Abstract
Purpose:
Treatment of patients with oncogene-addicted cancers with tyrosine kinase inhibitors (TKI) is biologically and clinically different than with cytotoxic chemotherapy. We have observed that some patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib (RECIST progression after initial benefit) have accelerated progression of disease after discontinuation of TKI. To examine this observation and define the course of patients following TKI discontinuation, we systematically evaluated patients enrolled on clinical trials of agents to treat acquired resistance to erlotinib or gefitinib.
Methods:
We evaluated patients with EGFR-mutant lung cancer who participated in trials for patients with acquired resistance which mandated TKI discontinuation prior to administration of study therapy. Disease flare was defined as hospitalization or death attributable to disease progression during the “washout” period.
Results:
Fourteen of 61 patients (23%; 95% CI 14-35%) experienced a disease flare. The median time to disease flare after TKI discontinuation was 8 days (range 3-21). Factors associated with disease flare included shorter time to progression on initial TKI (p=0.002) and the presence of pleural (p=0.03) or CNS disease (p=0.01). There was no association between disease flare and the presence of T790M at the time of acquired resistance.
Conclusion:
In patients with EGFR-mutant lung cancer and acquired resistance to EGFR TKIs, discontinuation of erlotinib or gefitinib prior to initiation of study treatment is associated with a clinically significant risk of accelerated disease progression. Clinical trials in this patient population must minimize protocol mandated washout periods.
Keywords: EGFR, adenocarcinoma of lung, drug resistance
Statement of Translational Relevance
We systematically evaluated an observed phenomenon of rapid and symptomatic disease progression that occurs in patients with EGFR-mutant lung cancer and acquired clinical resistance to gefitinib and/or erlotinib shortly after discontinuation of the EGFR kinase inhibitor. By using the standard practice of a mandated drug washout period during clinical trials, we evaluated the disease course and associated clinical and molecular factors of patients enrolled on clinical trials to treat acquired resistance. We found that 23% of patients experienced a symptomatic disease flare, defined as hospitalization and/or death attributable to disease progression after discontinuation of erlotinib or gefitinib but before initiation of study drug. While this analysis has the obvious limitations of a retrospective study, we feel that the risk of disease flare in this patient population is significant enough to merit modification of clinical trial design to abbreviate standard washout periods in trials for patients with EGFR-mutant lung cancer and acquired resistance.
Introduction
The treatment of non-small cell lung cancer (NSCLC) has been dramatically altered in the past decade with the identification of somatic gene mutations that underlie tumor initiation and maintenance. EGFR mutations were first identified in lung cancer after clinical benefit to EGFR tyrosine kinase inhibitors was observed(1-4). Evaluation of tumor specimens in these patients led to the identification of two common mutations in EGFR, the exon 19 deletion and the exon 21 L858R missense mutation, which lead to constitutively activated EGFR kinase readily inhibited by gefitinib and erlotinib.
In patients with NSCLC whose tumors harbor an EGFR mutation, first-line TKI therapy is recommended(5-7). Patients who initially benefit from erlotinib or gefitinib and then develop progression of disease, are described as having acquired resistance(8). There is no genotype-directed standard therapy for patients with acquired resistance to erlotinib or gefitinib. Cytotoxic therapies are generally used(9). Studies of second-generation EGFR inhibitors, as well as other strategies, are ongoing.
Even after the development of acquired resistance to gefitinib and/or erlotinib, EGFR-mutant lung cancer has a unique biology, with a median post-progression survival of 19 months in patients with an acquired T790M resistance mutation and 12 months in those without an identified T790M, though it is important to note that in the reported series TKI therapy was continued in 91% and 83% of these patients, respectively(10). Despite this remarkable post-progression survival, we have noted that discontinuation of EGFR inhibition causes some patients to experience more rapid progression of symptoms, or a disease flare. Nonetheless, anti-cancer treatment discontinuation is usually mandated as part of the eligibility criteria for enrollment in clinical trials. We used this practice of a trial-mandated erlotinib and gefitinib washout period as an opportunity to estimate the frequency of disease flare upon EGFR inhibitor discontinuation and to evaluate clinical and molecular characteristics that may be associated with disease flare.
