Abstract
A recent clinical study demonstrated that damage to the insular cortex can disrupt tobacco addiction. The neurobiological mechanisms for this effect are not yet understood. In this study we used an animal model of nicotine addiction to examine the possibility that changes in insular cortex levels of dopamine (DA)- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32), a phosphoprotein enriched in DA neurons containing DA D1 receptors, may be associated with changes in vulnerability to nicotine addiction. Once rats acquired self-administration, they were given unlimited access to nicotine (0.01 mg/kg/infusion) for 23 h/day for a total of 10 days. Each infusion was paired with a visual cue (stimulus light) and auditory cue (sound of pump). Nicotine seeking, as assessed under a cue-induced reinstatement paradigm, and markers of DARPP-32 signaling, as assessed using western blot analysis, were examined in separate groups of rats at two different abstinent intervals: 1 and 7 days. Consistent with findings with other drugs of abuse, rats in the 7-day abstinence group took longer to extinguish and responded at higher levels during reinstatement testing as compared with rats in the 1-day reinstatement group. Relative to saline controls, rats in the 7-day but not the 1-day abstinence group had higher levels of DARPP-32 phosphorylated at the protein kinase A site in the insular cortex. These results demonstrate incubation of drug seeking following extended access to nicotine self-administration and suggest that enhanced protein kinase A signaling in the insular cortex via phosphorylation of DARPP-32 at Thr34 is associated with this effect.
Keywords: addiction, dopamine- and cAMP-regulated phosphoprotein of 32 kDa, rat, reinstatement, self-administration
Introduction
It was recently reported that smokers who have suffered damage to the insular cortex, usually from a stroke, were more likely to experience a decrease in, and in some circumstances elimination of, tobacco addiction (Naqvi et al., 2007). This result suggests that changes in the insular cortex may underlie changes in vulnerability to tobacco addiction, although very few studies have examined the role of the insular cortex in addiction studies. Results from imaging and inactivation/lesioning studies implicate the orbital and insular cortex in modulating the incentive value of reinforcers (Gallagher et al., 1999), including drugs of abuse (Volkow & Fowler, 2000; Fuchs et al., 2004; Contreras et al., 2007; Wang et al., 2007), and recent results show a strong association of insular cortex activation and abstinence-induced cravings to smoke (for review see Naqvi & Bechara, 2009). Parallel findings have recently been reported in a preclinical study on nicotine addiction with results showing that hypocretin transmission in the insula regulates nicotine reward and thus may be a potential neurobiological substrate for maintaining tobacco addiction (Hollander et al., 2008).
Another potential substrate is dopamine (DA)- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32). DARPP-32 is a bifunctional signal transduction molecule that is critical for the amplification of dopaminergic signaling. When phosphorylated by protein kinase A (PKA) at Thr34 it acts as a potent protein phosphatase-1 (PP-1) inhibitor which in turn can enhance or prolong the phosphorylation state of several other phosphoproteins and ultimately alter dopaminergic transmission (for review see Nairn et al., 2004). When phosphorylated at Thr75 [the brain-specific serine/threonine kinase-cyclin-dependent kinase 5 (CDK5)-site], it has an inhibitory effect on the phosphorylation of Thr34 by PKA that appears to serve as a negative feedback modulator of DA signaling through the DARPP-32/PP-1 signaling cascade. Although the majority of the work on DARPP-32 has been conducted on studies with cocaine, an accumulating body of evidence indicates that DARPP-32 is a major target for all drugs of abuse, including nicotine, and is known to be critically involved in other brain regions (e.g. nucleus accumbens) in mediating both drug-taking and drug-seeking behaviors (for review see Svenningsson et al., 2005). We do know that the insular cortex contains DARPP-32 (Berger et al., 1990; Ouimet et al., 1992) and recent work with cocaine suggests that PKA/DARPP-32 signaling in the insular cortex may play a key role in modulating drug seeking in response to drug-associated stimuli (Di Pietro et al., 2006, 2008). The possibility that alterations in DA PKA-regulated signaling of DARPP-32 in the insular cortex are associated with persistent changes in tobacco addiction is not yet certain.
Tobacco addiction has been modeled in animals using the self-administration paradigm. Self-administration of nicotine, the primary reinforcing component of tobacco, is an animal model with good face validity for human tobacco use (Perkins, 1999). When animals are allowed unrestricted access to nicotine, the levels that are self-administered are comparable to those observed in human smokers (Valentine et al., 1997). Importantly, after chronic nicotine self-administration, drug-abstinent animals, like humans, exhibit drug-seeking behaviors in response to cues that were formerly associated with the drug, a measure that is thought to correspond to relapse vulnerability (Shaham et al., 2003). Like the human situation where craving for the drug is reported to increase over the first few weeks of abstinence (Gawin & Kleber, 1986), drug seeking or reinstatement responding also increases, or incubates, over an abstinence period in animals (Grimm et al., 2001). Although numerous studies have been conducted describing the ‘incubation effect’ for cocaine- and heroin-seeking behavior (for review see Bossert et al., 2005), very little is known with regard to the neurobiological mechanisms of nicotine relapse.
