Abstract
The body's elemental/metal loads are known to exert essential influence in maintaining normal and abnormal metabolism leading to eventual pathology of some forms of cancer phenotypes. Accumulation of potentially toxic or nonessential trace metals has been observed but not highly noted as an active factor in toxicogenesis and in the development of many diseases including cancers. The compositional balance and distribution of trace metals in various body tissues are essential key players in homeostasis in life. To this end the etiology of diseases including cancer has been linked with the accumulation of potentially toxic or nonessential trace metals. However, scarce literature/experimental evidence exist as a scientific proof that metal concentrations play important role in the etiology and development of cancer phenotypes. The aim of this study was to investigate the differential relationship of metal concentrations and profiles in cancer and normal tissues from cadavers of humans. The originated hypothesis was that elemental/metal concentrations and profiles seen in post mortem will show significant differences between normal and cancer-derived tissues as well as between various tissue types in humans. This study also establishes critical elemental/metal profiles that may be relevant in providing correlations with the development of three major cancers. Normal human and tumor tissues of cadaverous lung, breast and liver tissues used in this study were obtained from US Biomax Company. Tissue samples were prepared using standardized digestion procedures necessary for use with the Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES). This equipment was utilized to determine the concentrations and profiles of 21 elements including Ag, Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Sb, Se, Sr, Tl, V, and Zn. Twelve major elements of Al, Ba, Ca, Cr, Cu, Fe, Mg, Na, Pb, Se, Sr, and Zn were found to be significantly different in term of their concentrations/profiles in normal and tumor tissues of human lung, breast and liver. These critical elements appeared to be respectively five to ten times more abundant in human lung and breast tumor than in their respective normal tissues. In contrast Ba, Cr, Cu, Fe, Zn, concentrations were shown to be lower in liver tumors than in normal liver tissues, and that Ca and Na appeared to be higher in human liver tumors than in normal liver tissues. Data analysis showed significant variations in elemental concentrations and profiles consistent with the hypothesis. It is concluded that metal/elemental homeostasis is essential for normal tissue function and that elemental variations and distributions are tissue specific as well as carcinoma specific. These results are promising and warrant further studies to confirm/exploit the possibility of manipulating elemental distribution and content as means for diagnosing/utility as therapeutic modalities in chronic human disease as well as cancer management.
Keywords: essential, non essential metal load, metal profile, cancer tissues, breast, lung, liver, ICP analysis, homeostasis
Introduction
Lung, liver, and breast cancers are highly prevalent in the United States [1-2]. Many studies have shown that homeostasis of metals/trace elements are very essential to the development of the cancer phenotype in many cancers. Essential trace elements are engaged in four major functions as stabilizers, elements of structure, essential elements for hormone function and cofactors in enzymes. Studies have also indicated a role for Ba through its toxicity and involvement in chronic disease with no established evidence in human cancers [3-6]. Calcium is recognized as a messenger in cell signaling and studies have established a role for Ca2+ as regulators and Ca channels were found to be different in their distribution and expression in cancer and normal human tissues [7-10. Elevated Fe causes free radicals that are linked to cancer, heart disease, aging, as well as liver and pancreatic damage [11-12]. It has also been found that Ni, Cu and Fe concentrations in cancerous human stomach to be significantly higher than those in non-cancerous stomach tissue samples, and also high Zn concentrations were found in both paired stomach samples.
Another evidence for the importance of metals and elemental discrepancy is provided through the upregulation of metallothioneins in many cancers [13, 37]. Sodium (Na) regulates blood pressure as well as having an established role in cellular homeostasis, however, there was no established role for Na and Vanadium (V) in human cancer [14-18]; in contrast a role for Zn was established in prostate cancer [19-21]. Generally literature review shows inconsistency regarding any correlation between metal concentrations and distribution of varieties of metals in normal and cancerous tissues in human subjects [23-26]. This study was intended to test the hypothesis that impaired element/metal homeostasis is important in the development and progression of cancer phenotypes and that metal body loads/profiles of cancer and normal phenotypes differ significantly in distribution and content of various tissues. It addresses two objectives: 1. To obtain experimental data from normal and cancer phenotypes and 2. To analyze and compare metal profiles from normal tissues to various tumor tissues obtained from human cadavers aimed at showing differential profiles for the two tissue types.
