoclonal VLR antibodies also bound to other PBMC populations. Because of the comparatively weak T cell-specific signal obtained with VLR6 and VLR97 (data not shown) we selected VLR32 for further analysis (Fig. 1A). This monoclonal VLR antibody contains 3 LRRV segments and is thus slightly larger than the average VLR protein (Fig. 1B). To facilitate purification and manipulation of this reagent in further studies, we engineered the 6xHis tag and the HA epitope tag into the invariant C-terminus of the VLR molecule. Inclusion of these sequences did not alter its binding specificity (data not shown).
Table 1.
Screening results of monoclonal VLR antibodies to PBMCs
| VLR clone | T cells | B cells | non B/T cells | monocytes |
|---|---|---|---|---|
| VLR6 | + | − | − | − |
| VLR18 | + | + | + | − |
| VLR25 | + | + | + | − |
| VLR32 | + | − | − | − |
| VLR33 | + | − | +1 | − |
| VLR37 | + | − | +1 | − |
| VLR73 | + | − | +1 | − |
| VLR87 | + | + | + | − |
| VLR97 | + | − | − | − |
| VLR99 | − | − | − | + |
| VLR109 | + | − | +1 | − |
| VLR139 | + | + | + | − |
T cells: CD3+, B cells: CD19+, non B/T cells: CD3−/CD19−, monocytes identified by FSC/SSC profile.
indicates partial staining of the cell population.
Figure 1.

Representative staining patterns of monoclonal VLR antibodies and VLR32 protein sequence. (A) Immunofluorescence staining of PBMC with monoclonal VLR32, VLR37 and VLR87. VLR binding to B cells (CD19+), T cells (CD3+) and non-B/T cells (CD19−/CD3−) is depicted by a black histogram. Staining with negative control VLR4 (specific for B anthracis BclA) is indicated by a filled grey histogram. (B) Schematic and protein sequence of human T cell binding VLR32. Note the inclusion of the HA and 6xHis epitope tags that were engineered into the invariant stalk region to facilitate purification and detection of recombinant VLR antibodies.
We further defined the binding specificity of the monoclonal VLR32 antibody by immunophenotyping panels of cell lines and primary cells with this reagent. Among the different cell lines tested, only T lineage cell lines stained positive for VLR32 binding (Fig. 2A). Similarly, T lineage cells from peripheral blood reacted with this antibody (Fig. 1A). When the reactivity of this VLR antibody was examined against tissue-based lymphocytes in the tonsils, the VLR32 antibody recognized all of the tonsilar CD3+ cells (Fig. 2B). In addition, a small population of tonsilar B lineage cells also bound VLR32 (Fig. 2B top row, center), a potentially informative observation in that a subpopulation of tonsilar B cells co-expresses the CD5 antigen (Fig. 2B, top row, right) (Dono et al., 1996; Dono et al., 2007). Indeed, B cell reactivity of the VLR32 antibody was found to be restricted to CD5 expressing cells (Fig. 2C). Furthermore, when we tested reactivity of monoclonal VLR32 with primary malignant CLL cells, which characteristically express the CD5 antigen, all CD5+ CLL cells from two patients were detected (Fig. 2C, right panel). These results demonstrated selective reactivity of monoclonal VLR32 antibody with cells expressing the CD5 antigen.
Figure 2.
Reactivity of VLR32 with cells expressing human CD5. (A) Immunofluorescence staining of human hemopoietic cell lines with VLR32. VLR32 binding is depicted by a black histogram. Staining with the control lamprey VLR4 antibody is indicated by a filled grey histogram. (B) Staining of tonsilar CD19+ B cells (top row) and CD3+ T cells (bottom row) with negative control VLR4 (left), VLR32 (center) or monoclonal mouse anti-human CD5 antibody (right). Shown is a representative of 4 analyzed tonsil samples. (C) Co-staining of VLR32 or VLR4 with monoclonal mouse anti-human CD5 antibody. Shown are representatives of 4 analyzed tonsilar tissue samples and 2 analyzed B-CLL samples.
