Abstract
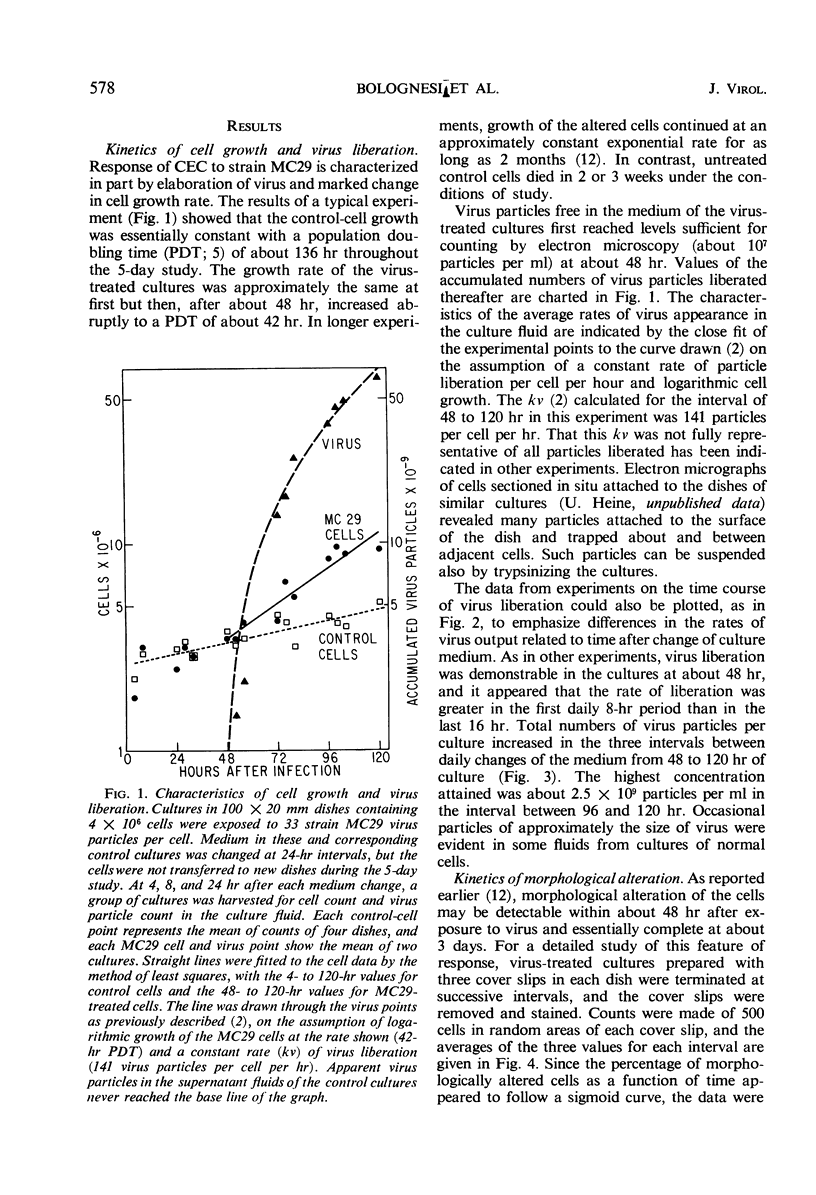
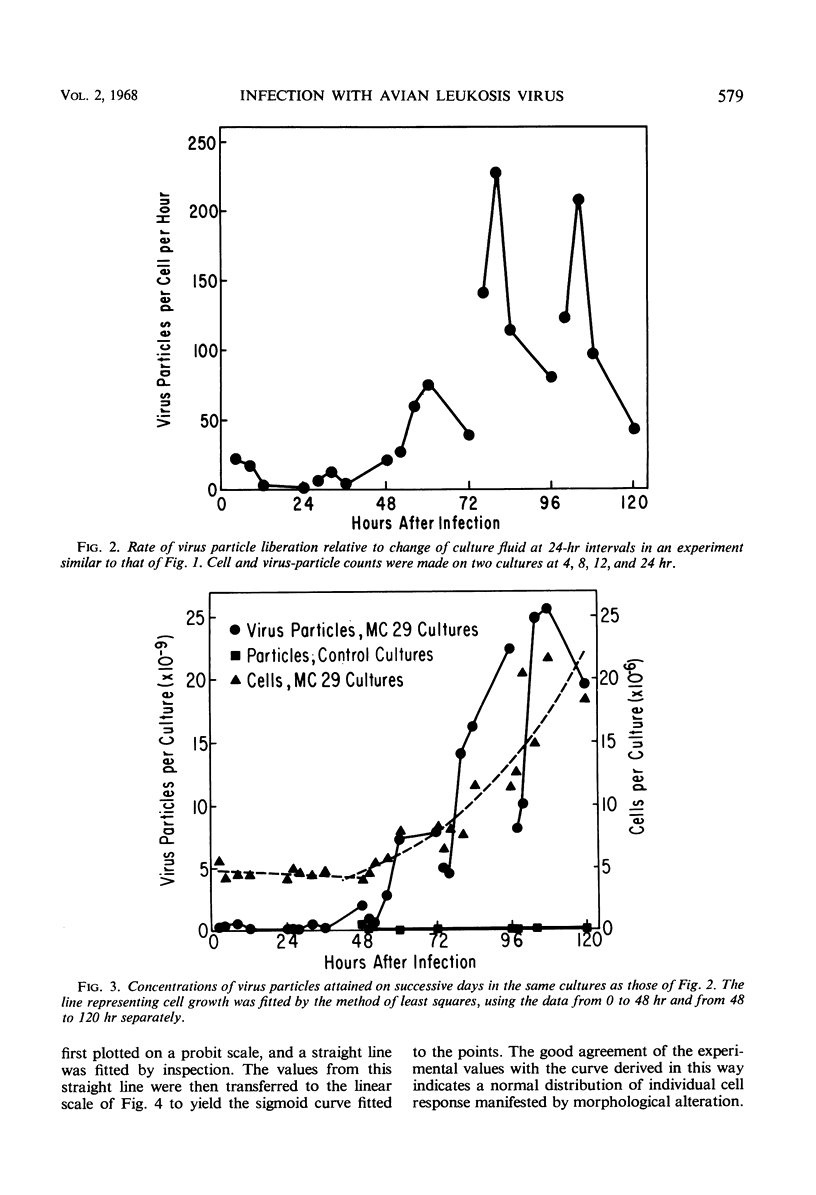
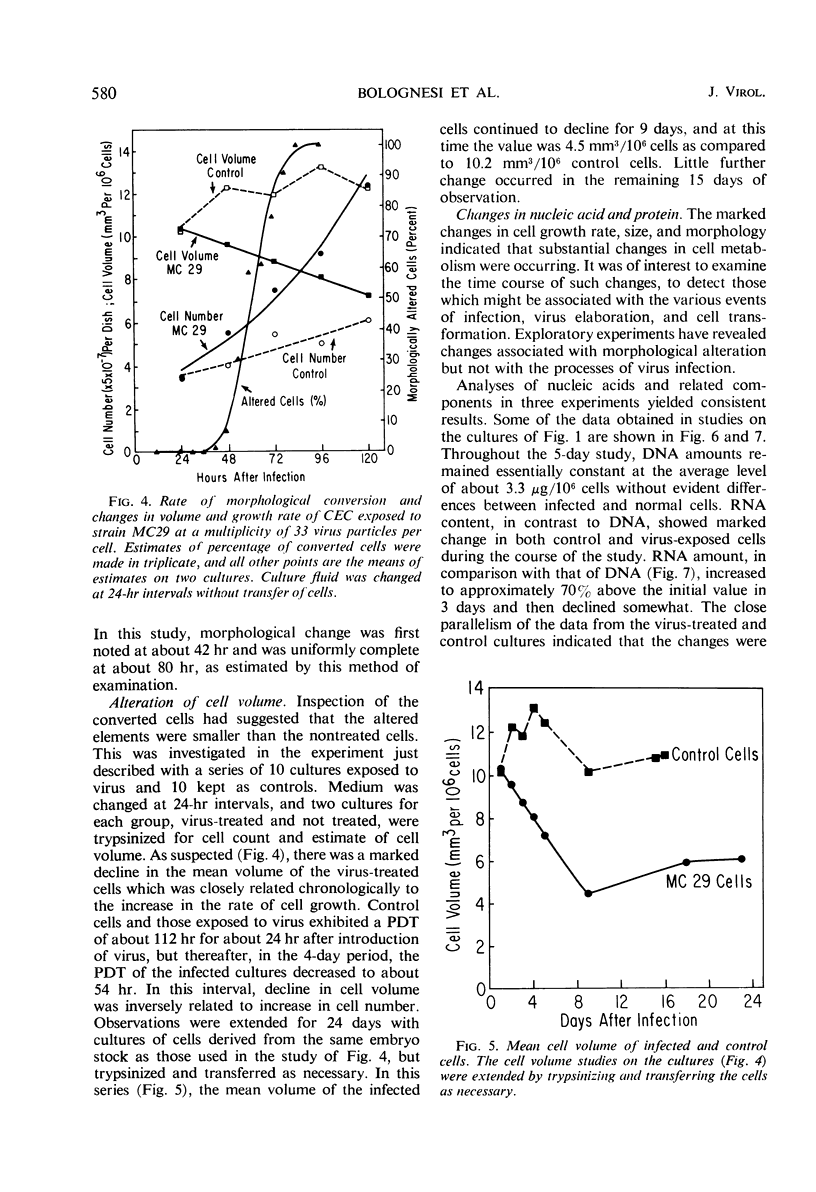
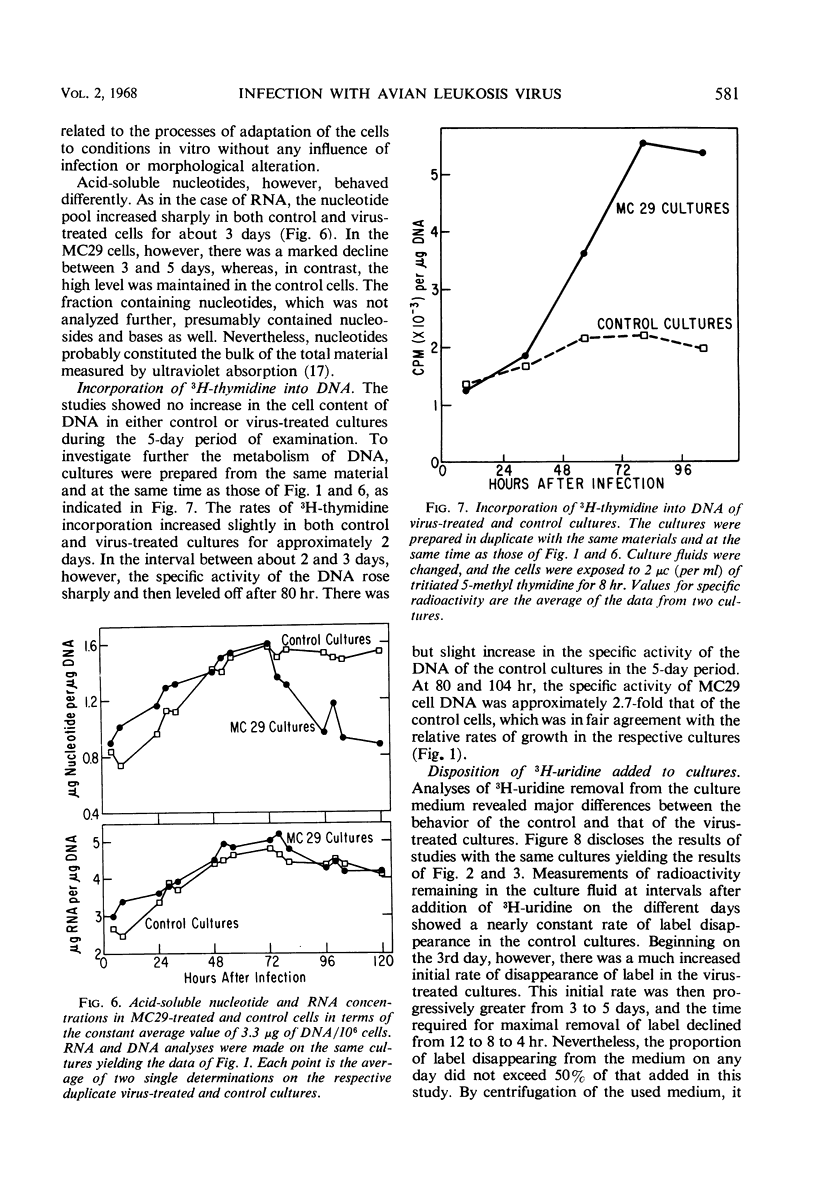
Strain MC29 avian leukosis (myelocytomatosis) virus induced infection, elaboration of virus, and morphological alteration in chick embryo cells in vitro. Virus liberation began within 18 hr, morphological change was detectable at about 40 hr, and the cultures could be completely altered within 80 hr after infection. Altered cells were about half the volume and grew at approximately twice the rate of uninfected elements. The output of virus estimated by electron microscopy was about 140 particles per cell per hr. Deoxyribonucleic acid remained constant, but ribonucleic acid increased in both infected and control cells in adjustment to culture environment. The rates of uptake and incorporation of 3H-uridine and the incorporation of 3H-thymidine increased in the infected cells with onset of morphological change but were unaffected by processes of infection and virus elaboration per se. Incorporation of a 14C-amino acid mixture was slightly greater in the infected than in control cells. The speed of continuity of infection and massive morphological alteration constitute a unique response to avian tumor viruses, and the system gives promise of singular value for detailed studies of the processes of infection and morphological change.