Abstract
The goal of this study was to determine the role of integrin-mediated adhesion of bone-marrow-derived progenitor cells (BMPCs) as a requirement for the endothelial barrier protection in a lung injury model. C57BL mice were used as the source for BMPCs, which were characterized as CD34+ and fetal liver kinase-1 (Flk1)+ and also an expression of a repertoire of integrins. We used a mouse model of bacterial lipopolysaccharide (LPS)-induced lung vascular injury and edema formation to test the effects of BMPC integrin expression in preventing endothelial barrier injury. Adhesion of BMPCs to purified extracellular matrix proteins induced focal adhesion kinase (Fak) phosphorylation and formation of branching point structures in a α4 and α5 integrin-dependent manner. BMPCs expressing red fluorescent protein (RFP) were administered via the retro-orbital venous route in mice treated intraperitonially with LPS (7.5 mg/kg body weight). We observed increased retention of RFP-labeled Flk1+ and CD34+ BMPCs for up to 8 weeks in mice injured with LPS. BMPC transplantation increased survival by 50% (at 72–96 hours after LPS) and reduced lung vascular injury and extravascular water content induced by LPS. However, blocking with anti-α4 or anti-α5 integrin antibody or shRNA-mediated silencing of α4 or α5 integrins in donor BMPCs failed to prevent the vascular injury or edema formation and mortality. Thus, α4 and α5 integrin-dependent adhesion of BMPCs in lung tissue plays a critical role in preventing lung vascular injury and increasing survival in a mouse model of LPS-induced acute lung injury.
Keywords: Acute lung injury, Endothelial progenitor cells, Endothelial barrier, Integrins, Stem Cells
Introduction
The repair of tissue in adults following injury requires trafficking and differentiation of both resident and nonresident progenitor stem cells [1–4]. Bone marrow (BM) serves as a reservoir for both hematopoietic and nonhematopoietic stem/ progenitor cells that are central to tissue repair [1–4]. Under normal steady-state conditions these cells remain relatively dormant in their BM niche but can migrate, sense survival cues, attach, and differentiate in response to injury [2–4]. In experimental transplantation studies using BM-derived mononuclear cells (MNCs), both hematopoietic and nonhematopoietic stem cells were detected in lungs, heart, and liver [5–7]. A subset of MNCs differentiated into CD34+ and Flk1+ progenitor cells, which have been defined as endothelial progenitor cells (EPCs) [8–10]. EPCs can traffic to and localize at sites of injury or ischemia where they differentiate into vascular endothelial cells in limb ischemic disease, myocardial infarction, and tumors and thereby promote neovascularization [8–14]. Results from these studies suggest two possible mechanisms of benefit: (a) by intercalating directly with endothelial monolayer or accumulating behind the vascular wall; (b) by secreting growth factors and cytokines, which then induce neovessel formation by activating resident stem/progenitor cells.
There are considerable uncertainties, however, in defining EPC characteristics in human subjects [15–18]. Several methods of EPC preparations have been described [15–18]. Classical EPCs isolation procedure relies upon peripheral blood MNCs [8, 15, 16]. These EPCs are characterized by their ability to form colonies (colony-forming unit, CFU-Hill colony) and may also contain nonadherent myeloid progenitor cells, monocytes, and T lymphocytes and function as accessory cells to promote neovascularization [6, 16, 18, 19]. A short-term culture of peripheral blood MNCs for 4–7 days gives rise to non-colony-forming adherent EPCs [16, 20, 21]. However, peripheral blood MNCs cultured for 6–21 days in endothelial cell conditions give rise to adherent late outgrowth endothelial colony-forming cells (ECFCs) with a characteristic cobblestone appearance [16, 20, 21]. Although studies are limited, human ECFCs may contribute to neovascularization [16, 20, 21]. In the present studies, we used mouse BM-derived CD34+ and Flk1+ cells, we refer to this set of cells as mouse BMPCs, and we specifically addressed the role of integrin expression in these cells in the mechanism of endothelial barrier protection.
