Abstract
Although kappa opioid receptor (KOP-r) antagonists are known to reduce reinstatement of cocaine, alcohol and nicotine seeking induced by a variety of stressors, the role of KOP-r in yohimbine-induced reinstatement of heroin seeking has not been investigated. Yohimbine, used as a stressor, increases the hypothalamic-pituitary-adrenal (HPA) hormones, causes anxiety and induces heroin craving in humans. The present experiments were undertaken to assess the effects of yohimbine on reinstatement of heroin seeking and associated changes in preprodynorphin (ppDyn) expression and HPA hormonal levels; and to determine whether these effects could be reduced by pretreatment with the selective KOP-r antagonist nor-binaltorphimine (nor-BNI). After heroin self-administration for 12 days (3h/day, 0.05 mg/kg/infusion, i.v.) and extinction for 8 days, reinstatement included the first baseline test after vehicle injection, the second test of yohimbine-induced reinstatement (1.25 mg/kg, i.p.), pretreatment with vehicle or nor-BNI (20 mg/kg, i.p.), the third baseline test after vehicle injection, and the final test of yohimbine-induced reinstatement. Immediately after the last test, several mesolimbic regions and plasma were collected for analyses of ppDyn and KOP-r mRNA levels and HPA hormones. Yohimbine-induced reinstatement was fully blocked by nor-BNI pretreatment. Furthermore, yohimbine elevated plasma HPA hormones, and this increase was blunted by nor-BNI. Finally, rats pre-treated with yohimbine displayed increased ppDyn mRNA levels in the nucleus accumbens shell and central nucleus of the amygdala. These data suggest that the stress responsive ppDyn/KOP-r system is a critical component of the neural circuitry underlying the effect of yohimbine stress on heroin seeking behavior and HPA activity.
Keywords: dynorphin, kappa opioid receptor, nucleus accumbens shell, central nucleus of the amygdala, heroin self-administration, yohimbine-induced reinstatement
One well-documented neurobiological change in response to chronic exposure to opiates, psychostimulants, alcohol and nicotine is the increased activity of the dynorphin (Dyn) and kappa opioid receptor (KOP-r) systems within brain circuits involved in motivated behavior [Shippenberg et al., 2007]. KOP-r agonists or natural Dyn peptide dose-dependently reduce basal and drug-induced dopamine release in the striatum of rodents [Spanagel et al., 1992; Zhang et al., 2004]. Activation of KOP-r produces depressive-like behaviors in rodents [Todtenkopf et al., 2004], whereas KOP-r blockade can result in antidepressant-like effects [Newton et al., 2002]. KOP-r antagonists exhibit anxiolytic activity in rodents [Knoll and Carlezon, 2010]. In recent years, KOP-r antagonists have been extensively investigated for their effects on the addictive properties of cocaine. KOP-r agonists can, under some circumstances, potentiate the reinforcing effects of cocaine [McLaughlin et al., 2006], whereas KOP-r antagonists can prevent stress-induced reinstatement of cocaine-seeking behavior [Beardsley et al., 2005; Aldrich et al., 2009]. In addition, a selective KOP-r antagonist nor-binaltorphimine (nor-BNI) attenuates drug self-administration in rats dependent on alcohol [Walker and Koob, 2008] or cocaine [Wee et al., 2009], and block nicotine withdrawal [Jackson et al., 2010].
Many stress-related peptides, and their receptors, are involved in drug cravings in humans [Sinha et al., 2006; Koob and Kreek, 2007] and in reinstatement of lever pressing in animals triggered by exposure to various stressors [Leri et al., 2002; Shaham et al., 2003; Zhou et al., 2008]. Although the ability of KOP-r antagonists to prevent stress-induced reinstatement of cocaine, alcohol and nicotine seeking behavior has been established, the effects of such compounds on reinstatement of heroin seeking have not been explored. Our hypothesis of the research described here is that a hyper-responsivity within the mesolimbic Dyn/KOP-r system is one cause of heroin seeking behavior. Therefore, the first aim of this study was to investigate the effect of the KOP-r antagonist nor-BNI on reinstatement of heroin seeking induced by yohimbine (Yoh). Laboratory studies in humans found that Yoh increased hypothalamic-pituitary-adrenal (HPA) activity, as well as subjective anxiety in normal subjects [Rosen et al., 1999] and drug craving in abstinent opiate addicts [Stine et al., 2002]. Yoh enhances central noradrenergic activity by acting as an antagonist at alpha-2 adrenergic autoreceptors and reinstates methamphetamine, cocaine, heroin, alcohol and food seeking [see recent reviews by See and Waters, 2011; Sinha et al, 2011]. The second aim was to explore alterations in Dyn activity, as well as plasma HPA hormonal levels, associated with Yoh-induced reinstatement. Therefore, male Sprague-Dawley rats were trained to self-administer heroin intravenously, followed by a period of withdrawal/extinction, and then tested for Yoh-induced reinstatement of heroin seeking. Following the behavioral test, specific brain regions were collected, and preprodynorphin (ppDyn) mRNA levels were quantified in the nucleus accumbens (NAc) core and shell, caudate-putamen (CPu), central nucleus of the amygdala (CeA), and medial/basolateral amygdala (Me/BLA). To allow for the correct interpretation of neurochemical data obtained in the Yoh-treated rats after heroin self-administration, alterations of ppDyn mRNA and HPA hormonal levels were also analyzed in heroin naïve rats treated with Yoh. Detailed methods are provided in the Supporting Information (SI), along with the plasma HPA hormone levels.