Among the 21 elements that were detected, Twelve (Al, Ba, Ca, Cr, Cu, Fe, Mg, Na, Pb, Se, Sr, and Zn) major elements were found to be statistically different in their concentrations and distribution between tumors and normal tissues. In the lung tumors samples six elements (Al, Cr, Cu, Fe, Na, and Zn) were found to be higher compared with cadaverous but normal tissues. In contrast to lung and breast tumors, liver tumors showed lower metal content for five elements (Ba, Cr, Cu, Fe and Zn) and higher concentrations of Ca, and Na in comparison to normal liver tissues. Based on experimental data we surmise and support the notions that trace metal element concentration and distribution play a substantial role in the homeostasis of normal tissue function to impact the development of various human cancers. We conclude that our studies showed statistically significant differences in the accumulation, combination and distribution of metals including trace elements within human tumor tissues that may pave the way in targeted interventions in diagnostic and therapeutic management of cancers.
Methods
Samples were obtained as processed tissue blocks representing human normal and tumor tissues from liver, breast and lung from US Biomax Inc., Rockville, MD. To avoid contamination, all handling of samples, liquids and open tubes was done in a clean air bench following OSHA regulations. Before use, the digestion beakers were immersed in 5% HNO3 24 hrs prior procedure [31]. Digestion procedures involved formalin-fixed blocks obtained from US Biomax. Tissue blocks were heated in oven at 60°C for 4 hours to melt paraffin wax. After that, tissue were immersed in xylene for 10 minutes twice followed by immersing them in 95% ethanol for 5 minutes twice and then tissues were dried at room temperature for 24 hours. For first digestion, all collected dried samples were weighted, divided and placed in 50ml beakers. Ten mls of concentrated 65 % Nitric Acid were added. Beakers were placed on hot plates for at least 3 hours or until all tissues in the beakers were completely dissolved and all liquid in the breakers were evaporated. For second digestion, a few drops of 30 % hydrogen peroxide were added when the beaker was still on the hot plate. All beakers were removed from the hot plate and allowed to cool down and dry completely. Ten mls of 5% Nitric Acid was added in each beaker, and transferred to test tubes (10 mls). for Inductively Coupled Plasma (ICP) analysis. A 5% solution of HNO3 was used for standard curves and instrument calibrations. Statistical analysis for levels of significance and normalization of data for comparisons used the SAS statistical Analysis Software (SAS 2008).
Results
Table 1 shows data for normal lung, liver and breast tissues; demonstrating no significant differences in Ba, Ni, Pb, Se, Mn and V. Nickel and V were detected at very low concentration and were respectively absent in liver and breast tissues. Calcium was detected at high levels and showed a 2-4 fold increase in the lung and liver tissues compared to the breast tissue. Chromium showed 2-5 fold increase in the liver and breast tissues compared to the lung tissues. Copper showed 2-4 fold increase in the breast and liver tissues compared to the lung tissue. Iron was detected at very high level and showed 12 fold increases in the liver compared to the lung and breast tissues. Magnesium was also detected at high levels and showed 5-14 fold increase in the lung and liver compared to the breast tissue. Sodium was detected at the highest level and showed 2-3 fold increase for the breast compared to liver and lung tissues and Sr showed 2-5 fold increase for the lung and liver compared to the breast tissues. Zinc was detected at the moderated level and showed a 2 fold increase in liver compared to both lung and breast tissues. Out of the 15 elements detected in normal human tissues. Al, Cr, Na, Ni and Pb were found to be highest in breast tissues. Barium, Ca, Cu, Fe, Mg, Mn, Sr, and Zn were highest in the liver. However, Se and V were found to be the highest in the lung tissue.