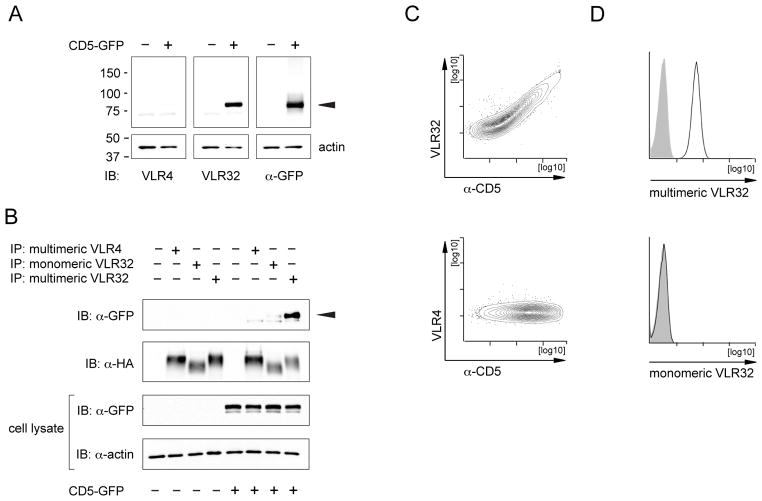
Our analysis thus indicated that the antigen detected by this VLR antibody is either CD5 or an antigen that is co-expressed with the CD5 antigen. To determine the identity of the antigen recognized by VLR32, we used this VLR antibody to immunoprecipitate the antigen from Jurkat T cells followed by tandem mass spectrometry. The precipitates were digested with trypsin and the resulting tryptic peptides were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for identification. A total of 10 peptide fragmentation spectra from 9 different peptides were assigned to CD5. These peptides corresponded to protein sequence coverage of 26%, allowing its identification with a confidence value of over 99%. Importantly, CD5 was one of only two proteins identified with at least 2 peptides specifically in the VLR32-containing IP compared to the ‘minus-VLR32’ negative control. The other identified protein, myosin-9 was only identified with 2 spectra from 2 unique peptides, corresponding to a sequence coverage of only 1%. Using this approach we determined that VLR32 immunoprecipitations contained the CD5 antigen (Table 2). We verified these results in parallel experiments testing the reactivity of VLR32 with cells transfected with CD5-GFP fusion constructs, by western blot analysis and immunoprecipitation experiments. VLR32, but not the negative control VLR4, was able to detect CD5-GFP fusion proteins in cell lysates from transiently transfected HEK293T cells (Fig. 3A). This reactivity was limited to lysates separated under non-reducing conditions as separation of cell lysates under reducing conditions abolished VLR32 binding (data not shown). In additional experiments, we demonstrated that VLR32 but not the negative control VLR4 precipitated CD5-GFP fusion proteins from cell lysates of transiently transfected HEK293T cells (Fig. 3B) and that VLR32 but not VLR4 stained HEK293T cells transfected with CD5 expression constructs in flow cytometry experiments (Fig. 3C). These experiments demonstrate the CD5-specificity of VLR32.
Table 2.
Mass spectrometric analysis of VLR32 immunoprecipitates.
| Rank | Access. No. | Protein | Unique peptides | Total peptides | ||
|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | |||
| 1 | IPI000253 | CD5 T-cell glycoprotein | 9 | 0 | 10 | 0 |
| 2 | IPI000195 | Isoform 1 of Myosin-9 | 2 | 0 | 2 | 0 |
Numbers of identified peptides. (+), Immunoprecipitates performed in the presence of VLR32; (−), Immunoprecipitates performed in the absence of VLR32
Figure 3.