In acute lung injury (ALI) patients, an increase in the number of circulating EPCs has been shown to correlate with better prognosis and survival [22]. Cell adhesion molecules including integrins likely play a key role in trafficking and adhesion of progenitor cells to the site of injury and tissue repair. The role of progenitor cells β2 and α4β1 integrins have been investigated with regard to homing and neovascularization [23–25]; however, the role of integrins in general in the mechanism of BMPC retention, thereby in promoting stable BMPC sequestration at the site of injury and mediating vascular repair, has not been described. Thus, here we ascertained the profile of integrin expression in adult mouse BM-derived CD34+ and Flk1+ adherent BMPC and role of donor BMPC integrins in mediating vascular repair in a mouse model of lipopolysaccharide (LPS)-induced lung vascular injury.
Materials and Methods
Antibodies, Extracellular Matrix, and shRNA Constructs
For EPC markers and anti-integrin antibodies, see supporting information Table 1. Anti-caveolin-1, anti-eNOS (clone 3), anti-Grb2, and Vitronectin (Vn) were purchased from BD Bioscience (Franklin Lakes, NJ, http://www.bdbiosciences.com). Goat anti-mouse vascular endothelial growth factor (VEGF) (AF-493-NA), goat anti-mouse Tie-2 (AF762), laminin-1 (Lm-1), and human plasma fibronectin (Fn) were purchased from R&D Systems Inc. (Minneapolis, http://www.rndsystems.com). Anti-Fak (2A7), anti-paxillin, anti-phosphotyrosine (4G10), anti-phospho-Erk1/2, anti-phospho-Fak (Y397), and anti-αvβ3 (LM609) antibodies, and laminin-4 (Lm-4) were purchased from Millipore (Temecula, CA, http://www.millipore.com). Type I collagen solution was purchased from Allergan (Irvine, CA, http://www.allergan.com). Non-silencing control shRNA (SHC002V), constructs for α2 (SHVRS-NM_002203) integrin, α5 (SHVRS-NM_002205) and α4 (SHVRS-NM_000885) integrin shRNA driven by lentiviral vectors, cycloheximide, monensin, tetramethylrhodamine isothiocyanate (TRITC)-phalloidin, and anti-vinculin mAb were purchased from Sigma (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com). Rabbit anti-vWF antibody (H300) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, http://www.scbt.com). Anti-lipid phosphate phosphatase-3 (LPP3) antibody was prepared in authors’ laboratory [26, 27]. Rabbit β1-cyto integrin pAbs were a kind gift of Dr. Guido Tarone (University of Torino, Torino, Italy). For blocking experiments, antibodies were dialyzed overnight in sterile 1× Tris-buffered saline (pH 7.5) at 4°C to remove any traces of impurities including sodium azide [26–28].
Model of Endotoxin-Induced Murine Microvessel Lung Injury
Four- to six-week-old C57BL6/J male (25–30 g body weight (BW); Charles River Laboratories International, Inc., Wilmington, MA, http://www.criver.com) were used for the in vivo experiments [29–31]. Mice were housed under pathogen-free conditions at the University of Illinois Animal Care Facility in accordance with institutional guidelines. Prior to cell therapy, mice were anesthetized with intramuscular injection of ketamine (60 mg/kg) and xylazine (2 mg/kg) in phosphate-buffered saline (PBS), pH 7.4.
