Abstract
Bacille Calmette-Guerin (BCG) is an attenuated strain of Mycobacterium bovis that is used widely as a vaccine for tuberculosis and is used as an effective treatment for superficial bladder carcinoma. Despite being the most successful cancer biotherapy, its mechanism of action and response determinants remain obscure. Here we establish a model system to analyze BCG interaction with bladder cancer cells, using it to show that these cells vary dramatically in their susceptibility to BCG infection. Unexpectedly, the uptake of BCG by bladder cancer cells occurs by macropinocytosis rather than phagocytosis. BCG entry into bladder cancer cells relied upon Rac1, Cdc42 and their effector kinase Pak1. The difference in susceptibility between BCG-permissive and BCG-resistant bladder cancer cells was due to oncogenic activation of signaling pathways that activate macropinocytosis, with PI3K activation stimulating BCG uptake independently of Akt. Similarly, activated Ras strongly activated Pak1-dependent uptake of BCG. These results reveal that oncogenic activation of macropinocytosis determines BCG uptake by bladder cancer cells, implying that tumor responsiveness to BCG may be governed by the specific mutations present in the treated cancer cell.
Introduction
Bladder cancer is among the most common tumors diagnosed in the United States, with an estimated annual incidence of 70,530 new cases and 14,680 deaths in 2010 (1). Approximately 70% of bladder tumors are classified as superficial (non-muscle-invasive). Treatment of superficial bladder cancer by transurethral resection alone is associated with a 40-80% risk of recurrence and a 10-27% chance of progressing to muscle-invasive, regional or metastatic disease (2).
BCG is an attenuated strain of Mycobacterium bovis that was derived by prolonged in vitro passage of virulent M. bovis. Apart from its longstanding use as a vaccine against tuberculosis, BCG is also used as intravesical therapy for carcinoma in situ (CIS) of the bladder. BCG administration plays a central role in managing CIS as well as high grade Ta (papillary) and T1 (lamina propria invasive) lesions after transurethral resection (3). However, up to 30% of treated patients experience recurrence or progression of disease (3).
Despite over 30 years of clinical experience with intravesical BCG for bladder cancer, its mechanism of antitumor effect remains unknown and no markers exist to predict response. Direct and indirect immune mechanisms have been postulated to play a role (4), as have direct cytotoxic effects on the tumor cell (5). BCG attachment to tumor cells, leading to internalization and processing of the mycobacterium, likely plays a crucial role in activation of BCG-mediated anti-tumor activity (6-8).
The usual host cells of pathogenic mycobacteria, including M. tuberculosis and M. bovis, are macrophages. Macrophages internalize mycobacteria through phagocytosis, which is facilitated by attachment of the bacterium to receptors at the plasma membrane. However, M. tuberculosis is not known to actively promote its uptake in order to be internalized. In contrast, bacteria that invade nonphagocytic host cells often elaborate effector functions that induce their own uptake. One such strategy, which has been termed “zippering”, involves expression of invasion proteins on the bacterial surface that interact with cellular receptors, initiating signaling cascades that result in actin polymerization and membrane extensions with subsequent tight engulfment and internalization of the bacteria. This mechanism is utilized by bacteria such as L. monocytogenes and Y. pseudotuberculosis(9). An alternative strategy, termed “triggering”, uses a type III secretion system to inject bacterial proteins into the host cytoplasm that trigger actin polymerization and membrane ruffling, leading to co-uptake of bacteria and extracellular fluid in a pathway that mimics macropinocytosis. “Triggering” is used by S. typhimurium and S. flexneri for cellular entry (10, 11). BCG is not known to use either of the above uptake mechanisms, and the pathway by which it enters bladder cancer cells, which are derived from non-phagocytic epithelial cells, is unknown. In this study, we investigated the mechanism and molecular requirements for BCG entry into bladder cancer cells.
Materials and Methods
Bladder cancer cell lines
Bladder cancer cell lines were a gift from Dr. Dan Theodorescu. Cells were grown in Eagle minimal essential medium (MEM) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine and 1% non-essential amino acids, and with 100 U/ml penicillin, and 100μg/ml streptomycin (except where noted). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air. All cells were confirmed to be mycoplasma free by MycoAlertTM assay.
To ensure authenticity, all cell lines underwent mass spectrometry-based genetic fingerprinting using 42 highly variable single nucleotide polymorphisms across all chromosomes as previously described (12).
BCG, Mycobacterium smegmatis and Escherichia coli
GFP-expressing BCG (BCG-GFP) was created by transforming BCG Pasteur strain with pYUB921 (episomal plasmid encoding GFP and conferring kanamycin resistance). mCherry-expressing BCG (BCG-mCherry) was created by transforming BCG Pasteur with pMSG432 (episomal plasmid encoding mCherry and conferring hygromycin resistance). GFP-expressing E. coli was created by transforming DH5α strain of E. coli with pGFP(ASV) vector (Clontech) which encodes GFP and confers ampicillin resistance. BCG strains were grown at 37°C in Middlebrook 7H9 media supplemented with 10% albumin/dextrose/saline, 0.5% glycerol and 0.05% Tween 80, and in the presence of 20μg/ml kanamycin (BCG-GFP) or 50μg/ml hygromycin (BCG-mCherry). GFP-expressing M. smegmatis was created by transforming M. smegmatis with pYUB921. M. smegmatis was grown in LB media supplemented with 0.5% glycerol, 0.5% dextrose, and 0.05% Tween 80, in the presence of 20μg/ml kanamycin. E. coli was grown in LB media in the presence of 100μg/ml carbenicillin.
Pharmacologic inhibitors
Cells were pre-treated with the inhibitors in serum free media at the specified concentrations for one hour prior to infection with BCG, and kept in the media for the duration of infection. In all experiments utilizing chemical inhibitors, the highest concentration of DMSO (0.1%) was used as vehicle control. The sources of the pharmacologic inhibitors used in this study are listed in the supplementary methods.
Plasmids and transfections
PLK0.1-PTEN and PLK0.1-SC were a gift from Dr. Xuejun Jiang. PLK0.1 shRNA constructs for knock-down of Pak1, Pak2, PDK1 and clathrin heavy chain (CHC) were purchased from the Memorial Sloan Kettering High-Throughput Screening core facility. The scrambled shRNA lentivirus PLK0.1-SC was used as control for shRNA knockdown. Lentivirus for shRNA knockdown of PTEN, Pak1 or CHC was made by co-transfecting the respective plasmids with Mission Lentiviral Packaging Mix (Sigma) into 293T cells in 10cm2 plates, using lipofectamine 2000 (Invitrogen) as per the manufacturer's instructions. Lentivirus for overexpression of PTEN, Pak1, Cdc42, Rac1, K-ras and H-ras was made by co-transfecting the respective constructs with the packaging plasmids VSV-G and pCPG into 293T cells in 10cm2 plates, using lipofectamine 2000. After transfection, cells containing the lentiviral insert were selected using 1.5 μg/ml puromycin (Invitrogen) for 4 days. Please see Supplementary Methods for complete list of shRNA sequences and sources of cDNAs.