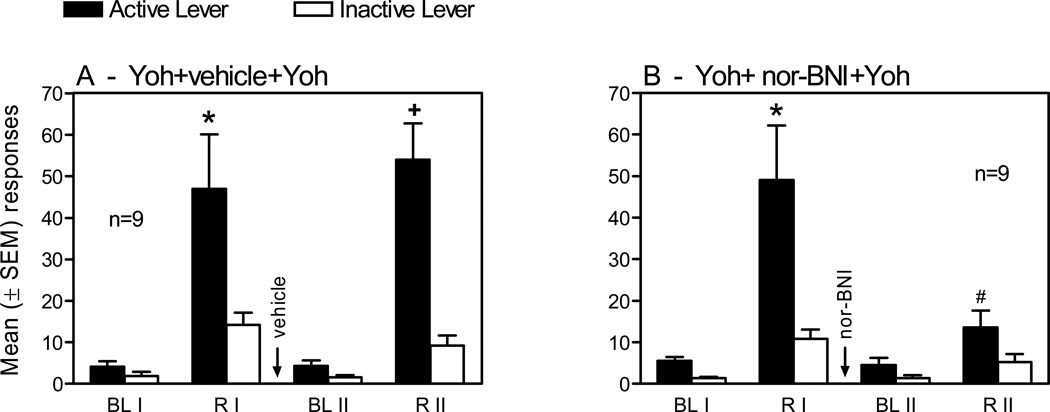
Behavior. Over the 12 sessions of heroin self-administration, there was an increase in responding on the active lever (Table S1A), and this was subsequently reduced by 8 days of extinction. Fig. 1 represents responding on the active and inactive levers during the reinstatement phase of the experiment in animals treated with Yoh (1.25 mg/kg, i.p.) alone in Reinstatement test I (R I) and with either 0 or 20 mg/kg nor-BNI prior to the second Yoh-induced Reinstatement test II (R II) (see Fig. S1 for a detailed timeline). The ANOVA revealed significant Group × Test × Lever interaction [F(3,48)=3.31, p<0.05], Group × Test interaction [(3,48)=3.56, p<0.05], and Test × Lever interaction [F(3,48)=9.36, p<0.01]. There were also significant main effects of Test [F(3,48)= 21.1, p<0.01] and of Lever [F(1,16)=19.9, p<0.01]. In the group that did not receive nor-BNI, Yoh enhanced responding on the active and inactive levers in both the R I and R II tests (Fig. 1A), but the increase was significant only on the active lever (p<0.01 for both the R I and RII, Newman-Keuls post-hoc tests). In the group pre-treated with nor-BNI prior to the R II test, however, Yoh produced significant reinstatement of responding only on the active lever in the RI test (p<0.01) (Fig. 1B). The inhibiting effect of nor-BNI on Yoh-induced reinstatement was further revealed by a significant difference (p<0.01) in active lever responding between groups on the R II test (compare Fig. 1A and 1B).
Fig 1.
Mean (±SEM) responses on the active and inactive levers during 3 hours after exposure to yohimbine (Yoh) in reinstatement tests following extinction in heroin self-administering (SA) Sprague-Dawley rats. Twenty min prior to the reinstatement tests I and II (R I and R II), rats were injected with Yoh (1.25 mg/kg, i.p.). Before each reinstatement test, a separate 3-hour extinction served as baselines I and II (BL I and BL II). Two days before Yoh-induced R II, rats were pretreated with an injection of nor-BNI (20 mg/kg, i.p.) or vehicle (saline). See Fig. S1 in SI section for a detailed timeline. * p<0.01 vs. active lever in BL I and inactive lever in R I; + p<0.01 vs. active lever in BL II and inactive lever in R II; # p<0.01 vs. active lever in R I and active lever in R II in the vehicle-pretreated Yoh group.