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAUDREAU G. S., BECKER C., BONAR R. A., WALLBANK A. M., BEARD D., BEARD J. W. Virus of avian myeloblastosis. XIV. Neoplastic response of normal chicken bone marrow treated with the virus in tissue culture. J Natl Cancer Inst. 1960 Feb;24:395–415. [PubMed] [Google Scholar]
- BEAUDREAU G. S., BECKER C., STIM T., WALLBANK A. M., BEARD J. W. Virus of avian myeloblastosis. XVI. Kinetics of cell growth and liberation of virus in cultures of myeloblasts. Natl Cancer Inst Monogr. 1960 Sep;4:167–187. [PubMed] [Google Scholar]
- Dulbecco R. Production of Plaques in Monolayer Tissue Cultures by Single Particles of an Animal Virus. Proc Natl Acad Sci U S A. 1952 Aug;38(8):747–752. doi: 10.1073/pnas.38.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKERT E. A., BEARD D., BEARD J. W., BURMESTER B. R. Dose-response relations in experimental transmission of avian erythromyeloblastic leukosis. II. Host-response to whole blood and to washed primitive cells. J Natl Cancer Inst. 1953 Apr;13(5):1167–1184. [PubMed] [Google Scholar]
- GROUPE V., MANAKER R. A. Discrete foci of altered chicken embryo cells associated with Rous sarcoma virus in tissue culture. Virology. 1956 Dec;2(6):838–840. doi: 10.1016/0042-6822(56)90064-2. [DOI] [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., WU M. L., HIXON W. S., CRAWFORD E. J. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954 Mar;207(1):19–37. [PubMed] [Google Scholar]