The goals of the present study were to determine whether incubation of drug seeking occurs following extended access to nicotine self-administration and to determine whether changes in PKA-regulated signaling of DARPP-32 may be associated with this incubation effect. Changes were examined in the insular cortex and in two other brain regions implicated in drug addiction, the medial prefrontal cortex and nucleus accumbens. These mesolimbic structures are of particular interest in the long-lasting neurobiological alterations that may underlie vulnerability to relapse. Imaging studies in cocaine- and tobacco-dependent humans have associated changes in the prefrontal cortex and nucleus accumbens with abstinence-induced craving (Kalivas et al., 2005; Volkow et al., 2005; Wang et al., 2007) with parallel findings from rodent studies examining reinstatement and the incubation effect (Grimm et al., 2003; Conrad et al., 2008; Freeman et al., 2008; Koya et al., 2009). Based on previous work on the incubation effect with cocaine and heroin self-administration, changes were examined at two different abstinence intervals (1 and 7 days). We hypothesized that cue-induced nicotine seeking would be higher in rats in the 7-day abstinence group as compared with rats in the 1-day abstinence group and that PKA-regulated signaling of DARPP-32 would be associated with this behavioral effect.
Materials and methods
Subjects and surgery
Thirty-five adult male Sprague Dawley rats (Charles River Laboratories, Portage, ME, USA) weighing approximately 325 g at the start of the study were used as subjects. Upon arrival at the facility, rats were housed in individual operant conditioning chambers (ENV-018M; Med Associates, St Albans, VT, USA) with free access to food and water, and maintained on a 12-h light/dark cycle (room and houselights on from 07:00 to 19:00 h). After a 2-day acclimation period, rats were trained to lever-press for sucrose pellets (45 mg) during daily 23-h sessions beginning at 12:00 h. using methods previously described by Lynch (2008). Briefly, training sessions were initiated by the introduction of the left lever into the operant chamber and responding was reinforced under a fixed-ratio 1 schedule with sessions continuing until rats obtained a minimum of 50 pellets in a session (this typically occurred within the first three sessions). At this point, the left lever was retracted and remained retracted until self-administration training began. Each rat was then prepared with an indwelling jugular catheter (Silastic tubing; 0.51 and 0.94 mm o.d.; Dow Corning Corporation, Midland, MI, USA) in the right jugular vein under ketamine (60 mg/kg) and pentobarbital (5 mg/kg) anesthesia using methods previously described by Lynch & Carroll (2000). Upon recovery, rats were replaced in their individual operant chambers and, once grooming and eating behaviors resumed, behavioral testing was initiated (typically after 24 h of recovery). Rats were weighed every Monday, Wednesday and Friday, and for two consecutive days after surgery, and health was monitored daily. Catheter patency was tested for two consecutive days following surgery and thereafter every Monday, Wednesday and Friday by flushing the catheter with a small amount of heparinized saline and then pulling back until blood appeared in the line. If a catheter was not patent (i.e. no blood appeared in the line), a new one was implanted into the left jugular vein and testing resumed a minimum of 24 h later. The experimental protocol was approved by the University of Virginia Animal Care and Use Committee and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals, set by the National Institutes of Health.
Drugs
Nicotine bitartrate was purchased from Sigma-Aldrich (St Louis, MO, USA) (dose expressed as free base weight). Nicotine was mixed in sterile 0.9% saline (pH adjusted to 7.4 ± 0.5 with NaOH) and then passed through a microfilter. Nicotine infusions (0.01 mg/kg) were delivered at a rate of 0.1 mL/s and the infusion duration was adjusted according to the body weight of each rat. This dose of nicotine was selected based on previous research showing that, under extended access conditions, this dose of nicotine maintained high levels of self-administration with daily levels comparable to those observed in human smokers (Valentine et al, 1997). Nicotine solutions were made weekly and refrigerated but they were added to the drug syringes at room temperature (68–72°F).
Experimental procedures
Nicotine self-administration training
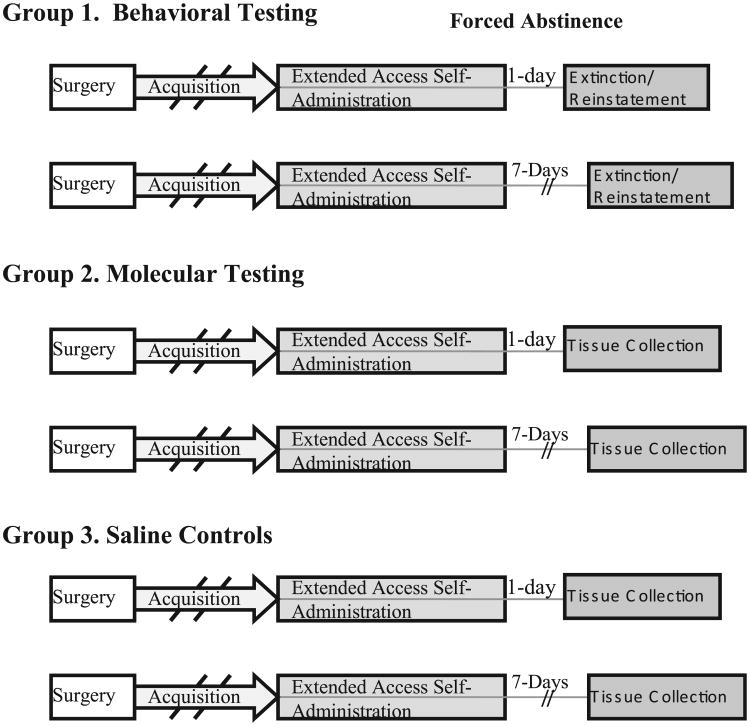
Because extinction and reinstatement responding can in itself produce changes in the brain (Sutton et al, 2003), the behavioral and molecular components of this study were conducted in independent groups of rats (see Fig. 1). The first group was examined for behavioral changes after either 1 or 7 days of abstinence and the second group was examined for molecular changes at these same time-points. The second group was treated in exactly the same manner as the first group except that they were not subjected to extinction and reinstatement testing. A third group of rats was given access to saline infusions and used as a control group for the molecular component of this study. This group was trained at the same time as the rats given access to nicotine and matched with rats in the nicotine groups for the total number of self-administration training sessions prior to testing under extended access conditions (as described below).