BCG infection
Bladder cancer cells were plated a day prior to infection in antibiotic-free media to reach 50%-80% confluence on the day of infection. Cells were washed and plated in serum and antibiotic-free media one hour prior to infection. BCG was thawed and diluted in serum and antibiotic-free media to achieve a multiplicity of infection (MOI) of 10:1. Plates were incubated at 37°C for the indicated time period and then washed three times with PBS, three times with media containing 1% penicillin-streptomycin, rewashed with PBS, detached using trypsin, and resuspended in PBS for analysis by flow-cytometry. Although absolute difference in BCG uptake between BCG-resistant and BCG-permissive cell lines is maximal after 24 hours, a 4-hour infection time point was chosen for experiments with chemical inhibitors to account for short half life of inhibitors.
Antibodies
Antibodies against pAkt (Ser473, #4060), Akt (#4691), S6K (#9202), p-S6K (Thr389, #9205), pPak1/pPak2 (pPak1 Ser144 / pPak2 Ser141, #2606), Pak1 (#2602), Pak2 (#2608), Pak3 (#2609), PDK1 (#3062), β-actin (#3700), pMek1 (Ser298, #9128), Mek1 (#9124), pLIMK1 (Thr508, #3841), LIMK1 (#3842), CHC (#4796), and Myc-Tag (#2276) were purchased from Cell Signaling. pPAK1 antibody (Ser144, EP656Y, ab40795) was purchased from Abcam. PTEN antibody (clone 6H2.1) was purchased from Cascade BioScience.
Microscopy
Cells were plated on glass coverslips in 6-well plates and allowed to attach overnight. The following day the cells were infected with BCG as described above. Nuclei were stained using Hoechst (Invitrogen). Cells were then fixed with 4% PFA in room temperature and stained with Texas-Red Phalloidin (Invitrogen). Confocal images were obtained with a Leica Inverted confocal SP2 microscope. Phase contrast microscopy was conducted using a Zeiss AxioVert 200M microscope with a Coolsnap ES camera. For live imaging using fluorescent dextran, cells were plated in glass-bottom 35mm dishes (MatTek) and allowed to attach overnight. The following day the cells were infected with BCG as described above. Alexa Fluor 568-conjugated dextran MW 10,000 (Invitrogen) at a concentration of 0.1mg/ml was added to the media immediately following addition of BCG, and the cells were incubated at 37°C for the specified time period, and washed as described above. Live microscopy was performed on a Zeiss Axiovert 200M microscope, with a Yokogawa spinning disk (CSU-22) unit, and an incubation chamber set to 37°C with 5% CO2 in air. Microscopy images were adjusted for contrast using Volocity software (PerkinElmer).
Flow-cytometry
Cell suspensions were analyzed on an LSR II flow cytometer (BD Biosciences), using the FACS DiVa software (BD Biosciences) according to manufacturer's instructions. Data analysis was performed with the FlowJo software package (Tree Star). GFP was detected on the FITC channel using a 488nm laser. mCherry was detected on the PE-Texas Red channel using a 532nm laser. As the cells had a high degree of auto-fluorescence, an empty channel (Pacific Blue) was used to optimize gating of GFP-positive or mCherry positive events. Gating strategy is described in figure S1.
Apoptosis and cell death assay
Bladder cancer cells were infected with BCG-GFP for 4 or 24 hours, washed with PBS, detached with trypsin and resuspended in PBS. In order to evaluate apoptosis, cells were stained using Pacific Blue Annexin V (Invitrogen) as per the manufacturer's instructions. Proportion of apoptotic cells (positive for annexin V fluorescence) was determined by flow-cytometry. Unstained cells were used as gating controls.
Transferrin uptake
Cells were washed with serum-free media, and media was replaced with serum-free media one hour prior to addition of transferrin. Media was replaced with serum-free media containing 25μg/ml Alexa-568-conjugated transferrin (Invitrogen) for 15 minutes at 37°C. Cells were chilled on ice and washed three times with ice-cold PBS. Cells were then washed with 0.1M glycine, 0.1M sodium chloride, PH 3.0 to remove extracellular transferrin. Cells were detached using trypsin, resuspended in PBS, and analyzed by flow-cytometry.
Results
Bladder cancer cell lines derived from human tumors vary in their susceptibility to BCG infection
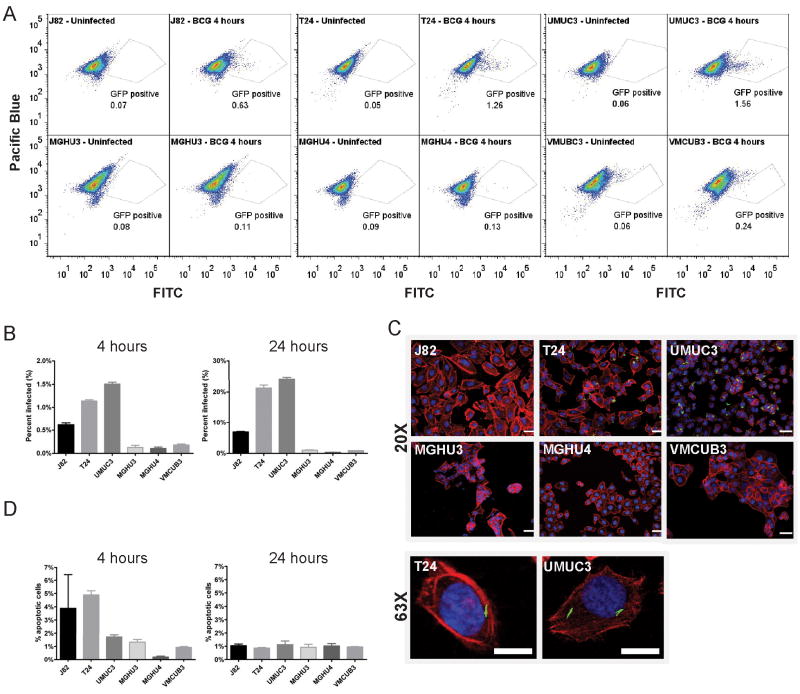
To study the mechanism of BCG infection of bladder cancer cells, we established an in vitro infection system using a BCG strain expressing GFP and a panel of bladder cancer cell lines derived from human tumors. We first asked whether the cell lines differ in their susceptibility to BCG infection. We infected the cell lines J82, T24, UM-UC-3, MGH-U3, MGH-U4, and VMCUB-3 with BCG-GFP, and determined uptake of BCG using flow-cytometry (figures 1A, S1). Cell lines could be categorized into two groups according to their susceptibility to BCG infection; three of the cell lines (UM-UC-3, T24 and J82) readily took up BCG, with up to 25% of the cells infected after 24 hours, while the others (MGH-U3, MGH-U4 and VMCUB-3) were resistant to BCG infection, with less than 2% of the cells infected after 24 hours (figure 1B). These finding were confirmed by confocal microscopy (figure 1C). To exclude the possibility that differences in BCG infection were due to apoptosis, we stained infected cells with annexin V, an early marker of apoptosis. The proportion of apoptotic cells was similar in the BCG-resistant compared to the BCG-sensitive lines, suggesting that apoptosis did not account for the differences in BCG permissiveness (figure 1D).