VLR32 specifically recognizes human CD5. (A) Western blot of lysates of 293T cells transfected with human CD5-GFP fusion constructs or untransfected control cells. Cell lysates were separated under non-reducing conditions and immunoblotted (IB) with the indicated antibodies. The position of the CD5-GFP fusion protein is indicated by an arrowhead. The bottom section of the membrane was probed with anti-actin antibodies to verify equal loading. (B) Immunoprecipitation of CD5-GFP fusion proteins with VLR32. Lysates of 293T cells transfected with human CD5-GFP fusion constructs or lysates of untransfected control cells were subjected to immunoprecipitation with multimeric VLR4, monomeric VLR32 or multimeric VLR32. The cell lysates (bottom panels) and immunoprecipitates (top panels) were separated by SDS-PAGE under reducing conditions and were probed with anti-GFP antibodies to detect CD5-fusion proteins, anti-HA tag antibodies to detect VLR proteins and anti-actin antibodies to verify loading of the whole cell lysates. An arrowhead indicates immunoprecipitated CD5-GFP fusion proteins. Shown is a representative of 4 independent experiments. (C) Detection of cell surface CD5 on transiently transfected HEK293T cells by VLR32. HEK293T cells were stained with VLR32, negative control VLR4, and mouse monoclonal anti-CD5 antibody to analyze binding specificity of VLR32. (D) Detection of cell surface CD5 on Jurkat T cells with multimeric but not monomeric VLR32. VLR32 binding is depicted by a black histogram. Staining of cells with multimeric negative control VLR4 is indicated by a filled grey histogram
Prior studies of VLR antibodies suggest that binding of the antibody to the antigen is avidity-based and that the affinity of the individual antigen-binding unit to the antigen is often comparatively low (Herrin et al., 2008; Kirchdoerfer et al., 2012). To investigate the affinity versus avidity-based binding of VLR32 to the CD5 antigen, we generated monomeric VLR32 antibodies by deleting the C-terminal 42 residues of the VLR antibody. As expected, the resulting individual VLR units displayed a slightly faster migration pattern compared to full-length VLR proteins (Fig. 3B). Only the multimeric VLR32 was able to bind to CD5 efficiently as shown for immunoprecipitation (Fig. 3B) and flow cytometry analyses (Fig. 3D). VLR binding was not detected by flow cytometry using the monomeric VLR32 (Fig. 3D) and only a weak signal was obtained for immunoprecipitated CD5-GFP using the monomeric VLR32 (Fig. 3B). These data indicate an avidity-based contribution to the binding of the VLR32 lamprey antibody to human CD5.
4. Discussion
In this study, we demonstrate for the first time that monoclonal lamprey VLR antibodies can be used for purification and mass spectrometry-based identification of cell surface expressed protein antigens. Unlike conventional immunoglobulin-based antibodies, VLR antibodies utilize their leucine-rich repeats as basic structural units, resulting in a fundamentally different protein architecture of antigen receptors. Structural analyses of monoclonal VLR antibodies specific for the erythrocyte H-trisaccharide, HEL and Bcl-A coat protein display antibody-antigen interactions that are distinct from antigen-antibody interactions observed for conventional mammalian antibodies. A flexible loop-structure protruding from the C-terminal LRR capping unit of the VLR antibody forms a pocket for the relatively small H-trisaccharide antigen which interacts with residues in the located in the inner concave surface of the VLR antibody and the C-terminal loop. On the other hand, the C-terminal loop interacts with residues located in the active site of HEL, an epitope location to which it is notoriously difficult to raise conventional immunoglobulin-based antibodies, which preferentially interact with planar epitopes. We hypothesize that the unique origins and protein architecture of VLR antibodies will render these novel reagents uniquely suited for biomarker discovery. Key to using monoclonal VLR antibodies for this purpose will be their applicability for the capture and purification of protein antigens. Using monoclonal VLR32 antibody, we demonstrate that lamprey antibodies can be used effectively for immunoprecipitation applications followed by mass spectrometric protein identification. The inability of a monomeric form of the VLR antibody to bind to Jurkat T cells indicates its low affinity, in keeping with recent analyses indicating a Kd of 3.0×10−6M for monomeric units of the VLR4 antibody (Kirchdoerfer et al., 2012). However, our data show that a low affinity of the individual antigen-binding unit to the antigen does not impede the use of multimeric VLR antibodies for protein purification. We observed a weak signal for CD5-GFP fusion proteins in immunoprecipitation experiments using monomeric VLR antibodies, which is likely due to ‘artificial’ multimerization of these VLR units upon binding to protein G beads. This type of ‘artificial’ multimerization would not occur in flow cytometry assays where we detected no residual binding activity of the recombinant monomeric VLR32 units.