Fig. 1.
Schematic of the time-line of experimental events for the behavioral and molecular groups. The behavioral groups included nicotine self-administering rats that were tested at either 1 or 7 days of abstinence and the molecular groups included nicotine and saline self-administering rats from which tissue was obtained at either 1 or 7 days following the last self-administration session.
Rats were trained to self-administer nicotine infusions (0.01 mg/kg/infusion) under a fixed-ratio 1 schedule of reinforcement during daily sessions beginning at 12:00 h. At the start of each training session, the nicotine-associated lever (left lever) was extended into the operant chamber and each response on it produced an infusion of nicotine and the illumination of the stimulus light above the lever for the duration of the infusion (approximately 2.4 s). Each rat received one ‘priming’ infusion at the initiation of each training session. Sessions were terminated and the left levers retracted once rats had obtained all 20 infusions that were available. The right lever (inactive lever) was extended into the chamber for the duration of the experiment and responses on it were recorded but produced no consequence. Responding was assessed daily under the fixed-ratio 1 schedule until rats acquired nicotine self-administration, which was defined as two consecutive sessions where 20 infusions were delivered.
Extended access nicotine self-administration
Once rats acquired nicotine self-administration under the restricted access training conditions (e.g. the total number of infusions was capped at 20), they were given unrestricted access to nicotine infusions (under a fixed-ratio 1 schedule) for 23 h each day for a total of 10 days. At the beginning of each session, the left lever was extended into the operant chamber and remained extended until 1 h before the next extended access session. As in the training phase, a ‘priming’ infusion of nicotine was given at the beginning of each extended access session and, during the session, each infusion was paired with a stimulus light and the sound of the pump.
Following the 10th extended access nicotine self-administration session, rats were detached from their self-administration tethers but remained in their individual operant chambers. Rats in the behavioral groups were tested under the extinction/cue-induced reinstatement paradigm after either 1 day (N = 7) or 7 days (N = 8) of forced abstinence and rats in the molecular groups were killed after either 1 day (N = 8) or 7 days (N = 6) of forced abstinence without undergoing additional behavioral testing. Another group of rats was given access to saline infusions (N = 6) using the same training and extended access testing conditions that were used for nicotine self-administration with tissue obtained following 1 or 7 days of forced abstinence.
Extinction and cue-induced reinstatement following a 1- or 7-day forced abstinence period
After either a 1- or 7-day forced abstinence period, rats assigned to a behavioral group underwent extinction and reinstatement testing. These time-points were selected based on previous research with cocaine showing that cue-induced reinstatement responding is initially low following1 day offorced abstinence but increases significantly over a 7-day abstinence period (Grimm et al., 2001). Extinction testing consisted of a minimum of five 1-h sessions in which the left lever extended into the operant chamber for 60 min and retracted for 5 min before the start of the next session. Lever responses during these extinction sessions were recorded but did not produce any consequence. These extinction sessions continued until rats reached the extinction criterion of fewer than 15 responses per hour on the previously active lever (all rats reached the criterion within six sessions).
The 1-h cue-induced reinstatement session was initiated at 5 min after the last extinction session. At the beginning of the reinstatement session, the left active lever extended into the operant chamber and one non-contingent presentation of the cues formerly associated with nicotine (the light above the formerly active lever and the sound of the infusion pump) was given for 5 s to act as a prime. During this session, each response on the formerly active lever led to a 5-s presentation of these same cues.