Table 1. Distribution of 15 elements: lung, liver and breast normal and cancerous tissue samples respectively.
| Element | Normal Lung μg/g | Lung Tumor μg/g | p value | Normal Liver μg/g | Liver Tumor μg/g | p value | Normal Breast μg/g | Breast Tumor μg/g | p value |
|---|---|---|---|---|---|---|---|---|---|
| Al | 5.33 ± 0.56 | 28.9 ± 3.87 | <.0001 | 5.72 ± 0.73 | 5.24 ± 1.26 | 6.30 ± 0.62 | 5.61 ± 0.77 | 0.026 | |
| Ba | 8.21 ± 2.57 | 5.26 ± 1.82 | 9.44 ± 4.69 | 5.18 ± 0.97 | 6.24 ± 0.59 | 5.26 ± 1.15 | |||
| Ca | 54.1 ± 13.9 | 52.30 ±12.72 | <.0001 | 119 ± 5.6 | 187 ± 27 | 26.3 ± 5.29 | 112 ± 37.75 | 0.0673 | |
| Cr | 0.49 ± 0.38 | 1.05 ± 1.52 | <0.058 | 1.31 ± 1.39 | 0.34 ± 0.12 | <.0001 | 2.44 ± 0.23 | 14.31 ± 3.89 | 0.1562 |
| Cu | 0.44 ± 0.44 | 0.78 ± 0.37 | 1.80 ± 0.29 | 0.38 ± 0.13 | <.0001 | 0.78 ± 0.53 | 3.21 ± 1.86 | ||
| Fe | 11.3 ± 2.04 | 150 ± 18.1 | <.0001 | 135 ± 31 | 9.35 ± 1.41 | 14.8 ± 4.91 | 164 ± 52 | <.0001 | |
| Mg | 25.13 ± 5.94 | 29.3 ± 20.84 | 0.0021 | 71 ± 37 | 77 ± 14.4 | 4.97 ± 0.93 | 55.7 ± 30.1 | 0.0021 | |
| Mn | 0.19 ± 0.07 | 0.25 ± 0.11 | 0.3 6 ± 0.17 | 0.16 ± 0.05 | 0.15 ± 0.03 | 0.63 ± 0.44 | |||
| Na | 64.9 ± 13.52 | 880 ± 113 | <.0001 | 73 ± 12.8 | 176 ± 0.05 | <.0001 | 196 ± 28 | 607 ± 190 | 0.0095 |
| Ni | 0.07 ± 0.05 | 0.20 ± 0.15 | Not detected | Not detected | 0.08 ± 0.02 | 0.85 ± 0.68 | |||
| Pb | 2.24 ± 2.28 | 0.25 ± 0.29 | 0.0010 | 2.50 ± 1.42 | 2.18 ± 0.75 | 3.21 ± 2.15 | 0.65 ± 0.85 | ||
| Se | 0.38 ± 0.41 | 0.44 ± 0.08 | 0.25 ± 0.18 | 0.18 ± 0.15 | 0.22 ± 0.13 | 0.38 ± 0.09 | |||
| Sr | 2.42 ± 1.73 | 2.43 ± 0.79 | 3.96 ± 1.58 | 3.06 ± 0.15 | 0.0009 | 0.70 ± 0.22 | 1.73 ± 0.69 | 0.0057 | |
| V | 0.06 ± 0.03 | 0.10 ± 0.10 | ------- | 0.05 ± 0.03 | 0.002 ± 0.44 | Not detected | Not detected | ----- | |
| Zn | 6.48 ± 0.97 | 62.3 ± 4.04 | <.0001 | 12.5 ± 1.33 | 8.04 ± 1.42 | <.0001 | 7.98 ± 0.38 | 36.7 ± 1.93 | 0.0004 |
Comparing the three tissues for individual elements showed that there is a 5 fold increase in Al in the lung compared to liver and breast tumor tissues both of which showed no differences in their Al content. Ca showed more than 2 fold increases in liver and breast compared to lung tissue. For Cr there is more than 14 fold increase in the breast tumor tissue compared to lung and liver tumors. There is also 4-8 fold increase in Cu in breast tumor compared to lung and liver tissues. There is 15 fold increase in Fe content for lung and breast tumor tissues compared to liver tumor tissues. There is also 2-2.5 fold increase in Mg content for breast and liver compared to lung tumor tissues. Although, Mn content was low it showed 1.5-4 fold increase for lung and breast compared to liver tumor tissues. Sodium content