- Langlois A. J., Sankaran S., Hsiung P. H., Beard J. W. Massive direct conversion of chick embryo cells by strain MC29 avian leukosis virus. J Virol. 1967 Oct;1(5):1082–1084. doi: 10.1128/jvi.1.5.1082-1084.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov Z., Heine U., Beard D., Beard J. W. Strain MC29 avian leukosis virus. Myelocytoma, endothelioma, and renal growths: pathomorphological and ultrastructural aspects. J Natl Cancer Inst. 1967 Mar;38(3):251–285. [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SEBRING E. D. The source of poliovirus ribonucleic acid. Virology. 1961 Feb;13:258–260. doi: 10.1016/0042-6822(61)90062-9. [DOI] [PubMed] [Google Scholar]
- SCOTT J. F., FRACCASTORO A. P., TAFT E. B. Studies in histochemistry. I. Determination of nucleic acids in microgram amounts of tissue. J Histochem Cytochem. 1956 Jan;4(1):1–10. doi: 10.1177/4.1.1. [DOI] [PubMed] [Google Scholar]
- SHARP D. G., BEARD J. W. Counts of virus particles by sedimentation on agar and electron micrography. Proc Soc Exp Biol Med. 1952 Oct;81(1):75–79. doi: 10.3181/00379727-81-19782. [DOI] [PubMed] [Google Scholar]
- Sundelin P. Microphotometric determination of DNA and RNA in single chick embryo fibroblasts during morphological transformation induced by Rous sarcoma virus. Exp Cell Res. 1967 Jun;46(3):581–592. doi: 10.1016/0014-4827(67)90383-7. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. A kinetic study of infection of chick embryo cells in vitro by Rous sarcoma virus. Virology. 