Western blot analysis
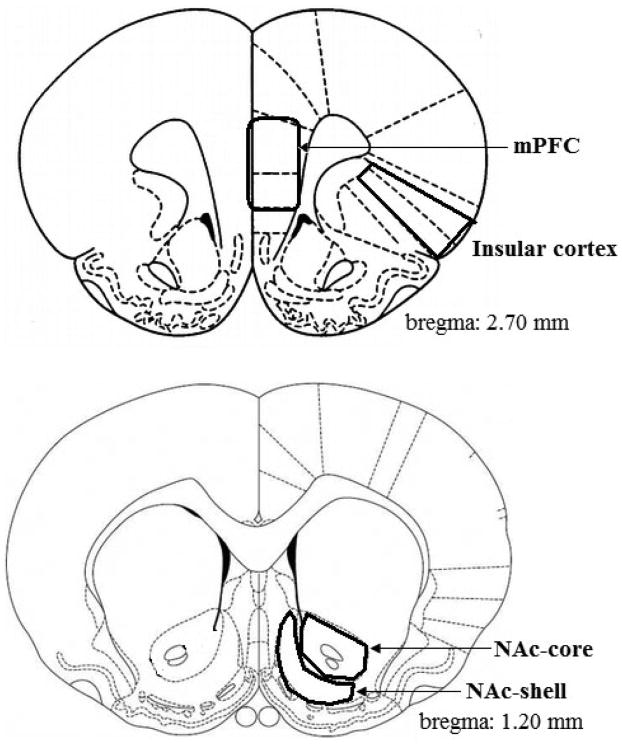
Following either 1 or 7 days of forced abstinence, rats assigned to a molecular group were killed by decapitation. Brains were quickly removed and placed in a prechilled brain slicer for dissection. The insular cortex and nucleus accumbens core and shell were dissected from 2-mm-thick coronal brain slices based on coordinates from Paxinos & Watson (1998) (see Fig. 2). Brain tissue was rapidly frozen in liquid nitrogen and stored in a freezer at −80°C until further processing.
Fig. 2.
Schematic illustration of brain regions used for molecular studies. The insular cortex and nucleus accumbens core and shell were identified and dissected from 2-mm-thick coronal brain slices as shown. mPFC, medial prefrontal cortex; NAc, nucleus accumbens.
Tissue punches were sonicated for 20–30 s while on ice in a cold solution of l× Radio Immuno Precipitation Assay (RIPA) Lysis Buffer (Upstate-Millipore, Bedford, MA, USA), 1 : 100 protease inhibitor (Thermo Scientific, Rockford, IL, USA) and phosphatase inhibitors (Cocktails 1 and 2; Sigma-Aldrich). Samples were centrifuged at 15 000 r.p.m. (16 g) for 10 min at 4°C and the supernatant (total protein lysate) was transferred to a new tube. Protein concentrations were calculated using the bicinochoninic acid protein assay kit (Pierce, Rockford, IL, USA) and diluted in 2× Laemmli sample buffer to achieve the equivalent final protein concentrations. Protein (15 μg) was loaded onto a 12% Tris–HCl gel, electrophoresed and transferred to a nitrocellulose membrane by electroblotting (10 V, overnight at 4°C) in a l× transfer buffer (BioRad, Hercules, CA, USA). Membranes were blocked in 50% Odyssey blocking buffer (LiCor, Lincoln, NE, USA) and 50% l× phosphate-buffered saline for 1 h at room temperature before being incubated with their respective primary antibodies overnight at 4°C [rabbit DARPP-32 #2302, phospho-DARPP-32 (Thr34) #2304 and phospho-DARPP-32 (Thr75) #2301; Cell Signaling Technology Boston, MA, USA]. Primary antibodies were diluted with 50% Odyssey blocking buffer and 50% wash buffer (1 : 1000, DARPP-32 antibodies; 1 : 500, phospho-DARPP-32 antibodies). Membranes were then incubated with red fluorescent Alexa Fluor 680 dye anti-rabbit secondary antibody (1 : 5000; Invitrogen, Carlsbad, CA, USA) for 1.5 h at room temperature covered in foil. Protein bands were visualized on an Odyssey Infrared Imaging System (LiCor). After the membranes were scanned, the same membranes were then reprobed with mouse monoclonal neuronal tubulin antibody (1 : 15 000; Upstate-Millipore) and using green fluorescence IRDye800 anti-mouse secondary antibody (1 : 10 000; Rockland, Gilbertsville, PA, USA) each for 30 min at room temperature. Samples from saline and nicotine self-administration groups were analyzed on the same immunoblots.
Data analysis
The mean number of infusions and responses on the active lever over the 10-day extended access period were compared between the nicotine self-administration groups and the saline group using repeated-measures ANOVA with group as the between-subjects factor and day as the within-subjects factor. Responses on the inactive lever were compared with responses on the active lever using the paired t-test. The mean number of responses on the active and inactive levers and daily nicotine intake (mg/kg) were averaged over the extended access period and compared between the four nicotine self-administration groups using ANOVA. The dependent measures for rats assigned to a behavioral group were active and inactive lever responses during each of the 1-h extinction tests and during the 1-h reinstatement test. Repeated-measures ANOVA was used to compare the number of responses during the five 1-h extinction sessions and to compare the number of responses during the last extinction session with those observed during the reinstatement session between the two abstinence groups. Posthoc comparison of extinction and reinstatement responding was made using an unpaired t-test.
The total and phosphorylated levels of DARPP-32 were quantified on an Odyssey Infrared Imaging System (LiCor). Values for each subject were obtained after removal of outliers (defined as > 2 SDs from the mean) and were expressed relative to the quantity value for tubulin, which was measured in the same sample on the same membrane. This value was used for subsequent ANOVA statistical analysis. One of the saline control rats did not have usable bands for DARPP-32 in the insular cortex and the data from this animal were not included in the insular cortex analysis. All statistical analyses were conducted using SPSS 17.0 and findings were considered statistically significant if P-values were < 0.05.