showed the highest content in lung tumor tissues. There is a 3-5 fold increase in content for breast and lung in comparison to liver tumor tissues. For Ni the content is generally low and is absent in the liver tumor tissues. There is a 4-8 fold increase in Ni content for breast compared to lung and liver tumor tissues. Lead showed 2-8 fold increase in breast and liver compared to lung tumor tissues. Selenium was detected at low concentration but did not show significant difference within the three tumor tissues and Sr was detected but showed no significant difference in content in all three tumor tissues. Vanadium was detected at very low concentration in liver and lung and was not detected in the breast tumor tissues. Zinc showed highest level and 4-8 fold increase in breast and lung compared to liver tumor tissues. Among 21 elements tested by ICP in normal and tumor human tissues, twelve (Al, Ba, Ca, Cr, Cu, Fe, Mg, Na, Pb, Sr, Zn) showed statistically significant differences, four (Mn, Ni, Se, V) were detected at the low level but showed statistically significant differences, and six (Ag, As, Cd, Co, Sb, Tl were variably detected at low level in various tissues but were not statistically significant.
Table 2 shows the ranking of element levels between tumor and normal tissues of the same and different organs. As can be seen, Na ranked highest in both lung and breast tissues as well as liver tumor tissues. In contrast, Fe ranked highest in the normal liver tissue and second in ranking in both lung and breast tumor tissues and third in the normal breast and liver tumor tissues. Calcium ranked first in the liver tumors and second in normal breast, normal liver and liver tumors and ranked third in the normal lung and breast tumor tissues. Zn ranked third in lung tumor tissues and fourth and fifth in normal breast, normal liver and liver tumor tissues. It also ranked fifth in breast tumor while Mg ranked fourth in this tumor tissue. Al ranked ninth and Cu ranked tenth in both normal and tumor lung tissues.
Table 2.
Comparative Ranking of Elements Low to Highest in Samples.
| Tissue Type | Metal Element Sequence |
|---|---|
| Normal Lung Lung Tumor |
Na, Fe, Ca, Mg Ba, Pb, Sr, Zn, Al, Cu, Cr Na, Fe, Zn, Ca, Mg, Al, Ba, Sr, Cu, Cr |
| Normal Breast Breast Tumor |
Na, Ca, Fe, Zn, Ba, Mg, Pb, Cr, Sr, Cu, Sr, Se Na, Fe, Ca, Mg, Zn, Cr, Al, Ba, Cu, Sr, Pb, Mn, Se |
| Normal Liver Liver Tumor |
Fe, Ca, Na, Mg, Zn, Al, Sr, Pb, Ba, Cr, Cu Ca, Na, Mg, Fe, Zn, Al, Ba, Se, Sr, Pb, Cu, Cr |
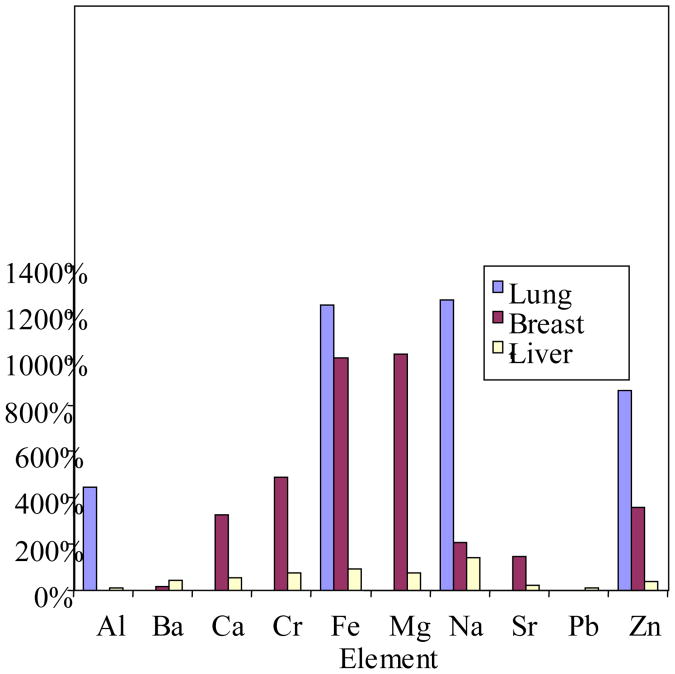