1959 Jun;8(2):209–222. doi: 10.1016/0042-6822(59)90005-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- TRAVNICEK M., BURIC L., RIMAN J., SORM F. THE NUCLEOTIDE COMPOSITION OF THE RNA OF THE AVIAN MYELOBLASTOSIS VIRUS (BAI, STRAIN A) AND OF THE NUCLEIC ACIDS OF LEUKAEMIC MYELOBLASTS. (A COMPARATIVE STUDY). Neoplasma. 1964;11:571–584. [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. 3. The differential effect of serum and polyanions on multiplication of uninfected and converted cells. J Natl Cancer Inst. 1966 Aug;37(2):167–175. [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]
- Trager G. W., Rubin H. Mixed clones produced following infection of chick embryo cell cultures with Rous sarcoma virus. Virology. 1966 Oct;30(2):275–285. doi: 10.1016/0042-6822(66)90102-4. [DOI] [PubMed] [Google Scholar]
- Trager G. W., Rubin H. Rous sarcoma virus production from clones of nontransformed chick embryo fibroblasts. Virology. 1966 Oct;30(2):266–274. doi: 10.1016/0042-6822(66)90101-2. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. A rapid quantitative hematocrit method for measuring increase in cell population of strain L (Earle) cells cultivated in serum-free nutrient solutions. J Natl Cancer Inst. 1956 Sep;17(3):305–313. [PubMed] [Google Scholar]
- Zamecnik P. C. The mechanism of protein synthesis and its possible alteration in the presence of oncogenic RNA viruses. Cancer Res. 1966 Jan;26(1):1–6. [PubMed] [Google Scholar]