Figure 1. Heterogeneous BCG susceptibility among bladder cancer lines.
A. Specified cell lines were incubated with BCG-GFP for 4 hrs and evaluated by flow-cytometry. For each cell line, representative flow-plots of uninfected and infected cells are shown. X-axis: GFP-fluorescence. Y-axis: pacific-blue fluorescence (empty channel used to facilitate gating). Number within gate represents percentage of GFP-positive events out of total events.
B. Specified cell lines were incubated with BCG-GFP for indicated time periods and BCG uptake measured by flow-cytometry.
C. Specified cell lines were incubated with BCG-GFP for 24 hours and evaluated by confocal microscopy. Nuclei are stained with Hoechst (blue), actin with Texas-red phalloidin (red) and GFP-expressing BCG is shown in green. Top: 20X magnification. Scale bar: 50 μm. Bottom: 63X magnification. Scale bar: 15 μm.
D. Specified cell lines were incubated with BCG-GFP for 4 or 24 hrs, stained with pacific-blue labelled annexin V and evaluated by flow-cytometry.
BCG uptake by bladder cancer cells is inhibited by cytochalasin D, EIPA and staurosporine
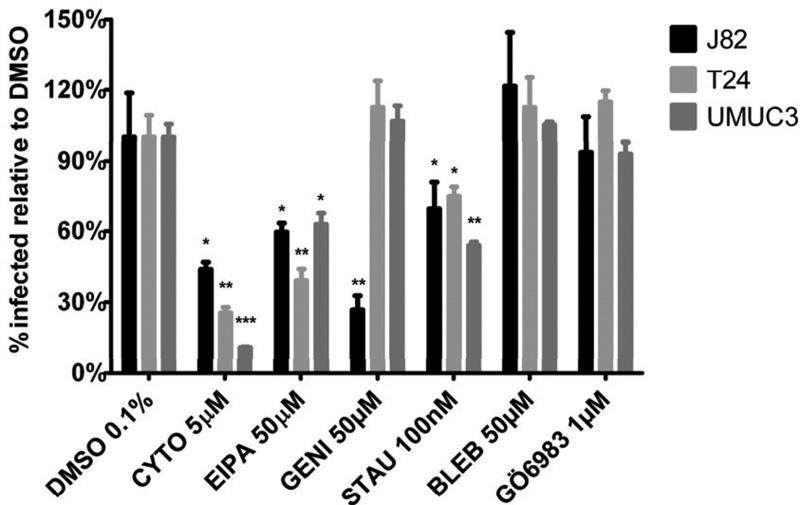
The data above indicates that a subset of bladder cancer cells efficiently take up BCG. This result is surprising insofar as bladder cells are non-phagocytic, and mycobacteria, in contrast to other bacterial pathogens such as Salmonella and Listeria, do not have pathogenic effector functions to invade epithelial cells. Thus, the mechanism of BCG uptake into bladder cancer cells susceptible to BCG infection is unclear. To determine the endocytic pathway mediating uptake of BCG in bladder cancer cells, we utilized a panel of small molecule inhibitors, which are commonly used to investigate mechanisms of pathogen internalization (13), and assessed their effect on BCG uptake. We first tested the actin polymerization inhibitor cytochalasin D and found that it diminished uptake of BCG by 64%-89% (figure 2). Additionally, the Na+/H+ pump inhibitor ethyl-isopropyl amiloride (EIPA), which has been used as an inhibitor of macropinocytosis (14), inhibited uptake of BCG by 47%-61%. The protein kinase inhibitor staurosporine inhibited uptake by 25%-46% and genistein (tyrosine-kinase inhibitor) inhibited uptake in J82. BCG uptake was not inhibited by the nonmuscle myosin inhibitor blebbistatin or Gö-6983 (a protein kinase C inhibitor). Taken together these data suggest that uptake of BCG by bladder cancer cells is dependent on the actin cytoskeleton and on protein kinases. The inhibition by EIPA is suggestive of, albeit not specific for, uptake by macropinocytosis (14).
Figure 2. BCG uptake requires actin and protein kinases.
Specified cell lines were pretreated with the indicated small molecule inhibitors and incubated with BCG-GFP for 4 hours in the presence of the inhibitors. BCG uptake was measured by flow-cytometry. BCG uptake is shown as percent of BCG uptake in presence of DMSO. DMSO: Dimethylsulfoxide. CYTO: Cytochalasin D. EIPA: 5-(N-Ethyl-N-isopropyl) amiloride. GENI: Genistein. STAU: Staurosporine. BLEB: Blebbistatin
*, P<0.05; **, P<0.005; ***, P<0.0005
To verify that the action of the inhibitors was not mediated through a direct effect on BCG, we measured uptake of paraformaldehyde-fixed BCG-GFP by bladder cancer cells in the presence of the same panel of small molecule inhibitors. The same effects seen with live BCG were seen with fixed BCG, confirming that the effect of the inhibitors was due to a bladder cell target and not through a direct effect on BCG such as compromising bacterial viability (figure S2). Similar results were obtained with M. smegmatis, a nonpathogenic mycobacterium (figure S3A) and Escherichia coli (figure S3B). However, the efficiency of infection with E. coli was lower than seen with either BCG or M. smegmatis. Thus, the uptake of BCG by bladder cancer cells reflects activation of a generalized uptake pathway for bacterial particles.