Materials and Methods
We identified all patients enrolled in therapeutic clinical trials at our institution for patients with lung cancer who had developed acquired resistance to erlotinib and/or gefitinib. Trials were only included if they mandated discontinuation of the TKI for at least 7 days. Patients were included in this analysis if their tumors harbored a sensitizing EGFR mutation (exon 18 point mutation G719X , exon 19 deletion, or exon 21 point mutations L858R and L861Q) and met the consensus criteria for acquired resistance(8). Patients who were enrolled in more than once such clinical trial were evaluated only during the initial washout period. Testing for EGFR mutations was as previously described(11, 12).
The primary endpoint of this analysis was frequency of disease flare after discontinuation of TKI, defined as hospitalization or death attributable to disease progression after stopping the TKI and prior to initiation of study therapy. Hospitalizations due to infection, venous thromboembolism and other non-oncologic issues were not considered a disease flare. Clinical characteristics and disease course were reviewed for all patients under an Institutional Review Board/Privacy Board waiver. Time to progression (TTP) on initial TKI was calculated from date of first TKI until date of physician documented progression. Time on TKI was calculated from date of first TKI until date of discontinuation for trial washout. For patients who had a disease flare, time to flare was calculated from date of TKI discontinuation to date of hospitalization or death. Correlative variables that are binary were evaluated with the Fisher exact test. For time to progression on TKI, distribution plots were compared with Wilcoxon rank tests.
Results
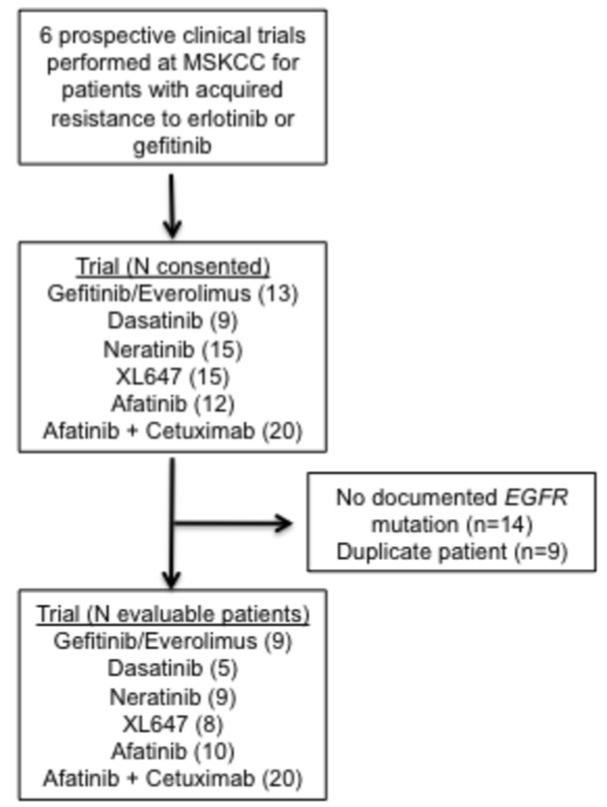
Six clinical trials studying patients with acquired resistance to EGFR TKIs were identified, accruing patients between August of 2005 and January 2011. The 6 trials included in this analysis and the numbers of patients who participated in each trial are included in Figure 1(13-17). Of the 84 patients enrolled in the 6 trials studied, 14 were excluded due to lack of a documented sensitizing mutation in EGFR. Seven patients were enrolled in multiple trials (three trials for 2 patients and two trials for 5 patients). These patients were included only during their first washout period. Patient characteristics are presented in Table 1.
Figure 1.