Al was detected in both breast and liver tissues. Ba was detected only in both liver tissues. Fe and Na as well as Cu and Cr were reversed in rank between normal and tumor liver tissues. Lead (Pb) was detected in both breast tissues and as well, Se, Mg, and Sr. Na, Fe, Zn and Cu were detected at various concentrations in all tissues. Cr was detected in five tissues excluding normal lung tissues. Fig 1 showed the normalized profile of three different tumor tissues in comparison to each other. As can be seen, each tumor tissues has peculiar pattern of elemental profile indicating that tumor development in various tissues depends on a different pattern of elemental distribution/content and also indicates different mechanisms of metal metabolism within these three tumor tissues.
Figure 1. Percentage differences of significant metal Concentrations in lung, breast, and liver tissues (Normalized).
Discussion
The main aim of this study was to evaluate the levels of metal element concentrations in the lung, breast and liver tissues as possible factors involved into the development or support the progression of lung, breast, and liver cancer. Cancer progression is a complex multi-step process that consists of transformation, tumor growth, invasion and metastasis. Processes involved in element metabolism and homeostasis are related to cancer development and progression. The clinical/biological testing and interpretation of the role of trace elements in cancer and analytical technology are currently available to measure and profile elements in human tissues. Our data showed six elements (Al, Cr, Cu, Fe, Na, and Zn) were found in extremely higher concentrations in human lung tumor tissues compared with normal human lung tissues. Literature data [27-30] support that lung cancer patients tend to have a significant increase in serum Cu and Cu/Zn ratio levels and decrease in serum Zn and Fe concentrations. Thus lowers of copper and higher amounts of Zn and Fe are measured in tumor tissues.
Epidemiological studies also confirm that human exposure to soluble chromates also lead to significantly increased risk of lung cancer; this is confirmed in our studies where elevated Cr was noted [22-29]. Our results on the human breast tumor tissues have also shown that concentration of seven elements (Ca, Cr, Fe, Mg, Na, Sr, and Zn) were higher in breast tumor tissues. However, Pb concentration was lower compared with normal breast tissues. Samples of normal tissue (5 cm away from tumor) were also taken from cadavers with malignant tumors. The malignant tissue had the highest levels of four elements to support our findings for Fe and Zn [33-32 ]. Literature also supported that the regulation of intracellular calcium is an important signaling mechanism for cell proliferation in both normal and cancerous cells to support our findings with Ca [36-37].