BCG uptake is dependent on Cdc42, Rac1 and Pak1
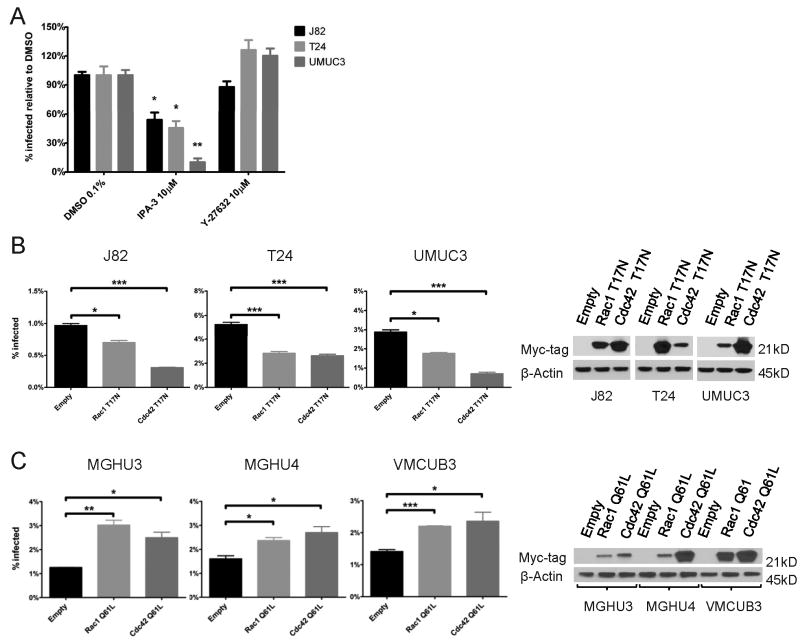
Rho-family GTPases, including Rac1, Cdc42 and RhoA, are involved in actin cytoskeletal organization and in various pathways of endocytosis. Rac1 and Cdc42 control lamellipodia formation and membrane ruffling, and are essential for macropinocytosis and for Fc-receptor-mediated phagocytosis (14-16), as is their downstream effector, p21-activated kinase 1 (Pak1) (17). RhoA, through its downstream effector RhoA Kinase (ROCK), is required for complement receptor-mediated phagocytosis (16). To determine the role of Rho-family GTPases in BCG uptake, we initially used two small molecule inhibitors, Y-27632, an inhibitor of ROCK, and IPA-3, an inhibitor of group 1 PAK family kinases, including Pak1. Y-27632 did not have a significant effect on BCG uptake (Figure 3A), but IPA-3 inhibited BCG uptake by 46%-90% (figure 3A), an effect that was also observed with fixed BCG, confirming an effect on the bladder cell (figure S2).
Figure 3. BCG uptake requires CDC42, Rac1, and Pak1.
A. Specified cell lines were pretreated with IPA-3 or Y-27632 and incubated with BCG-GFP for 4 hours in the presence of the inhibitors. BCG uptake was measured by flow-cytometry. BCG uptake is shown as percent of BCG uptake in presence of DMSO.
B. Specified cell lines were stably transduced with empty vector or with DN-Rac1(T17N) or DN-Cdc42(T17N) (both with N-terminal myc-tag). BCG uptake was measured by flow-cytometry 4 hours after infection. Western blot shows expression of myc-tagged Rac1(T17N) or Cdc42(T17N).
C. Specified cell lines were stably transduced with empty vector, Rac1(Q61L) or Cdc42(Q61L) (both with N-terminal myc-tag). BCG uptake was measured by flow-cytometry 8 hours after infection. Expression of myc-tagged Rac1(Q61L) or Cdc42(Q61L) was demonstrated by Western blotting.
*, P<0.05; **, P<0.005; ***, P<0.0005
To further investigate the role of Rac1 and Cdc42, we stably transduced BCG-permissive cell lines with the dominant negative Rac1(T17N) and Cdc42(T17N (18), and measured BCG uptake. We observed that either construct inhibited BCG uptake; Cdc42(T17N) by 50%-75% and Rac1(T17N) by 28%-46% (figure 3B). To test whether Rac1 and/or Cdc42 are sufficient to activate BCG uptake in a BCG-resistant cell lines, we stably transduced MGH-U3, MGH-U4 and VMCUB-3 with Rac1(Q61L) and Cdc42(Q61L), activated forms of Rac1 and Cdc42 respectively (19). Either construct significantly increased BCG uptake; Rac1(Q61L) by 48%-141% and Cdc42(Q61L) by 67%-100% (figure 3C). These results indicate that Rac1 or Cdc42 are both necessary and sufficient to determine BCG uptake by bladder cancer cell lines.
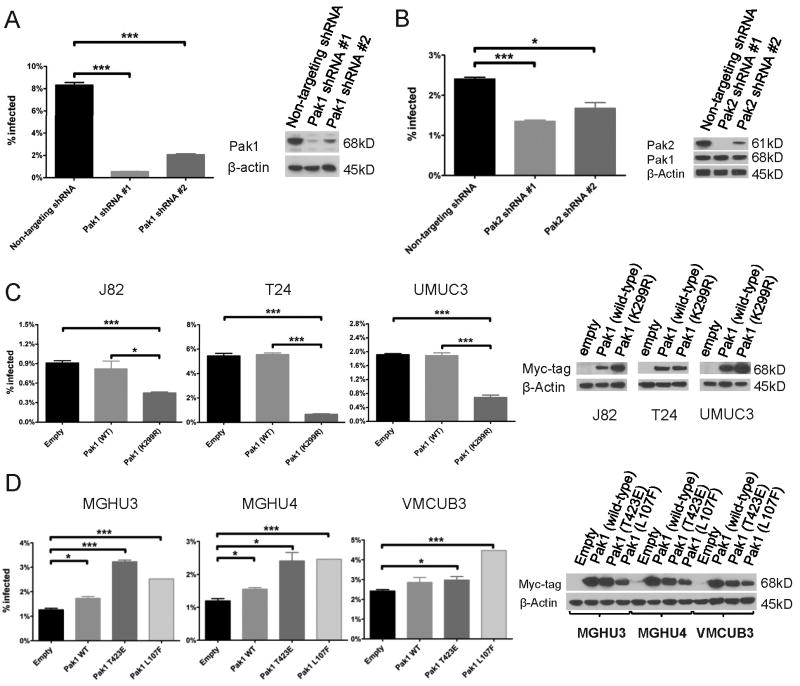
We next investigated the role of Pak1, downstream effector of Rac1 and CDC42. We depleted Pak1 protein in the cell line UM-UC-3 by lentiviral delivery of two shRNAs targeting Pak1, and verified depletion by Western blotting of whole cell lysates with anti-Pak1 antibodies (figure 4A). Depletion of Pak1 by either shRNA resulted in striking inhibition of BCG uptake by a factor of 4 to 15 (figure 4A), an effect similar to that seen with the Pak1 inhibitor IPA-3. Depletion of Pak2, another group I Pak (20) resulted in a 31%-44% decrease in BCG uptake (figure 4B). We verified depletion of Pak2 by Western blotting, and additionally confirmed that the Pak2 shRNAs did not affect Pak1 protein levels (figure 4B). Pak3, the third member of the group I PAK family, was not expressed in these cell lines (data not shown).
Figure 4. BCG uptake by Bladder Cancer Cells requires activated Pak1.
A. UM-UC-3 was stably transduced with non-targeting or two Pak1 shRNAs. BCG uptake was measured by flow-cytometry 4 hours after infection. Knock-down of Pak1 was demonstrated by Western blotting.
B. UM-UC-3 was stably transduced with non-targeting or two Pak2 shRNA. BCG uptake was measured by flow-cytometry 4 hours after infection. Knock-down of Pak2 and lack of significant effect on Pak1 expression was demonstrated by Western blotting.
C. Specified cell lines were stably transduced with an empty vector, wild-type Pak1, or DN-Pak1(K299R) (N-terminal myc-tagged). BCG uptake was measured by flow-cytometry 4 hours after infection. Expression of myc-tagged Pak1 was demonstrated by Western blotting.
D. Specified cell lines were stably transduced with an empty vector, wild-type Pak1, Pak1(T423E) or Pak1 (L107F) (N-terminal myc-tagged). BCG uptake was measured by flow-cytometry 8 hours after infection. Expression of myc-tagged Pak1 was demonstrated by Western blotting.