Results
Nicotine self-administration
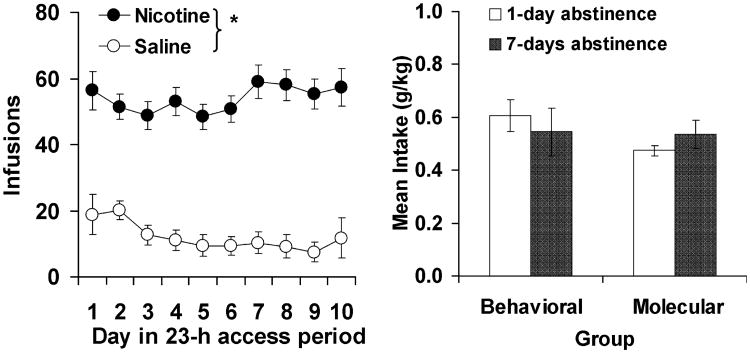
Under extended access conditions, rats self-administered high levels of nicotine and intake was relatively constant throughout the 10-day access period (Fig. 3, left panel). The number of infusions obtained by rats in the saline control group was minimal throughout the testing period. A repeated-measures ANOVA comparing the number of infusions between the saline and nicotine groups revealed a significant overall effect of group (F1,33 = 87.3, P < 0.001) but a non-significant effect of day (F9,297 = 0.45, P = 0.91). Levels of inactive lever responding in the nicotine groups were similar to levels of responding for saline (data not shown) and were significantly lower than levels of active lever responding for nicotine (t27 = 15.6, P < 0.001). Importantly, prior to subsequent behavioral and molecular testing, each of the nicotine groups was similar with regard to average nicotine intake (Fig. 3, right panel) and levels of active and inactive lever responding (data not shown; P-values > 0.05).
Fig. 3.
Extended access nicotine self-administration. (Left panel) Mean (±SEM) number of infusions self-administered as a function of day during the extended access self-administration period averaged across the four nicotine self-administration groups and the saline control group. *Significant difference between nicotine and saline (P < 0.05). (Right panel) Mean (±SEM) nicotine intake (g/kg) averaged over the 10-day extended access period for each of the four nicotine self-administration groups.
Extinction/reinstatement testing
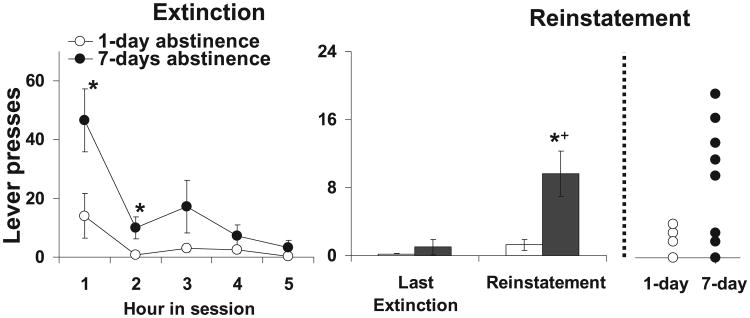
Rats in the 7-day abstinence group responded at higher levels under extinction conditions and took longer to extinguish as compared with rats in the 1-day abstinence group (Fig. 4, left panel). Repeated-measures ANOVA comparing the number of responses during the five 1-h extinction sessions revealed a significant mean effect of hour (F4,54 = 9.8, P < 0.001) and group (F1,13 = 8.1, P = 0.01) and a significant interaction of hour by group (F4,54 = 2.6, P = 0.04). Posthoc comparison of responding within each hour revealed a significant group difference for hours 1 and 2 (t13 = 2.4, P = 0.02; t13 = 2.2, P = 0.03, respectively) and a trend for a difference for hour 3 (t13 = 1.5, P = 0.08) but no difference for hours 4 and 5 (P-values> 0.05). Thus, prior to reinstatement testing, the two groups did not differ significantly on levels of extinction responding.
Fig. 4.
Incubation of nicotine-seeking behavior. (Left panel) Extinction. Mean (±SEM) number of responses on the lever formerly associated with nicotine for each of the five 1-h extinction sessions for rats in the 1- and 7-day abstinence groups. *Significant difference between the 1- and 7-day abstinence groups (P < 0.05). (Right panel) Reinstatement. Mean (±SEM) number of responses made during the 1-h reinstatement session as compared with the last extinction session for rats in the 1- and 7-day abstinence groups. *Significant difference between the number of responses made during the reinstatement session as compared with the number made during the last extinction session (P < 0.05). +Significant difference between the 1- and 7-day abstinence groups (P < 0.05).