In normal epithelial cells, free calcium concentration is essential for cells to enter and accomplish the S and M phases of the cell cycle. Pasha [9] demonstrated that the average concentrations of Cd, Co, Cr, Cu, Fe, Mn, K, Ca, and Zn were noted to be significantly higher in the malignant human breast tissues compared with the benign human breast tissues. In human liver tumor tissues, we found the concentration of Cr, Fe and Zn noticeably low compared to normal human liver tissues. Also, Cu and Ba are found to be lower in human liver tumor than in normal human liver. Only Na was found to be high in all human tumor tissues of the breast, lung and liver. The interaction between TAMs and cancer cells could enhance cancer cell growth, invasion, metastasis and angiogenesis by stimulating cancer cells or express multiple gene products that are involved in the regulation of tumor-associated angiogenesis, cell cycle, inflammation, signal transduction, invasion, and activities of protease and adhesion molecules [33-35]. While we have detected ten elements Na, Fe, Ca, Mg, Zn, Cr, Sr, Pb, Se, Cu, five of which (Ca, Cr, Cu, Fe, and Zn) were perfectly matched with the literature in term of their concentrations [36]. Moreover, it was clearly demonstrated that the concentration of various metal elements is relatively high in most human tumors. The decreasing ratio of Zn/Fe in human breast tumor was matched with the literature and that Cr was present in lung diseases and tumors in people who were exposed to this element [37].
Conclusion
Our studies targeted the analysis element/metal profiles of 21 elements using normal and tumor human tissues of lung, liver, and breast. Standardized digestion and ICP procedures were used to detect 21 elements including Ag, Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Sb, Se, Sr, Tl, V, and Zn. Analysis showed that the most represented elements (12) in human tumor tissue with high concentration were Al, Ba, Ca, Cr, Cu, Fe, Mg, Na, Pb, Se, Sr, and Zn content in most organs tested. These elements play essential physiological roles in homeostasis that is very essential to the development of the cancer phenotype in many cancers. Among elements with toxic properties, Na showed the highest concentration levels in all three of the human tumor tissues. Tumor metal profile in human lung tumor has shown that the concentrations of Al, Cr, Cu, Fe, Na, and Zn were significantly higher compared to human normal lung. Ten elements (Ca, Cr, Cu, Fe, Mg, Na, Se, Sr, Mn, and Zn) were found to be significantly high in human breast tumor compared to normal human breast. Ca and Na were found to be higher in the human liver tumors. Only Na was found to be high in all human tumor tissues of breast, lung and liver. Al, Na, and Zn were found to be at highest concentration in human lung tumor. However, Fe was highest in the human breast tumor and Ca had highest concentrations compared to the other human tumors, and Vanadium was not detected in human breast tumor, as well, nickel was also not detected in human liver tumors. We concluded that significant differences in the accumulation, combination and distribution of metals within human tumor tissues is elucidated and that it presents a great potential for exploiting this differential to establish diagnostic tools as well as targeted metal interference therapies for human cancers.
Supplementary Material
Acknowledgments
This research is supported by a grant from the National Institutes of Health (Grant # 1G12RR13459) through the NCRR-RCMI Center for Environmental Health at Jackson State University (JSU).
References
- 1.American Cancer Society. 2009 www.cancer.org.Inc.