*, P<0.05; **, P<0.005; ***, P<0.0005
The data suggested that activated Pak1 was the main group I PAK family member stimulating BCG uptake by bladder cancer cells. To confirm this result, we stably transduced the BCG-permissive cell lines with Pak1(K299R), a dominant negative form of Pak1 (21). Consistent with our results using shRNA, DN-Pak1 decreased BCG uptake by 50%-88% when compared to an empty construct or wild-type Pak1 (figure 4C). Furthermore, transduction of BCG-resistant cell lines with either of two activated forms of Pak1 (22) resulted in a 23%-156% (for Pak1 T423E) and an 85%-106% (for Pak1 L107F) increase in BCG uptake (figure 4D). Taken together, these data indicate that BCG entry into bladder cancer cells occurs through activation of a pathway that involves Rac1, Cdc42, and Pak1.
Uptake of BCG is independent of dynamin and clathrin
Receptor mediated pathways for uptake of large particles, such as phagocytosis, or the zippering-type endocytosis used to internalize pathogens like Listeria, are dependent on the GTPase dynamin (23, 24). To investigate the role of Dynamin in BCG uptake, we transiently transfected the BCG-sensitive cells lines T24 and UM-UC-3 with wild-type dynamin2, or DN dynamin2(K44A) (25). As the C-terminus of dynamin in these constructs is fused to GFP, we used BCG-mCherry for these experiments. As shown in Figure S4A, transfection with either construct did not significantly alter BCG uptake. Similarly, neither construct had an appreciable impact on BCG uptake by MGH-U4 (figure S4B). Conversely, transfection with dynamin2(K44A), but not wild-type dynamin2, significantly inhibited uptake of transferrin, a process that is known to be dynamin-dependent (26) (figure S5A). Clathrin has also been shown to be essential for the “zippering”-type endocytosis of pathogens like Listeria (24). Despite effective knockdown of Clathrin heavy chain (CHC) by three different shRNas, no reduction in BCG uptake was observed (figure S4C). Uptake of transferrin, a known clathrin-dependent process (27), was inhibited by CHC knockdown (figure S5B).
Internalized BCG co-localizes with fluid phase fluorescent dextran
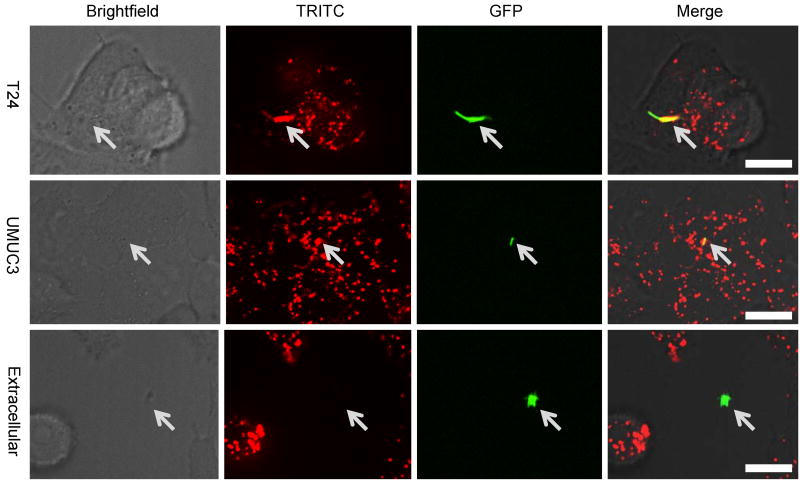
The molecular requirements for BCG uptake, namely the involvement of Rac1/Cdc42/Pak1, the inhibition of uptake by EIPA, and the lack of dependence on dynamin or clathrin, suggest that uptake occurs through macropinocytosis. Particles internalized by macropinocytosis are taken up together with extracellular fluid, whereas particles internalized by receptor-mediated uptake pathways, such as phagocytosis or “zippering”, exclude extracellular fluid (28). To determine whether extracellular fluid is being internalized with BCG, we infected the cell lines T24 and UM-UC-3 with BCG-GFP in the presence of red-fluorescent dextran (MW 10,000) in the medium. Red fluorescent dextran could be seen in the same vesicle as BCG-GFP (Figure 5). We excluded the possibility that dextran was attaching to BCG before uptake by observing that fluorescent dextran did not co-localize with extracellular BCG (Figure 5).
Figure 5. Internalized BCG colocalizes with fluid phase markers.
Confocal microscopy of T24 and UM-UC-3 incubated with BCG-GFP for 4 hours in the presence of red-fluorescent dextran (MW 10,000) in the media. Arrows point to location of BCG. Scale bar: 15 μm.
The PI3K-PTEN pathway determines BCG uptake by bladder cancer cells
The data presented above indicate that the mechanism of entry of BCG into some bladder cancer cells is via macropinocytosis. However, some bladder cancer cells are resistant to BCG uptake, suggesting that they do not have an activated macropinocytosis pathway. We hypothesized that the pattern of mutations present in the BCG-resistant and BCG-sensitive cell lines may determine their permissiveness for BCG uptake. Of the cell lines used in this study, two BCG-permissive cell lines (J82 and UM-UC-3) are reported to have a deletion of PTEN (29), and two (T24 and UM-UC-3) have activating mutations in Ras (30). We investigated the causal relationship between these mutations and BCG susceptibility, focusing first on the PTEN/PI3K/Akt pathway.