CD5 positive human B cells have been described previously in tonsilar tissues (Fischer et al., 1997) and other reports indicate a comparable proportion of peripheral blood B cells (10–25%) expressing the CD5 antigen (Gadol and Ault, 1986; Ebeling et al., 1993). While tonsilar CD5-positive B cells were readily detected using monoclonal VLR32, we did not detect B cells that bound VLR32 in our initial screen of the VLR library on PBMCs. However, in subsequent experiments we observed a significant inter-person variability of CD5+ cells in blood and found that VLR32 can recognize CD5+ B cells in blood (data not shown), suggesting that the lack of VLR32-binding B cells in our original screen is likely reflective of a donor sample devoid of a substantial CD5+ B cell population.
In conclusion, we present monoclonal VLR antibodies as novel reagents for proteomics-based biomarker identification. The unique origins and protein architecture of these reagents raise the possibility that VLR antibodies may recognize antigens against which conventional antibodies cannot readily be generated due to structural or tolerogenic reasons. VLR antibodies may thus serve as valuable reagents for biomarker discovery and as complements in existing panels of conventional antibodies.
Acknowledgments
This study was supported in part by Canadian Cancer Society grant 2012-701054 to G. Ehrhardt, NIH grant 5U19AI096187-02 to G. Ehrhardt and M. Cooper and NIH grant 2R01AI072435-07 to M. Cooper.
References
- Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–3. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–22. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Dono M, Burgio VL, Colombo M, Sciacchitano S, Reverberi D, Tarantino V, Cutrona G, Chiorazzi N, Ferrarini M. CD5+ B cells with the features of subepithelial B cells found in human tonsils. Eur J Immunol. 2007;37:2138–47. doi: 10.1002/eji.200636887. [DOI] [PubMed] [Google Scholar]
- Dono M, Burgio VL, Tacchetti C, Favre A, Augliera A, Zupo S, Taborelli G, Chiorazzi N, Grossi CE, Ferrarini M. Subepithelial B cells in the human palatine tonsil. I. Morphologic, cytochemical and phenotypic characterization. Eur J Immunol. 1996;26:2035–42. doi: 10.1002/eji.1830260911. [DOI] [PubMed] [Google Scholar]
- Ebeling SB, Schutte ME, Logtenberg T. Peripheral human CD5+ and CD5- B cells may express somatically mutated VH5- and VH6-encoded IgM receptors. J Immunol. 1993;151:6891–9. [PubMed] [Google Scholar]
- Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–91. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstad J, Good RA. The Evolution of the Immune Response. 3. Immunologic Responses in the Lamprey. J Exp Med. 1964;120:1151–68. doi: 10.1084/jem.120.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Klein U, Kuppers R. Molecular single-cell analysis reveals that CD5-positive peripheral blood B cells in healthy humans are characterized by rearranged Vkappa genes lacking somatic mutation. J Clin Invest. 1997;100:1667–76. doi: 10.1172/JCI119691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadol N, Ault KA. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986;93:23–34. doi: 10.1111/j.1600-065x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–7. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, Boydston JA, Turnbough CL, Jr, Cooper MD. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci U S A. 2008;105:2040–5. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin BR, Cooper MD. Alternative adaptive immunity in jawless vertebrates. J Immunol. 2010;185:1367–74. doi: 10.4049/jimmunol.0903128. [DOI] [PubMed] [Google Scholar]
- Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci U S A. 2010;107:14304–8. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer RN, Herrin BR, Han BW, Turnbough CL, Jr, Cooper MD, Wilson IA. Variable lymphocyte receptor recognition of the immunodominant glycoprotein of Bacillus anthracis spores. Structure. 2012;20:479–86. doi: 10.1016/j.str.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–80. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–56. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, Aravind L, Pancer Z, Mariuzza RA. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol. 2009;16:725–30. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]