- 2.Williams C. Lung Cancer: The Facts 2. Oxford University Press; 1995. Wikipedia. Lung Cancer. 22 May 2008. 22 May 2008. < http://en.wikipedia.org/wiki/Lung_cancer>. [Google Scholar]
- 3.De Palma G, Goldoni M, Catalani S, et al. Metallic elements in pulmonary biopsies from lung cancer and control subjects. Acta biomed. 2008;79(1):43–5. [PubMed] [Google Scholar]
- 4.IARC, International Agency for Research on Cancer. Chromium, nickel and welding IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 49. World Health Organization; Lyon, France: 1990. [Google Scholar]
- 5.Johnson CH, Van Tassell VJ. Ann Emerg Med. 10. Department of Internal Medicine, Maricopa Medical Center; Phoenix, Arizona 85010: 1991. Acute barium poisoning with respiratory failure and rhabdomyolysis; pp. 1138–42. [DOI] [PubMed] [Google Scholar]
- 6.Kerger BD, Finley BL, Corbett GE, Dodge DG, Paustenbach DJ. Ingestion of chromium (VI) in drinking water by human volunteers: Absorption, distribution, and excretion of single and repeated doses. J Tox Environ Health. 1997;50:67–95. doi: 10.1080/009841097160618. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso TF. Chromium as an industrial carcinogen: Part I. Am J Ind Med. 1997;31:1291–39. doi: 10.1002/(sici)1097-0274(199702)31:2<129::aid-ajim1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Nadadur SS, Srirama K, Mudipalli A, Badmaev Vladimir, Prakash Subbalakshmi, Majeed Muhammed. Iron transport & homeostasis mechanisms: their role in health & disease. The Journal of Alternative and Complementary Medicine. 2008;128(4):533–44. [PubMed] [Google Scholar]
- 9.Pasha Q, Malik Salman A, Iqbal Javed, Shaheen Nazia, Shah Munir H. Comparative Evaluation of Trace Metal Distribution and Correlation in Human Malignant and Benign Breast Tissues. Indian Med J. 2008:1559–0720. doi: 10.1007/s12011-008-8158-z. [DOI] [PubMed] [Google Scholar]
- 10.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–75. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 11.Sorahan T, Burges DC, Hamilton L, Harrington JM. Lung cancer mortality in nickel/chromium platers, 1946–95. Occup Environ Med. 1998;55:236–242. doi: 10.1136/oem.55.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xining He,1 Paul Hahn,1 Jared Iacovelli,1 Robert Wong,1 Chih King,1 Robert Bhisitkul,2 Mina Massaro-Giordano,1 and Joshua L. Dunaief Iron homeostasis and toxicity in retinal degeneration, F. M. Kirby Center for Molecular Ophthalmology, Scheie Eye Institute. 2007.07.004.
- 13.Siddiqui MK, Jyoti S, Singh S, Mehrotra PK, Singh K, Sarangi R. Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions: An Indian study. Environment International. 2006 Jul;32(5):630–637. doi: 10.1016/j.envint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Margalioth EJ, schenker IG, chevion M. Copper and Zinc Levels in Normal and Malignant Tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.wang X, Tian J, yin XM, Zhang X, Qin ZH, Wang Z. Distribution of Trace Elements in Normal and Tumor-bearing mice using the Multitracer Technique. Biological Trace Element Research. 2001;81:177. doi: 10.1385/bter:81:2:177. [DOI] [PubMed] [Google Scholar]
- 16.Geraki K, Farquharson MJ, Bradley DA. Concentrations of Fe, Cu and Zn in breast tissue, a synchrotron XRF study. Phys Med Biol. 2002;47:2327–2339. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 17.Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, et al. Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ Health Perspect. 2002;5:797–9. doi: 10.1289/ehp.02110s5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breast Cancer Metals Ionescu JG, Novotny J, Stejskal VD, Latsch A, Blaurock-Busch E, Eisenmann-Klein M. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol Lett. 2006;27(Suppl 1)
- 19.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9:1081–1084. doi: 10.1038/nm902. [DOI] [PubMed] [Google Scholar]
- 20.Kilic E, Saraymen R, Demiroglu A, Ok E. Chromium and manganese levels in the scalp hair of normal and patients with breast cancer. Biol Trace Elem Res. 2004;102(13):19–25. doi: 10.1385/BTER:102:1-3:019. [DOI] [PubMed] [Google Scholar]