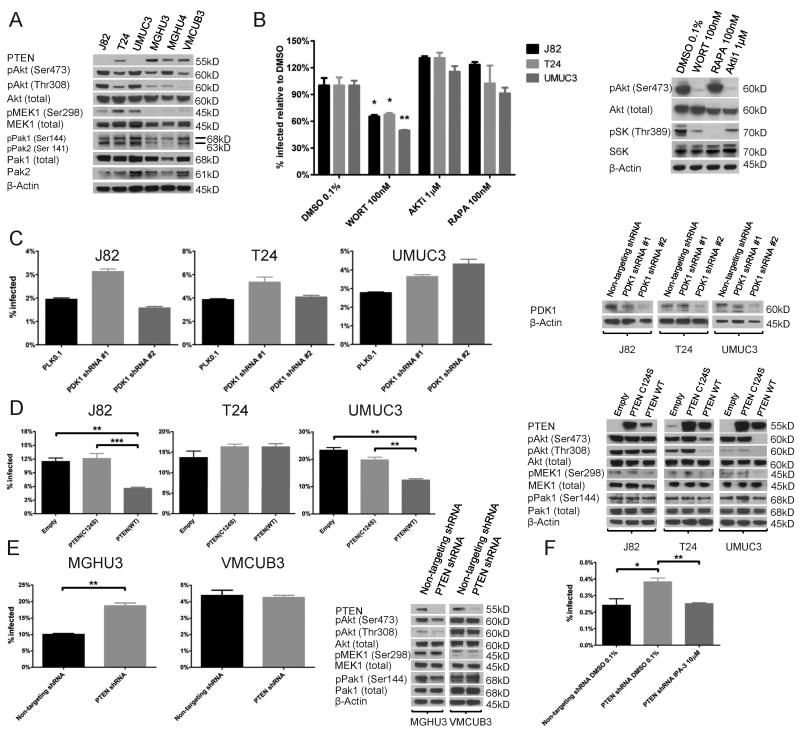
J82 and UM-UC-3, the two cell lines with reported PTEN deletion, showed no detectable PTEN protein (Figure 6A). All three sensitive cells (J82, T24, and UMUC3) had elevated levels of phospho-Pak1(Ser144) and pMEK1(Ser298), the latter being a direct phosphorylation target of Pak1 (31). We could not detect expression of pLimk1/pLimK2, also a downstream target of Pak1 phosphorylation (32), nor of Pak3 (data not shown). Wortmannin (Figure 6B) or BKM120 (a selective Pan-PI3K inhibitor, figure S6) (33), inhibited BCG uptake by 33%-50%. In contrast, inhibitors of Akt or mTOR did not decrease BCG uptake, despite clear inhibition of their downstream targets (Figure 6B). We deduced that these inhibitors were not acting through a direct effect on BCG by showing the same effects on uptake of fixed BCG (figure S2). To further determine whether the effect of PI3K on BCG uptake is mediated through the canonical PI3K-PDK1-Akt pathway, we depleted PDK1 using two different lentiviral-delivered shRNAs, and confirmed depletion by Western blotting (figure 6C). PDK1 depletion did not inhibit BCG uptake (figure 6C), confirming that the effect of PI3K on BCG uptake is not mediated through the PDK-1-Akt arm of the PI3K pathway.
Figure 6. Activation of PI3K stimulates BCG uptake through Pak1 dependent macropinocytosis.
A. Western blot of extracts from specified cell lines showing expression of the indicated proteins.
B. Specified cell lines were pretreated with wortmannin, Akti XIII or rapamycin and incubated with BCG-GFP for 4 hours in the presence of the inhibitors. BCG uptake is shown as percent of BCG uptake in presence of DMSO. Western blotting of UM-UC-3 after 1 hour treatment with DMSO, wortmannin, Akti XIII, or rapamycin.
C. Specified cell lines were stably transduced with non-targeting or one of two PDK1 shRNAs. BCG uptake was measured by flow-cytometry 4 hours after infection. PDK1 Knock-down was confirmed by Western blotting.
D. Specified cell lines were stably transduced with empty vector, PTEN(C124S) or wild-type PTEN. BCG uptake was measured by flow-cytometry 24 hours after infection. Expression of PTEN and downstream pathways was demonstrated by Western blotting.
E. Specified cell lines were transduced with non-targeting or one of two PTEN shRNAs. BCG uptake was measured by flow-cytometry 24 hours after infection. Knock-down of PTEN and its effect on downstream pathways was demonstrated by Western blotting.
F. MGH-U4 stably transduced with PTEN shRNA was pretreated with DMSO or IPA-3 and incubated with BCG-GFP for 4 hours in the presence of the inhibitor. BCG uptake was measured by flow-cytometry.
*, P<0.05; **, P<0.005; ***, P<0.0005; DMSO: Dimethylsulfoxide. WORT: Wortmannin. AKTI: Akti XIII. RAPA: Rapamycin
To study the role of PTEN in BCG uptake, cDNAs encoding PTEN (wild-type) or PTEN (C124S), a PTEN protein deficient for lipid phosphatase activity (34), were stably transduced into the BCG-sensitive cell lines (Figure 6D). Restoration of wild-type PTEN, but not PTEN(C124S), resulted in approximately 50% reduction in BCG uptake in the cell lines J82 and UM-UC-3 (Figure 6D), but not in T24, which has a missense mutation of PTEN (29). Although expression of wild-type PTEN did not clearly decrease phosphorylation of Pak1-S144, we did observe decreased phosphorylation of Mek1 at the serine in position 298 in the cells induced with wild-type PTEN. Knockdown of PTEN in MGH-U3 resulted in approximately 2-fold increase in uptake of BCG compared to a non-targeting shRNA control, but the same phenomenon was not seen in VMCUB-3, despite PTEN depletion as evidenced by Western blotting (Figure 6E).
To confirm that PTEN knockdown was activating the same pathway of BCG uptake observed in permissive cell lines, we tested whether the increase in BCG uptake following PTEN knockdown in the cell line MGH-U4 could be abrogated by inhibition of Pak1. IPA-3 completely abrogated the increase in BCG uptake stimulated by PTEN knockdown (Figure 6F), indicating that hyperactivation of the PI3K pathway in a resistant cell line activates BCG uptake through the same pathway as in susceptible cell lines with loss of PTEN function. Overall, these data demonstrate that the PTEN-PI3K signaling pathway modulates BCG uptake by bladder cancer cells. Activation of the PI3K pathway activates macropinocytotic uptake of BCG, but this effect is independent of the downstream kinases PDK1, Akt and mTOR.
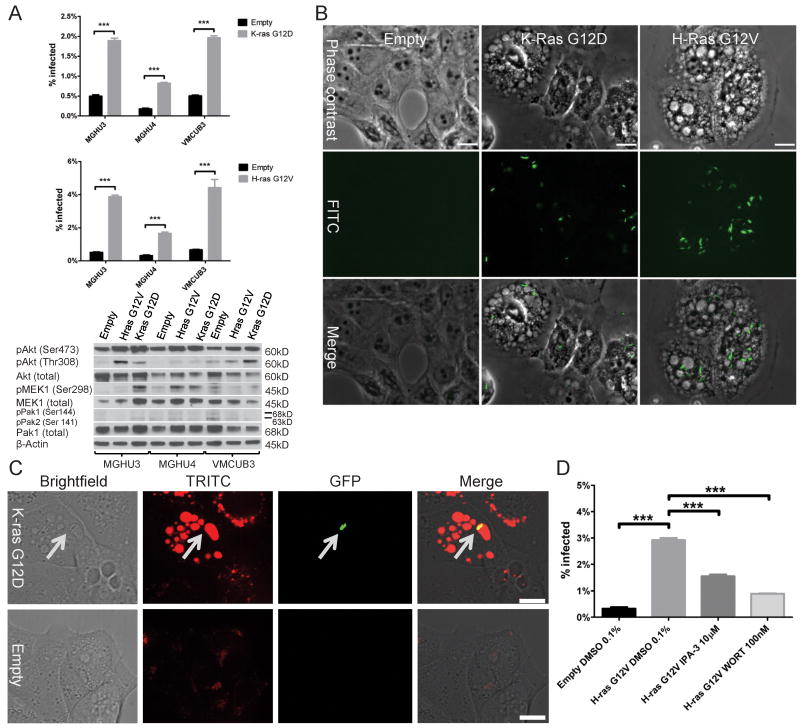
Activated Ras activates macropinocytotic BCG uptake
We next investigated the role of Ras in uptake of BCG by bladder cancer cells. We stably transduced BCG-resistant cell lines with cDNAs encoding K-ras G12D and H-ras G12V, constitutively-activated forms of K-ras and H-ras, respectively. Both activated forms of Ras caused a dramatic increase in BCG uptake, up to 7-fold higher than control cells (Figure 7A). In 2 of 3 cell lines (MGHU3 and MGHU4), activated Ras stimulated phosphorylation of Mek1 at the Pak1 target site serine 298, indicating activation of the Pak1 pathway. Fluorescence microscopy confirmed increased BCG uptake by Ras-transformed cell lines, and revealed striking morphologic changes in these cells, including numerous cytoplasmic vacuoles (Figure 7B). BCG-GFP localized in dextran-containing macropinosomes in cells expressing K-Ras G12D, but not control cells (Figure 7C), indicating uptake by macropinocytosis. Activation of BCG uptake by K-Ras G12D could be abrogated by either IPA-3 and wortmannin suggesting that the action of Ras occurs upstream of PI3K and Pak1 (Figure 7D).