Similarly, under reinstatement testing conditions, rats in the 7-day abstinence group responded at higher levels on the formerly active lever as compared with rats in the 1-day abstinence group (Fig. 4, right panel). In fact, only three of the seven rats in the 1-day abstinence group responded one or more times during the reinstatement test as compared with seven out of eight rats in the 7-day abstinence group (Fig. 4, far panel). A repeated-measures ANOVA comparing the number of responses in the last extinction session with those observed in the last reinstatement test revealed significant main effects of hour (F1,13 = 7.2, P = 0.02) and group (F1,13 = 13.0, P = 0.003). Subsequent comparison within each group revealed an increase in responding from the last extinction session to the reinstatement session for the 7-day abstinence group (t7 = 6.3, P = 0.03) and a trend for an increase for the 1-day abstinence group (t6 = 4.1, P = 0.09). Thus, rats in the 7-day abstinence group took longer to extinguish and responded at higher levels under both extinction and reinstatement conditions as compared with rats in the 1-day abstinence group.
Changes in phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa
Cortex
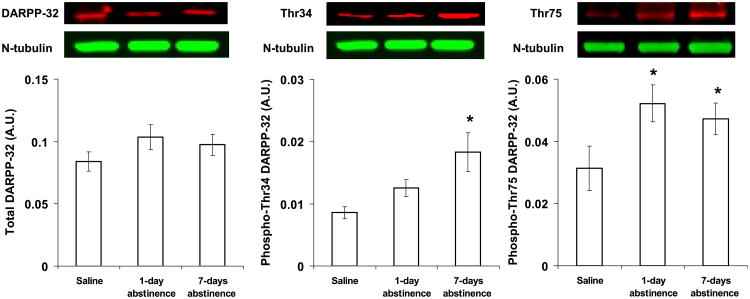
Although total levels of DARPP-32 in the insular cortex did not differ between the saline and nicotine groups (Fig. 5, left panel; F2,16 = 1.1, P = 0.38), phosphorylated levels of DARPP-32 at Thr34 were regulated following extended abstinence from nicotine self-administration (Fig. 5, middle panel; F2,16 = 4.4, P = 0.03). Subsequent pairwise comparison revealed that phospho-Thr34 levels were significantly higher in the 7-day abstinence group as compared with the saline group (t9 = 2.4, P = 0.01) with a trend for a difference between the 7- and 1-day abstinence group (t12 = 1.2, P = 0.06). Levels of phospho-Thr34 did not differ significantly between the saline group and the 1-day abstinence group (t9 = 0.4, P = 0.24). Phosphorylated levels of DARPP-32 at Thr75 were also regulated following abstinence from nicotine self-administration (Fig. 5, right panel; F2,16 = 4.2, P = 0.03). However, in contrast to phospho-Thr34, levels of phospho-Thr75 were higher in both the 1- and 7-day abstinence groups as compared with the saline control group (t11 = 5.5, P = 0.02 and t9 = 5.3, P = 0.02, respectively). Thus, relative to controls, levels of phospho-Thr34 in the insular cortex were higher at 7 days but not 1 day of abstinence, whereas levels of phospho-Thr75 were higher at both 1 and 7 days of abstinence. Notably, total and phosphorylated levels of DARPP-32 did not differ between groups in the medial prefrontal cortex (see Table 1).
Fig. 5.
Effect of abstinence length on total and phosphorylated levels of DARPP-32 in the insular cortex. Total DARPP-32 (left panel) and phosphorylated levels of DARPP-32 at Thr34 (middle panel) and Thr75 (right panel) were quantified by densitometry, and the data were normalized to tubulin. Immunoblots for the detection of total DARPP-32 and phospho-Thr34 and phospho-Thr75 are shown above each respective panel. *Significant difference as compared with saline (P < 0.05). Data represent means ± SEM. A.U., arbitrary unit.
Table 1. No effect of nicotine abstinence on total and phosphorylated levels of DARPP-32 in the medial prefrontal cortex or nucleus accumbens shell.
| Saline controls | 1 day abstinent | 7 days abstinent | P-value | |
|---|---|---|---|---|
| Medial prefrontal cortex | ||||
| Total DARPP-32 | 0.157 ± 0.040 | 0.134 ± 0.015 | 0.111 ± 0.016 | > 0.05 |
| Phospho-Thr34 | 0.026 ± 0.003 | 0.020 ± 0.002 | 0.019 ± 0.002 | > 0.05 |
| Phospho-Thr75 | 0.058 ± 0.004 | 0.083 ± 0.010 | 0.071 ± 0.008 | > 0.05 |
| Nucleus accumbens shell | ||||
| Total DARPP-32 | 0.037 ± 0.009 | 0.029 ± 0.008 | 0.031 ± 0.006 | > 0.05 |
| Phospho-Thr34 | 0.012 ± 0.003 | 0.013 ± 0.001 | 0.012 ± 0.001 | > 0.05 |
| Phospho-Thr75 | 0.018 ± 0.002 | 0.014 ± 0.001 | 0.017 ± 0.002 | > 0.05 |
Values are presented as mean ± SEM.