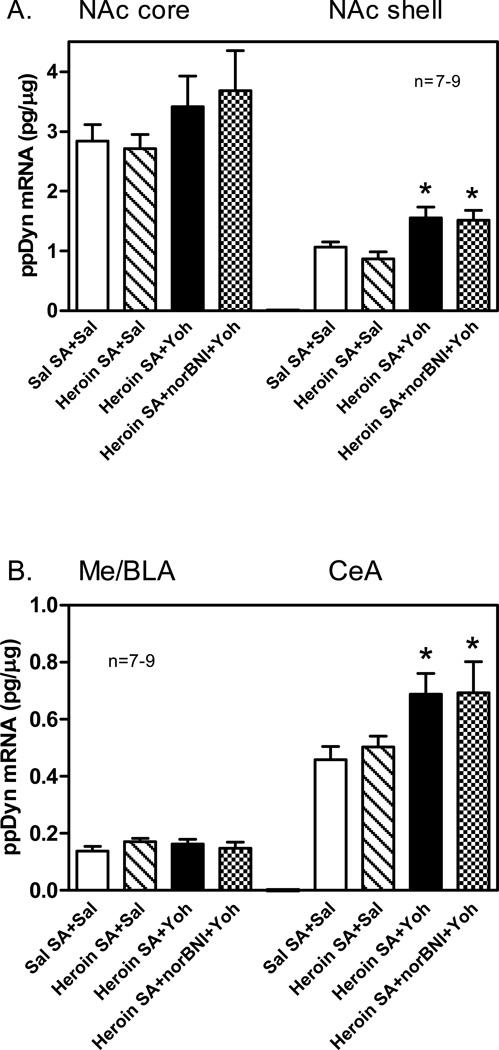
Neurochemistry. Levels of ppDyn mRNA were measured in the NAc core and shell, CPu, CeA, Me/BLA and lateral hypothalamus (LH) in rats that: (1) self-administered saline and then received vehicle (saline) prior to both R I and R II sessions; (2) self-administered heroin and received saline prior to both R I and R II; (3) self-administered heroin and received Yoh prior to both R I and R II, and (4) self-administered heroin, received Yoh prior to both R I and R II, and nor-BNI pretreatment before R II. In the NAc shell (Fig. 2A), one-way ANOVA revealed a significant effect of Group [F(3,29)=5.28, p<0.01], and Newman-Keuls post hoc tests revealed that, compared to saline self-administration rats, there were significant increases in the ppDyn mRNA levels in the rats treated with either Yoh alone (p<0.05) or Yoh and nor-BNI (p<0.05) after heroin self-administration. No significant group differences were found in the NAc core (Fig. 2A). In the CeA (Fig. 2B), one-way ANOVA revealed a significant effect of Group [F(3,28)=3.09, p<0.05] and the ppDyn mRNA levels were increased in the rats treated with either Yoh alone [df(1,28)=5.71, p<0.05, Planned comparison] or Yoh and nor-BNI [df(1,28)=5.33, p<0.05, Planned comparison] after heroin self-administration. No significant group differences were found in the Me/BLA (Fig. 2B). Finally, no significant group differences were found in the CPu and LH (see Table S2A in the SI). Analysis of KOP-r mRNA levels in the same groups and regions did not identify significant group differences in the NAc shell or core or Me/BLA (see Table S2B in the SI). In a separate experiment, levels of ppDyn mRNA were also compared in heroin naïve rats after Yoh (1.25 mg/kg) or saline treatment. As shown in Table S3 in the SI, there were no significant changes in ppDyn mRNA levels in the NAc shell or core, CeA or CPu.
Fig 2.
Preprodynorphin (ppDyn) mRNA levels in nucleus accumbens (NAc) core and shell (A), medial/basolateral amygdala (Me/BLA) and central nucleus of amygdala (CeA) (B) in saline (Sal) or heroin self-administering (SA) Sprague-Dawley rats after yohimbine (Yoh, 1.25 mg/kg, i.p.) with KOP-r antagonist nor-BNI pretreatment (20 mg/kg, i.p.). Animals were sacrificed 10 min after R II test, and tissues processed as described in the SI. Significant differences are indicated: *p<0.05 vs. Sal SA+Sal group.
KOP-r activation modulates drug-induced dopamine release in the NAc and CPu [Spanagel et al., 1992] and may potentiate or attenuate the reinforcing action of drugs depending on the stage of drug exposure or stress [McLaughlin et al., 2006; Maiya et al., 2009]. KOP-r antagonists have been investigated for their effects on the reinforcing action of drugs of abuse, depression and stress [Aldrich and McLaughlin, 2009; Knoll and Carlezon, 2010]. The present study provides initial evidence for the involvement of the ppDyn/KOP-r systems in heroin seeking. In fact, in rats trained to self-administer heroin, Yoh-induced reinstatement was blocked by pre-treatment with nor-BNI, and in heroin self-administration rats treated with Yoh, there were increases in ppDyn mRNA levels in the NAc shell and CeA.