Figure 7. Activated Ras stimulates BCG uptake via macropinocytosis.
A. Specified cell lines were stably transduced with empty vector, K-ras(G12D) or H-ras (G12V). BCG uptake was measured by flow-cytometry 4 hours after infection. Effect of activated Ras on downstream pathways was demonstrated by Western blotting.
B. Phase-contrast and fluorescence microscopy of VMCUB-3 transduced with empty vector, K-Ras(G12D) or H-Ras(G12V), and infected with BCG-GFP for 24 hours. Scale bar: 25 μM.
C. Confocal microscopy of VMCUB-3 transduced with empty vector or K-Ras(G12D). Cells were incubated with BCG-GFP for 3 hours in the presence of red-fluorescent dextran (MW 10,000) in the media. Arrows point to location of BCG. Scale bar: 15 μM.
D. VMCUB-3 transduced with H-Ras(G12V) was pretreated with DMSO, IPA-3 or wortmannin and incubated with BCG-GFP for 4 hours in the presence of the inhibitor. BCG uptake was measured by flow-cytometry.
*, P<0.05; **, P<0.005; ***, P<0.0005; DMSO: Dimethylsulfoxide. WORT: Wortmannin
Discussion
Our data shows that bladder cancer cell lines vary considerably in their propensity to take up BCG. We found that this property is dependent on activation of several oncogenic signaling pathways, resulting in increased macropinocytosis and uptake of BCG. This is, to our knowledge, the first report showing that cancer cells may become susceptible to a microbial pathogen due to oncogenic mutations.
The pathways determining BCG uptake by bladder cancer cells, namely, PTEN-PI3K, Ras, and Cdc42-Rac1-Pak1, are known to be interconnected. The oncoprotein Ras can activate PI3K (35), and is also able to activate Rac1 through its action on the guanine nucleotide exchange factor (GEF) Tiam1 (36). Rac1 can also be activated by increased phosphatidylinositol (3,4,5)-triphosphate concentrations (37), which can occur through PI3K activation or through PTEN loss. Cdc42 can itself activate PI3K (38).
The same pathways determining BCG uptake are also involved in bladder cancer oncogenesis. Alterations in the PTEN/PI3K/Akt pathway are frequently present in human bladder cancers. These include decreased expression or deletion of the tumor suppressor PTEN, activating mutations of PI3K, and, rarely, activating mutations of Akt1 (39). A sizeable fraction of bladder cancers harbor activating mutations of Ras, most commonly H-ras mutations (40). Cdc42 also appears to have a role in bladder cancer: expression of Cdc42 has been shown to be higher in bladder cancer compared to normal urothelial cells, and RNA interference of Cdc42 was found to suppress growth of bladder cancer cells (41, 42). Pak1 is overexpressed in a large proportion of bladder cancers (43), and may also be a marker of recurrence after transurethral resection of superficial bladder cancer (44).
Our findings could thus explain why treatment with BCG is successful in most, but not all, patients with bladder cancer. BCG therapy would be expected to provide the most benefit for those patients whose cancer contains mutations activating the pathways of BCG uptake, such as decreased PTEN expression, activating Ras mutations, or activated Pak1. Our findings could also provide a mechanism of specificity for BCG infection of tumor cell compared to the normal urothelium, which does not contain mutations activating BCG uptake.
Epithelial cells are not the usual target of mycobacteria; the main cell type involved in M. tuberculosis infection is the macrophage, and the receptors utilized by macrophages for phagocytosis of M. tuberculosis have been comprehensively described (45). We define a novel mechanism underlying BCG uptake within epithelial cells, which is dependent on the actin cytoskeleton, inhibited by EIPA, and controlled by Cdc42, Rac1 and Pak1, but independent of dynamin or clathrin. Perhaps most importantly, BCG was taken up with fluid phase markers. Overall, these features are most consistent with uptake by macropinocytosis.
Others have shown that BCG attachment and uptake by bladder cancer cells is facilitated through attachment of BCG fibronectin attachment protein (FAP) to fibronectin on bladder cancer cells (46). Receptor-mediated uptake of large particles, via phagocytosis, or by the clathrin-dependent pathway that is utilized for uptake of Listeria, is typically dependent on dynamin (10, 23, 24). BCG uptake, however, was independent of dynamin. One explanation for this apparent discrepancy is that uptake of BCG does not occur through a classical receptor-mediated uptake pathway. Rather, BCG that is either adjacent to bladder cancer cells or attached to them through a receptor is internalized because of increased membrane ruffling that accompanies macropinocytosis. The presence of a receptor for BCG attachment, such as α5β1 integrin, would lead to more mycobacteria being in intimate contact with the cells, and would thus promote this process, resulting in increased uptake of BCG. Although macropinocytosis has traditionally been described as receptor-independent (16), there have been some recent reports describing receptor-dependent pathways of macropinocytosis in the uptake of some viruses (47, 48).
If validated in clinical settings, our findings could have implications for the treatment of patients with bladder cancer. Based upon our results, Ras and PTEN aberrations may represent predictive biomarkers of BCG efficacy. Prospective genetic profiling of TUR specimens for mutations within these signaling pathways may allow clinicians to restrict BCG therapy to those patients most likely to respond. Furthermore, as novel therapies targeting oncogenic pathways are being developed for bladder cancer treatment, such as inhibitors of PI3K (49), receptor tyrosine kinase inhibitors (50) and RNA-interference mediated silencing of Cdc42 (42), care should be taken to consider possible effects of these treatment on BCG uptake and efficacy. Finally, BCG therapy could possibly be improved by local administration of activators of these pathways, thereby potentially rendering BCG-resistant cells sensitive. The findings presented here will catalyze a direct examination of the role of specific macropinocytosis activating mutations in clinical response to BCG.