Trials evaluated
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
|
| |
| Male sex – N (%) | 13 (21) |
|
| |
| Age at diagnosis in years – Median (range) | 58 (26-78) |
|
| |
| Smoking Status | |
| Former/Current | 12 (20) |
| Never (<100 cigarettes lifetime) | 49 (80) |
|
| |
| EGFR mutation – N (%) | |
| Exon 19 deletion | 41 (67) |
| Exon 18 E709A and G719A | 1 (2) |
| Exon 21 L858R | 19 (31) |
|
| |
| Time on gefitinib or erlotinib (months) | |
| Median (range) | 19 (7-78) |
|
| |
| Age at enrollment in years – Median (range) | 61 (27-80) |
|
| |
| Karnofsky Performance Status at enrollment (%) | |
| 90% | 13 (21) |
| 80% | 37 (61) |
| 70% | 11 (18) |
|
| |
| Lines of therapy prior to gefitinib/erlotinib (N%) | |
| 0 | 37 (61) |
| 1 | 20 (32) |
| 2 | 4 (7) |
| Lines of therapy after acquired resistance | |
| 0 | 41 (67) |
| 1 | 12 (20) |
| 2+ | 8 (13) |
|
| |
| First-line gefitinib/erlotinib – N (%) | |
| Single-agent | 29 (48) |
| With chemotherapy | 8 (13) |
| Maintenance after chemo | 5 (9) |
|
| |
| Gefitinib/erlotinib/Both – N (%) | |
| Erlotinib | 50 (82) |
| Gefitinib | 6 (10) |
| Gefitinib followed by erlotinib | 5 (8) |
|
| |
| Acquired resistance to trial enrollment (months) | |
| Median (range) | 6 (0-48) |
Fourteen of the 61 patients included in this analysis (23%, 95% CI 14-35%) had a disease flare (hospitalization or death attributable to disease progression) after discontinuation of the TKI. The median time to disease flare was 8 days (range 3 - 21 days). Only 3 of the 14 patients (21%) continued on the therapeutic trial in which they had enrolled. The clinical characteristics of the 14 patients who had a disease flare are shown in Table 2. Of these, 9 had EGFR exon 19 deletions, 1 had two exon 18 point mutations and 4 had exon 21 L858R point mutations. EGFR T790M results at the time of acquired resistance were available for 52 patients including 11 of the 14 patients with disease flare. An EGFR T790M was mutation found in 6 patients (55%) who experienced a disease flare and 27 patients (59%) who did not.
Table 2.
Disease characteristics and course of patients with disease flare after discontinuation of TKI. TKI (tyrosine kinase inhibitor)
| Sex & Smoking |
EGFR
mutation |
T790M | Sites of Disease |
Days off TKI |
Description of Flare |
|---|---|---|---|---|---|
| 47 Female Never smoker |
Exon 19 Deletion |
No | Pleura Brain Liver |
21 days | Progressive liver metatases with liver failure and death |
| 64 Female Never smoker |
Exon 19 Deletion |
No | Pleura | 21 days | Dyspnea |
| 53 Female Never smoker |
Exon 19 Deletion |
Unknown | Pleura Brain Bone |
14 days | CNS progression |
| 60 Female Never smoker |
Exon 21 L858R |
Unknown | Pleura Brain Bone |
7 days | Hypoxia and Bone pain |
| 34 Male Never smoker |
Exon 19 Deletion |
No | Pleura Brain |
11 days | Dyspnea |
| 27 Female Never smoker |
Exon 19 Deletion |
Unknown | Brain Liver Bone Pericardium |
11 days | Bone pain |
| 47 Female Never smoker |
Exon 21 L858R |
Yes | Pleura | 7 days | New leptomeningeal disease |
| 49 Male Never smoker |
Exon 19 Deletion |
Yes | Bone Liver |
7 days | New brain metastases, seizure |
| 61 Female Never smoker |
Exon 21 L858R |
No | Pleura Brain Liver Peritoneum |
3 days | Abdominal pain |
| 45 Female Never smoker |
Exon 19 Deletion |
Yes | Pleura Bone |
8 days | New leptomeningeal carcinomatosis, seizure, death |
| 46 Female Former smoker |
Exon 19 Deletion |
Yes | Pleura Liver Bone |
12 days | Epidural progression |
| 62 Female Never smoker |
Exon 19 Deletion |
Yes | Pleura Bone Pericardium |
8 days | Pericardial tamponade, death |