- 22.Leonard S, Gannett PM, Rojanasakul Y, Schwegler-Berry D, Castranova V, Vallyathan V, et al. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J Inorg Biochem. 1998;70:239–244. doi: 10.1016/s0162-0134(98)10022-3. [DOI] [PubMed] [Google Scholar]
- 23.Leonard SS, Bower JJ, Shi X. Metal-induced toxicity, carcinogenesis, mechanisms and cellular responses. Mol Cell Biochem. 2004;255(1-2):3–10. doi: 10.1023/b:mcbi.0000007255.72746.a6. [DOI] [PubMed] [Google Scholar]
- 24.Martin MB, Reiter R, Pham T, Avellanet YR, Camara J, Lahm M, et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology. 2003;144(6):2425–3629. doi: 10.1210/en.2002-221054. Breast Cancer and Metals. [DOI] [PubMed] [Google Scholar]
- 25.Ostrakhovitch EA, Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–121. doi: 10.1007/s10495-005-6066-7. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui MK, Jyoti, Singh S, Mehrotra PK, Singh K, Sarangi R. Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions, an Indian study. Environ Int. 2006;32(5):630–7. doi: 10.1016/j.envint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Thompson HJ, Kennedy K, Witt M, Juzefyk J. Effect of dietary iron deficiency or excess on the induction of mammary carcinogenesis by 1- methyl-1-nitrosourea. Carcinogenesis. 1991;12(1):111–4. doi: 10.1093/carcin/12.1.111. [DOI] [PubMed] [Google Scholar]
- 28.Wong O, Harris F. Cancer mortality study of employees at lead battery plants and lead smelters, 1947-1995. Am J Ind Med. 2000;38:255–270. doi: 10.1002/1097-0274(200009)38:3<255::aid-ajim4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Majewska U, et al. Trace element concentration distributions in breast, lung and colon tissues. Phys Med Biol. 2007;52:3895–3911. doi: 10.1088/0031-9155/52/13/016. [DOI] [PubMed] [Google Scholar]
- 30.Silva M, Tomal CA, Perez A, Ribeiro-Silva A, Poletti ME. Determination of Ca, Fe, Cu, and Zn and their Correlations in Breast Cancer and Normal Adjacent Tissues. X-ray Spectrometry. 2008;38(2):103–111. [Google Scholar]
- 31.Millos M, Costas-Rodríguez M, Lavilla I, Bendicho J. Multiple Small Volume Microwave-assisted Digestions using Conventional Equipment for Multi-elemental Analysis of Human Breast Biopsies by Inductively Coupled Plasma Optical Emission Spectrometry. Talanta. 2009;77(4):1490–1496. doi: 10.1016/j.talanta.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 32.Alimonti A, Bocca B, Mattei D, Lamazza M, Fiori E. Composition of Essential and Non-essential Elements in Tissues and Body Fluids of Healthy Subjects and Patients with Colorectal Polyps. Int J Environment and Health. 2009;3, No. 2:224. [Google Scholar]
- 33.Da silva AB, Domingues OLA, Ribeiro-silva ZA, Poletti ME. Discriminant Analysis of Trace Elements in Normal, Benign and Malignant BreastTissues Measured by Total Reflection x-ray Fluorescence Spectrochimica Acta Part B: Atomic Spectroscopy. 2009;64(6):587–592. [Google Scholar]
- 34.Lavilla I, Costas M, Miguel P, Millos J, Bendicho C. Elemental Fingerprinting of Tumorous and Adjacent Non-tumorous Tissues from Patients with Colorectal Cancer using ICP-MS, ICP-OES and Chemometric Analysis. Biometals Springer Netherlands. 2009;22(6):863–875. doi: 10.1007/s10534-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 35.Farquharson MJ, Al-Ebraheem A, Geraki K, Leek R. Zinc Presence in Invasive Ductal Carcinoma of the Breast and its Correlation with Oestrogen Receptor Status. Phys Med Biol. 2009;54:4213–4223. doi: 10.1088/0031-9155/54/13/016. [DOI] [PubMed] [Google Scholar]
- 36.Moore AB, Shannon J, Chen C, Lampe JW, Ray M, Lewis SM, Mstalsberg H, Thoma DB. Dietary and Stored Iron as Predictors of Breast Cancer Risk: A Nested Case-Control Study in Shanghai. International Journal of Cancer Radiation Oncology Investigations. 2009;125(5):1110–1117. doi: 10.1002/ijc.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charian MG, Jayasurya A, Bay BH. Metallothioneins in Human Tumors and Potential Roles in Carcinogenesis. Mutat Res. 533(1-2):201–9. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.