In humans, heroin dependence is often characterized by negative affective states, including anhedonia (an inability to experience pleasure from rewarding stimuli), anxiety and disrupted stress responses during drug withdrawal [Koob and Kreek 2007]. Stress can potentiate negative affective states in heroin abstinent people and trigger craving and relapse [Sinha et al., 2011]. A relative hyper-responsivity of the Dyn system may represent one critical neuro-adaptation in response to stress during heroin withdrawal, and could be important for craving in humans. The present study utilized a drug-seeking behavioral model in which rats that underwent extinction after self-administering heroin displayed reinstatement of operant responding induced by Yoh. In the heroin-withdrawn rats, ppDyn mRNA levels were not different from the control (saline self-administration rats not treated with Yoh). But, in response to Yoh, the heroin self-administration rats displayed increased ppDyn mRNA level in the NAc shell and CeA, two brain regions known to play an important role in drug seeking and anxiety. Consistent with these results, Yoh has been found to potently induce neuronal activation in the NAc shell and CeA [Cippitelli et al., 2010]. Increased Dyn activity might be expected as a result of enhanced ppDyn gene expression, and thus this heightened Dyn tone may be one factor involved in reinstatement of heroin seeking caused by Yoh. Although interesting, the ppDyn result of the current study should be interpreted with caution. The heroin naïve rats treated with Yoh (Table S3) did not control for a possible interaction between acquisition of operant behavior and Yoh (Fig. 2), and such experiment could be conducted in animals trained to self-administer sucrose and then treated with Yoh.
The behavioral objective of these experiments was to investigate the potential of KOP-r receptor blockade to reduce heroin seeking when reinstatement was induced by Yoh. Although the main effects of Yoh on behaviours motivated by drugs of abuse are related to noradrenergic and HPA axis activation, Yoh-induced reinstatement of cocaine and alcohol seeking also has a considerable serotonergic component [see recent review by Sinha et al., 2011]. Nor-BNI (20 mg/kg, i.p.), a systemically active and selective KOP-r antagonist, significantly blocked Yoh-induced reinstatement. Consistent with our finding, KOP-r antagonists have been found to reduce drug seeking induced by a variety of stressors, like foot-shock or forced swim, but not by drug priming with drugs of abuse [Beardsley et al., 2005; Aldrich et al., 2009]. It is unlikely that this resulted from a suppression of general activity because the well-established anxiolytic profile of the compound is based on performance in tests where reductions in anxiety are indicated by enhanced motor activity [Knoll and Carlezon 2010]. Therefore, these results suggest that Dyn activation of KOP-r receptors in response to Yoh plays a critical role in modulating the effects of Yoh stress on reinstatement of heroin seeking. To our knowledge, this is the first demonstration of KOP-r involvement in heroin seeking behavior.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Vadim Yuferov for his comments during manuscript preparation. This work was supported by NIH NIDA Research Center Grant DA-P60-05130 (MJK), NET grant from CIHR (FL) and R01 DA 032928 (JVA). The authors would like to thank the NIDA Division of Drug Supply and Analytical Services, Drs. J Douglass and O Civelli for rat ppDyn cDNA, and Dr. L Yu for rat KOR cDNA.
References
- Aldrich JV, McLaughlin JP. Peptide kappa opioid receptor ligands: potential for drug development. AAPS J. 2009;11:312–322. doi: 10.1208/s12248-009-9105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci U S A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist JDTic on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology. 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiya R, Zhou Y, Norris E, Kreek MJ, Strickland S. Tissue plasminogen activator modulates cellular and behavioral response to cocaine. Proc Natl Acad Sci. 2009;106:1983–1988. doi: 10.1073/pnas.0812491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace T, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Kosten TR, Kreek MJ. The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol Psychiatry. 1999;45:1636–1645. doi: 10.1016/s0006-3223(98)00259-5. [DOI] [PubMed] [Google Scholar]
- See RE, Waters RP. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2010;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–231. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate mesolimbic dopaminergic pathway. Proc Natl Acad Sci. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine S, Southwick S, Petrakis I, Kosten T, Charney D, Krystal J. Yohimbine-induced withdrawal and anxiety symptoms in opioid dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–270. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–275. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine induced place preference in C57BL/6J mice. Psychopharmacology. 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.