| 42 Female Never smoker |
Exon 18 E709A & G719A |
Yes | Pleura Bone |
8 days | Acute pleural effusion requiring drainage |
| 67 Female Former smoker |
Exon 21 L858R |
No | Pleura Brain |
8 days | Dyspnea |
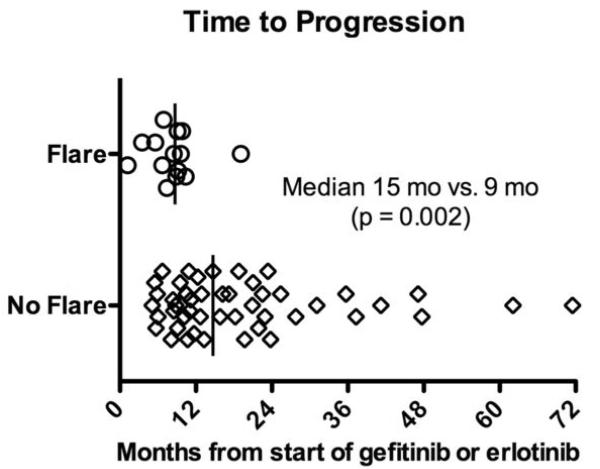
We compared clinical characteristics in patients who did and did not develop a flare after TKI discontinuation. Patients with disease flare had a shorter time to progression on TKI treatment (median 9 months, range 1-19 months) than patients who did not experience flare (median15 months, range 5-72 months, p = 0.002) (Figure 2). The time from documentation of acquired resistance to trial enrollment for all patients was a median of 6 months (range 0 – 48 months) and was not different between the groups (p=0.46). In the 30 days prior to TKI discontinuation, there were 4 disease-related hospitalizations, 2 in each group (p=0.2). Flare was associated with the presence of brain or pleural metastases prior to TKI discontinuation (p=0.01 and 0.03, respectively). Flare was not associated with type of EGFR sensitizing mutation, presence/absence of T790M, performance status, sex, tobacco use, or specific TKI (Table 3).
Figure 2.
Time to progression on initial tyrosine kinase inhibitor (TKI) in patients with and without flare after TKI discontinuation
Table 3.
Characteristics associated with flare following discontinuation of TKI. TKI (tyrosine kinase inhibitor)
| Clinical Characteristic | Number | % Flare | p-value |
|---|---|---|---|
|
| |||
| Exon 19 deletion | 41 | 22% | 1 |
| Exon 21 L858R | 19 | 21% | |
|
| |||
| T790M | 33 | 18% | 0.5 |
| No T790M | 19 | 26% | |
|
| |||
| First-line TKI alone | 29 | 34% | 0.06 |
| First-line chemo | 32 | 13% | |
|
| |||
| TKI only | 20 | 30% | 0.5 |
| Chemo | 41 | 20% | |
|
| |||
| Symptoms of disease progression | 20 | 40% | 0.05 |
| No symptoms | 41 | 15% | |
|
| |||
| Karnofsky Performance Status 70% | 11 | 36% | 0.4 |
| Karnofsky Performance Status 80-90% | 50 | 22% | |
|
| |||
| CNS involvement | 14 | 50% | 0.01 |
| No CNS disease | 47 | 15% | |
|
| |||
| Bone involvement | 31 | 26% | 0.8 |
| No bony disease | 30 | 20% | |
|
| |||
| Pleural involvement | 36 | 33% | 0.03 |
| No pleural disease | 25 | 8% | |
|
| |||
| Male | 13 | 15% | 0.7 |
| Female | 48 | 25% | |
|
| |||
| Erlotinib | 40 | 33% | 0.3 |
| Gefitinib | 11 | 9% | |
|
| |||
| Current/Former Smoker | 12 | 16% | 0.7 |
| Never smoker | 49 | 24% | |
Discussion
In this analysis of patients with acquired clinical resistance to EGFR TKI therapy, we observed a 23% flare rate during the EGFR TKI washout period, defined as hospitalization or death attributable to disease progression. After identifying the phenomenon of disease flare in a prior study(14), we had attempted to minimize harm by abbreviating the standard washout periods of 21-28 days to 14 days in more recent trials. Despite this precaution, patients continued to experience flare after a median of only 8 days off TKI. Characteristics associated with development of flare included a shorter time to progression on initial TKI, preceding symptoms of disease progression, and presence of CNS and pleural disease. We recently reported that when acquired resistance is attributable to a T790M point mutation, disease may follow a more indolent course than clinical resistance without T790M(10). However, in this study there was not a lower rate of disease flare in patients with T790M-mediated acquired resistance. We believe that the rate of rapid disease progression reported here is clinically significant and should alter the design of clinical trials in this patient population.