Nucleus accumbens
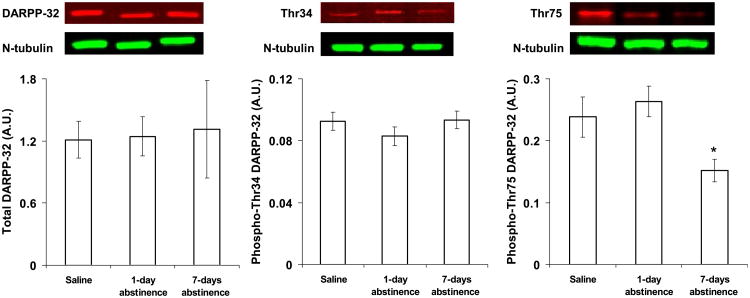
Although total levels of DARPP-32 in the nucleus accumbens core did not differ between groups (Fig. 6, left panel; F2,17 = 0.02, P = 0.98), phosphorylation of DARPP-32 at Thr75 (Fig. 6, right panel) but not Thr34 (Fig. 6, middle panel) was regulated following extended abstinence from nicotine self-administration (effect of group, F2,17 = 4.3, P = 0.03). Specifically, relative to saline controls, phospho-Thr75 was decreased in the 7-day (t10 = 2.3, P = 0.05) but not the 1-day (t12 = 0.6, P = 0.55) abstinence group. Levels of phospho-Thr75 were also significantly different between the 7- and 1-day abstinence groups (t12 = 3.2, P = 0.009). Thus, relative to controls, levels of phospho-Thr75 in the nucleus accumbens core were lower at 7 days but not 1 day of abstinence. Total and phosphorylated levels of DARPP-32 did not differ between groups in the nucleus accumbens shell (see Table 1).
Fig. 6.
Effect of abstinence length on total and phosphorylated levels of DARPP-32 in the nucleus accumbens core. Total DARPP-32 (left panel) and phosphorylated levels of DARPP-32 at Thr34 (middle panel) and Thr75 (right panel) were quantified by densitometry, and the data were normalized to tubulin. Immunoblots for the detection of total DARPP-32 and phospho-Thr34 and phospho-Thr75 are shown above each respective panel. *Significant difference as compared with saline and the 1-day abstinence group (P < 0.05). Data represent means ± SEM. A.U., arbitrary unit.
Discussion
The goals of this study were to determine whether drug seeking incubates over an abstinence period following extended access nicotine self-administration and to determine whether changes in DARPP-32 signaling in the insular cortex may be associated with this effect. Consistent with our hypothesis, rats in the 7-day abstinence group had higher levels of extinction responding, took longer to extinguish and responded at higher levels during reinstatement testing as compared with rats in the 1-day abstinence group. Relative to saline controls, rats in the 7-day but not the 1-day abstinence group had higher levels of DARPP-32 phosphorylated at the PKA site in the insular cortex. This effect is unlikely to be due to global alterations in DARPP-32 signaling throughout the cortex in that DARPP-32 was not regulated by nicotine in other cortical regions (e.g. the medial prefrontal cortex). Taken together, these results demonstrate incubation of drug seeking following extended access nicotine self-administration and suggest that enhanced PKA signaling in the insular cortex via phosphorylation of DARPP-32 at Thr34 is associated with this incubation effect.
The finding that nicotine-seeking behavior increased over an abstinence period is consistent with previous research with other drugs of abuse (for review see Bossert et al., 2005). This finding is also consistent with recent work with nicotine showing that levels of cue-induced reinstatement responding persist at high levels throughout an extended abstinence period when responding was assessed at 11, 26 and 41 days after nicotine self-administration (Liu et al., 2008). Although this previous study did not demonstrate an increase in reinstatement responding over an abstinence period, it is possible that the incubation effect would have been observed if early abstinence time-points had been included. It is also possible that extended access to nicotine is required to observe the increase in reinstatement responding over time. Indeed, Liu et al. (2008) found that the persistence of the reinstatement response depended on the level of access during the self-administration phase (i.e. the effect was persistent with rats that had access to high unit doses of nicotine but was not persistent in rats that had access to low unit doses of nicotine during short daily sessions).
The results from this study also reveal molecular changes in the insular cortex that were associated with abstinence from nicotine self-administration, a finding consistent with the idea that the insular cortex plays a critical role in addiction. In addition to the recent results from human smokers with lesions to the insular cortex, there is also preclinical evidence showing that hypocretin transmission in the insular cortex regulates nicotine's reinforcing effects (Hollander et al., 2008). The insular cortex has long been regarded as an area of the brain responsible for conscious urges and the results from this study suggest that it may be involved in modulating long-term changes in nicotine-seeking behavior during abstinence. This idea is consistent with recent work with cocaine self-administration in rats showing that intra-insular cortex infusion of DA D1 receptor agonists, which would stimulate PKA and increase phosphorylation of DARPP-32 at Thr34 (Hamada et al., 2005), modulates the motivational influence of drug-associated stimuli (Di Pietro et al., 2008). Thus, the results of this study extend previous findings from the insular cortex to include a role for PKA-regulated signaling of DARPP-32 in nicotine addiction.