In conclusion, we have shown that BCG uptake by bladder cancer cells is determined by some of the same pathways that lead to oncogenesis. Knowledge of the mechanism underlying responsiveness to BCG therapy will help tailor the treatment to individual patients based on their tumor genotype, and will hopefully lead to the development of more effective treatment options for bladder cancer.
Supplementary Material
Acknowledgments
We would like to thank Alan Hall for his insightful comments and critical reading of the manuscript. We thank Dr. Hakim Djaballah of the Memorial Sloan Kettering High-Throughput screening facility for providing some of the shRNA constructs used and the Memorial Sloan Kettering Molecular Cytology Core Facility for assistance with obtaining microscopy images. We thank Alan Hall, Jonathan Chernoff, Eric Holland, Xuejun Jiang, and Mark McNiven for reagents.
Grant Support: GRS is supported by an award from the Bladder Cancer Advocacy Network (BCAN) and by an award from the Lucille Castori Center for Microbes, Inflammation, and Cancer. This project was supported the Geoffrey Beene Cancer Research Center and the STARR Cancer Consortium.
Footnotes
The authors have no conflicting financial interests
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Gontero P, Bohle A, Malmstrom PU, O'Donnell MA, Oderda M, Sylvester R, et al. The role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. Eur Urol. 2010;57:410–29. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Bohle A, Brandau S. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol. 2003;170:964–9. doi: 10.1097/01.ju.0000073852.24341.4a. [DOI] [PubMed] [Google Scholar]
- 5.Pook SH, Rahmat JN, Esuvaranathan K, Mahendran R. Internalization of Mycobacterium bovis, Bacillus Calmette Guerin, by bladder cancer cells is cytotoxic. Oncol Rep. 2007;18:1315–20. [PubMed] [Google Scholar]
- 6.Becich MJ, Carroll S, Ratliff TL. Internalization of bacille Calmette-Guerin by bladder tumor cells. J Urol. 1991;145:1316–24. doi: 10.1016/s0022-5347(17)38622-6. [DOI] [PubMed] [Google Scholar]
- 7.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. The Journal of clinical investigation. 1990;85:62–7. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda N, Toida I, Iwasaki A, Kawai K, Akaza H. Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guerin (BCG): A role of BCG internalization into tumor cells. International journal of urology : official journal of the Japanese Urological Association. 2002;9:29–35. doi: 10.1046/j.1442-2042.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- 9.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–8. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 10.Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin AE, et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell host & microbe. 2007;2:340–51. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginocchio C, Pace J, Galan JE. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci U S A. 1992;89:5976–80. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janakiraman M, Vakiani E, Zeng Z, Pratilas CA, Taylor BS, Chitale D, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer research. 2010;70:5901–11. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–5. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 14.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. The Journal of cell biology. 2010;188:547–63. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 16.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nature reviews Molecular cell biology. 2008;9:639–49. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Molecular biology of the cell. 2000;11:3341–52. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. The Journal of cell biology. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K, et al. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. The Journal of biological chemistry. 2000;275:9725–33. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- 20.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. The international journal of biochemistry & cell biology. 2002;34:713–7. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, et al. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. The Journal of biological chemistry. 1995;270:23934–6. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 22.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. The Journal of biological chemistry. 2002;277:883–6. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 23.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nature cell biology. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 24.Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. The Journal of experimental medicine. 1999;190:1849–56. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. The Journal of cell biology. 2000;150:377–89. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. The Journal of cell biology. 1993;122:553–63. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanover JA, Willingham MC, Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–93. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein SC, Steinman RM, Cohn ZA. Endocytosis. Annual review of biochemistry. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- 29.Wang DS, Rieger-Christ K, Latini JM, Moinzadeh A, Stoffel J, Pezza JA, et al. Molecular analysis of PTEN and MXI1 in primary bladder carcinoma. International journal of cancer Journal international du cancer. 2000;88:620–5. doi: 10.1002/1097-0215(20001115)88:4<620::aid-ijc16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–25. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 31.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. The Journal of cell biology. 2003;162:281–91. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature cell biology. 1999;1:253–9. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 33.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Molecular cancer therapeutics. 2012;11:317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 34.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. The Journal of biological chemistry. 1998;273:13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 35.Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Current biology : CB. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 36.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nature cell biology. 2002;4:621–5. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 37.Missy K, Van Poucke V, Raynal P, Viala C, Mauco G, Plantavid M, et al. Lipid products of phosphoinositide 3-kinase interact with Rac1 GTPase and stimulate GDP dissociation. The Journal of biological chemistry. 1998;273:30279–86. doi: 10.1074/jbc.273.46.30279. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. The Journal of biological chemistry. 1994;269:18727–30. [PubMed] [Google Scholar]
- 39.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6008–17. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 40.Boulalas I, Zaravinos A, Karyotis I, Delakas D, Spandidos DA. Activation of RAS family genes in urothelial carcinoma. J Urol. 2009;181:2312–9. doi: 10.1016/j.juro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Volanis D, Zaravinos A, Kadiyska T, Delakas D, Zoumpourlis V, Spandidos DA. Expression profile of Rho kinases in urinary bladder cancer. Journal of BUON : official journal of the Balkan Union of Oncology. 2011;16:511–21. [PubMed] [Google Scholar]
- 42.Wu F, Chen Y, Li Y, Ju J, Wang Z, Yan D. RNA-interference-mediated Cdc42 silencing down-regulates phosphorylation of STAT3 and suppresses growth in human bladder-cancer cells. Biotechnology and applied biochemistry. 2008;49:121–8. doi: 10.1042/BA20070107. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YH, Xie D, Luo JH, Chen W, Chen LW, Xu QC, et al. The clinico-pathological significance of protein expression of PAK1 in bladder transitional cell carcinoma. Zhonghua yi xue za zhi. 2007;87:2710–3. [PubMed] [Google Scholar]
- 44.Ito M, Nishiyama H, Kawanishi H, Matsui S, Guilford P, Reeve A, et al. P21-activated kinase 1: a new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol. 2007;178:1073–9. doi: 10.1016/j.juro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infection and immunity. 1998;66:1277–81. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratliff TL, McCarthy R, Telle WB, Brown EJ. Purification of a mycobacterial adhesin for fibronectin. Infection and immunity. 1993;61:1889–94. doi: 10.1128/iai.61.5.1889-1894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amstutz B, Gastaldelli M, Kalin S, Imelli N, Boucke K, Wandeler E, et al. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. The EMBO journal. 2008;27:956–69. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, et al. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS pathogens. 2010;6:e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D, Tao J, Xu B, Qing W, Li P, Lu Q, et al. Phosphatidylinositol 3-kinase inhibitor LY294002 suppresses proliferation and sensitizes doxorubicin chemotherapy in bladder cancer cells. Urologia internationalis. 2011;86:346–54. doi: 10.1159/000322986. [DOI] [PubMed] [Google Scholar]
- 50.Dreicer R, Li H, Stein M, DiPaola R, Eleff M, Roth BJ, et al. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4090–5. doi: 10.1002/cncr.24467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.