This analysis has the inherent limitations of a retrospective study, which prevented us from identifying a matched comparison group to study the prevalence of disease flare in the absence of TKI discontinuation. Attempts to model an internal comparison group by comparing the patterns of disease progression within the same patient before and after TKI discontinuation were hampered by incomplete data on some patients prior to enrollment. This cohort was relatively fit in that all patients had a KPS of ≥70%. Furthermore, the median time from acquired resistance to discontinuation of TKI was 6 months and the hospitalization rate in the 30 days before TKI washout was not significantly different between the patients who experienced a disease flare and those who did not; this supports a causal relationship between TKI discontinuation and disease flare rather than a more aggressive underlying biology in the patients who experienced a disease flare. Due to the high event rate and no discernable difference in groups during the period between development of acquired resistance and trial enrollment, we anticipate that these observations are likely to be replicated if evaluated prospectively, although we do not advocate this as it may lead to an unacceptable risk to patients.
When acquired resistance occurs in oncogene-driven cancers with kinase activation, kinase activation persists or increases despite continued treatment with a kinase inhibitor. However, not every cell is resistant, as demonstrated in gastrointestinal stromal tumors where the resistant and clones can be visualized in a “matrix” of sensitive cells(18). If imatinib is stopped in these patients, growth is accelerated in the sensitive clone resulting in rapid and symptomatic progression that is associated with a PET flare(19). We believe the same mechanism occurs in EGFR-mutant lung cancers that have developed acquired resistance to erlotinib or gefitinib(20).
Based on preclinical studies that show EGFR-mutant cells made resistant to gefitinib can become sensitive once again by successive passages in the absence of the TKI(21) and clinical observations that after a period of TKI discontinuation patients can respond again to re-institution of the same agents(14), some have suggested withdrawal and retreatment with the same TKI after a “drug holiday” as a strategy to counter acquired resistance. Our data suggest that this approach is not suitable for all patients and that drug holidays can lead to more rapid tumor growth. A more appropriate approach would be to immediately substitute another therapeutic agent or add a new agent to the TKI.
As we believe that oncogene-addiction persists after the development of acquired resistance, clinical trials to investigate alternative treatment strategies are essential. The data presented here suggest that the usual trial-mandated EGFR TKI washout period in this patient population may be associated with an unacceptably high risk of more rapid disease progression prior to initiation of experimental agents. Mandated drug washout periods are designed to prevent interactions between drugs but are often broadly written to include all anti-neoplastic drugs. In early phase studies evaluating the safety and efficacy of erlotinib, the half-life (t1/2) was determined to be 8 hours(22). Therefore, in patients with normal hepatic function, a 24-48 hour washout period should be sufficient for drug clearance and will minimize the risk of significant disease flare. Only 1 of 14 patients in the flare group experienced the flare in 3 or fewer days after TKI discontinuation.
Oncogene addiction is a phenomenon recognized in many malignancies. Targeting the downstream effects (especially kinase activation) of these driver-mutations has been a successful strategy in chronic myelogenous leukemia, gastrointestinal stromal tumors, BRAF-mutant melanoma, and ALK-rearranged and EGFR-mutated lung cancer with dramatic and often durable responses(23-26). Gastrointestinal stromal tumors have a unique biology with rapid disease progression when the kinase inhibitor imatinib is removed after prolonged benefit(27). This series describes a similar flare phenomenon in the setting of acquired resistance in EGFR-mutant lung cancer when gefitinib or erlotinib are stopped because of radiographic disease progression and we anticipate that similar observations will be made in other oncogene driven malignancies treated with targeted inhibitors. As we investigate better treatments for patients with tumors that have developed acquired resistance to gefitinib and erlotinib, clinical trials should abbreviate trial mandated washout periods to minimize the risk of disease flare upon TKI discontinuation in patients with EGFR-mutant lung cancer.