Although the role of DARPP-32 in the insular cortex has not yet been examined in addiction studies, this signaling pathway is known to be regulated in other brain regions following exposure to drugs of abuse, including nicotine (Hamada et al., 2005; for review see Svenningsson et al., 2005). Most of the research in this area has been conducted with cocaine and the results from these studies have shown that repeated drug administration can increase the activity of PKA and subsequent phosphorylation of DARPP-32 at Thr34 in striatal and cortical areas (Nishi et al., 2000). Chronic drug exposure can also increase levels of CDK5 and subsequent phosphorylation of DARPP-32 at Thr75 that may act as a negative feedback modulator of PKA signaling. Consistent with this idea, inhibition of PKA signaling via intra-accumbens infusion of PKA inhibitors has been shown to attenuate drug-induced conditioned place preference (Cervo et al., 1997; Beninger et al., 2003) and conditioned locomotion (Sutton et al., 2000), and produce long-term decreases in motivation for drug (Lynch & Taylor, 2005; but see Self et al., 1998). Moreover, mice with a knock-in mutation that abolishes the Thr34 PKA phosphorylation site of DARPP-32 show a reduced sensitivity to the reinforcing effects of drugs of abuse (Zhang et al., 2006). In contrast, upregulation of this pathway via intra-accumbens infusion of PKA activators or infusion of CDK5 inhibitors has been shown to enhance the locomotor-activating and incentive motivational effects of drugs of abuse (Lynch & Taylor, 2005; Taylor et al., 2007). Although we did not observe changes in phosphorylated levels of Thr34 in the nucleus accumbens in the current study, we did find decreased levels of phospho-Thr75 at 7 days of abstinence, which is consistent with enhanced PKA-DARPP-32 signaling at a time when drug seeking is enhanced. The fact that we found this change in the nucleus accumbens core, but not the shell, is consistent with an emerging literature that suggests that, although DA signaling in the shell is preferentially activated over core regions during initial drug exposure, with repeated exposure, the shell : core ratio reverses such that DA signaling in the core is preferentially activated (Lecca et al., 2007). We also found that, in the insular cortex, phosphorylated levels of DARPP-32 at Thr75 were enhanced at both 1 and 7 days of abstinence, and that phosphorylated levels of DARPP-32 at Thr34 were enhanced at 7 days but not 1 day of abstinence. These findings suggest that signaling at phospho-Thr75 may be associated with abstinence from nicotine self-administration in general but that enhanced signaling at Thr34 may be associated with the heightened drug-seeking behavior that is observed following extended abstinence.
Conclusions
Nicotine dependence is the most common type of addiction in adults. Neuroadaptations caused by addictive drugs such as nicotine underlie changes in behavior and motivation that occur in addiction. The results from this study suggest that enhanced PKA signaling in the insular cortex via phosphorylation of DARPP-32 at Thr34 and in the nucleus accumbens core via decreased phosphorylation of DARPP-32 at Thr75 is associated with enhanced nicotine-seeking behavior following extended abstinence from nicotine self-administration. Thus, damage to PKA-DARPP-32 signaling in the insular cortex may, at least in part, explain the clinical findings of disruption of tobacco addiction in smokers with damage to this brain region.
Acknowledgments
We would like to thank Kristen Piehl and Will Hawkes for their technical assistance. This work was supported by the Virginia Youth Tobacco Project Small Grants Program and the University of Virginia. The authors have no financial relationships to disclose.
Abbreviations
- CDK5
cyclin-dependent kinase 5
- DA
dopamine
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- PKA
protein kinase A
References
- Beninger RJ, Nakonechny PL, Savina I. cAMP-dependent protein kinase and reward-related learning: intra-accumbens Rp-cAMPS blocks amphetamine-produced place conditioning in rats. Psychopharmacology. 2003;170:23–32. doi: 10.1007/s00213-003-1510-2. [DOI] [PubMed] [Google Scholar]
- Berger B, Febvret A, Greengard P, Goldman-Rakic PS. DARPP-32, a phosphoprotein enriched in dopaminoceptive neurons bearing dopamine D1 receptors: distribution in the cerebral cortex of the newborn and adult rhesus monkey. J Comp Neurol. 1990;299:327–348. doi: 10.1002/cne.902990306. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Cervo L, Mukherjee S, Bertaglia A, Samanin R. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775:30–36. doi: 10.1016/s0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychophar-macology (Berl) 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Hendrick JP, Ryan GR, Kuroiwa M, Higashi H, Tanaka M, Nairn AC, Greengard P, Nishi A. Nicotine regulates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa) phosphorylation at multiple sites in neostriatal neurons. J Pharmacol Exp Ther. 2005;315:872–878. doi: 10.1124/jpet.105.090852. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc NatlAcad Sci USA. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocainecraving. Neuropharmacology. 2009;56(Suppl. 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci. 2005;22:1214–1220. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl. 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immuno-cytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J Comp Neurol. 1992;323:209–218. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Perkins KA. Nicotine self-administration. Nicotine Tob Res. 1999;2:S133–S137. doi: 10.1080/14622299050011951. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sutton MA, McGibney K, Beninger RJ. Conditioned locomotion in rats following amphetamine infusion into the nucleus accumbens: blockade by coincident inhibition of protein kinase A. Behav Pharmacol. 2000;11:365–376. doi: 10.1097/00008877-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates theactions of multiple drugs of abuse. AAPS J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci USA. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Svenningsson P, Picetti R, Schlussman SD, Nairn AC, Ho A, Greengard P, Kreek MJ. Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32. J Neurosci. 2006;26:2645–2651. doi: 10.1523/JNEUROSCI.3923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]