Acknowledgments
Grant Support:
This work was supported in part by the grants P01-CA129243 (M.G. Kris) and NIH T32 CA009207 (D. Bajorin).
Financial Support: P01-CA129243 (M.G. Kris), T32 CA009207 (D. Bajorin)
Footnotes
Disclosures: G.J. Riely: consultant AstraZeneca, Boehringer-Ingelheim. M.G. Kris: consultant, AstraZeneca, Boehringer-Ingelheim, Pfizer. V.A. Miller: consultant Roche, Genentech, OSI.
References
- 1.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Kris MG, Lau CY, Ang D, Brzostowski E, Riely GJ, Rusch VW, et al. Initial results of LC-MAP: An institutional program to routinely profile tumor specimens for the presence of mutations in targetable pathways in all patients with lung adenocarcinoma. J Clin Oncol (Meeting Abstracts) 2010;28:7009. [Google Scholar]
- 3.Miller VA, Johnson DH, Krug LM, Pizzo B, Tyson L, Perez W, et al. Pilot trial of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib plus carboplatin and paclitaxel in patients with stage IIIB or IV non-small-cell lung cancer. J Clin Oncol. 2003;21:2094–100. doi: 10.1200/JCO.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–9. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Zhou C, Wu Y, Sun L, Zhang L, Zhang Y, et al. First ling Erlotinib vs Gemcitabline/Carboplatin in Activating EGFR Mutation-Positive Advanced Non-Small Cell Lung Cancer: Efficacy and Safety Results from Phsae III OPTIMAL Study in Chinese Patients. J Thorac Oncol. 2010;5:S373–4. [Google Scholar]
- 8.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–60. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 10.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Lung Cancer: Distinct Natural History of Patients with Tumors Harboring the T790M Mutation. Clin Cancer Res. 2011;17:1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li AR, Chitale D, Riely GJ, Pao W, Miller VA, Zakowski MF, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10:242–8. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin Cancer Res. 2011;17:1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price KA, Azzoli CG, Krug LM, Pietanza MC, Rizvi NA, Pao W, et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1623–9. doi: 10.1097/JTO.0b013e3181ec1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi N, Kris M, Miller V. A phase II study of XL647 in non-small cell lung cancer patients enriched for EGFR mutation. San Francisco, CA: 2007. al. e. [Google Scholar]
- 16.Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–83. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 17.Spicer J, Calvert H, Vidal L. Seoul: 2007. Activity of BIBW2992, an oral irreversible dual EGFR/HER2 inhibitor, in non-small cell lung cancer with mutated EGFR. al. e. [Google Scholar]
- 18.Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892–8. doi: 10.1148/radiol.2353040332. [DOI] [PubMed] [Google Scholar]
- 19.Van den Abbeele AD, Badawi RD, Manola J, Morgan JA, Desai J, Kazanovicz A, et al. Effects of cessation of imatinib mesylate (IM) therapy in patients (pts) with IM-refractory gastrointestinal stromal tumors (GIST) as visualized by FDG-PET scanning. J Clin Oncol. 2004;22:3012. [Google Scholar]
- 20.Foo J, Chmielecki J, Pao W, Michor F. A computational approach to optimize dosing strategies for EGFR tyrosine kinase inhibitors in EGFR mutant lung adenocarcinoma. AACR. 2009;50:594. [Google Scholar]
- 21.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling J, Johnson KA, Miao Z, Rakhit A, Pantze MP, Hamilton M, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos. 2006;34:420–6. doi: 10.1124/dmd.105.007765. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 24.Druker BJ. Imatinib: A Viewpoint by Brian J. Druker. Drugs. 2001;61:1775–6. doi: 10.2165/00003495-200161120-00009. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic Lymphoma Kinase Inhibition in Non,ÄìSmall-Cell Lung Cancer. New England of Medicine. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–9. doi: 10.1016/S1470-2045(10)70222-9. [DOI] [PubMed] [Google Scholar]