Cell Culture
We used C57BL/6J mice as a source for BM-MNCs, which were isolated using Ficoll gradient centrifugation. Buffy coat-containing MNCs were used as described [32]. To expand EPCs, cells were cultured on dishes coated with 0.2% gelatin in EGM-2 media (Cambrex, East Rutherford, NJ, http://www.cambrex.com) supplemented with 5% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.4 mmol/l glutamine + microvascular single-quote supplements. To induce expansion of Flk1+ cells, VEGF165 (100 ng/ml; R&D Systems, Inc.) was added to the media. Culture dishes were replenished with fresh complete media every second day. The media did not contain epidermal growth factor (EGF) as a supplement so that EGF receptor-expressing epithelial cells did not proliferate. At the end of 7 days (passage 0–1), cells were detached and re-plated onto dishes coated with 0.2% gelatin in complete media. Purified EPCs were expanded up to the fifth passage (5p), which is equivalent to 25 days in culture. For all experiments, we used late outgrowth EPCs that were passaged up to p3 (21–22 days in culture, equivalent to two population doublings). Methods for isolation of mouse lung microvascular endothelial cells have been described [33].
Exposure to Lipopolysaccharide (LPS), Lung Myeloperoxidase (MPO) Assay, and Survival Studies
To reduce the contribution of endogenous stem cells, mice were irradiated with 5.0 Gy total body irradiation (TBI). Following TBI, mice received autoclaved food and water for 72 hour prior to LPS challenge. For each group, at least eight mice were used. The experiment was performed three times to yield a total of 24 (n = 24) mice per group at each time point. Mice were left unirradiated or irradiated and thereafter treated with LPS (7.5 mg/kg BW) in sterile PBS (pH 7.4) via intraperitonial injection. This LPS concentration (7.5 mg/kg BW) was chosen as the lethal dose. All mice were administered in the retro-orbital (r.o.) vein with an equal volume of sterile PBS, endothelial cells (ECs), or EPCs. Lung tissue myeloperoxidase (MPO) activity as a measure of polymorphonuclear (PMN) cell sequestration in lungs was assessed [34–36].
Lung Capillary Filtration Coefficient (Kfc) Measurement
Lungs were isolated and perfused, and all experiments were carried out ex vivo. Kfc was measured to determine pulmonary microvascular permeability as described [34–36]. In brief, after 20 minutes of equilibration perfusion, the outflow pressure was raised by 8 cm-H2O for 3 minutes. Lung wet weight increased in a ramp-like fashion, indicating net fluid extravasation. At the end of each experiment, nonpulmonary tissues were removed from lungs. Lung dry weights were determined. Kfc was calculated from the slope of the recorded weight change normalized to the pressure change and lung dry weight. The control wet-to-dry lung weight ratio in control mouse lungs was 4.28 ± 0.24.
Results
Antigen Expression Profile of mBMPCs
We observed that the ex vivo expanded mBMPCs (as described in the Materials and Methods) were adherent and anchorage-dependent (supporting information Fig. S1). Thus, we carried out an extensive antigen expression profile of integrin polypeptides on mBMPCs by fluorescence-activated cell sorting (FACS) (supporting information Fig. S2, Table 1). We used isotype-matched anti-mouse IgG as control for mECs and mBMPCs (supporting information Fig. S2A). Both mECs and mBMPCs similarly expressed laminin/collagen-binding α1 and α2 integrins (supporting information Fig. S2B, S2C) and α3 integrin (supporting information Fig. S2D). However, mBMPCs α4 expression was greater than mECs (at the same population doubling as mEPCs) (supporting information Fig. S2E). mBMPCs and mECs had comparable expression of integrin α5 (supporting information Fig. S2F). Integrin α4 andα5 subunits combine with β1 subunit to form α4β1 and α5β1 integrins known to be fibronectin receptors. mBMPCs α6 integrin expression was low compared to α4 and α5 subunits (supporting information Fig. S2G). Expression of integrin β4 subunit on mBMPCs was not detected (supporting information Table 1). mBMPCs also had greater αv integrin expression than mECs (supporting information Fig. S2H) and also greater expression of β1 integrin than that of mECs (supporting information Fig. S2I). mBMPCs had far greater expression of CD34 than mECs (supporting information Fig. S2J). mECs and mBMPCs did not express β2 and β5 integrins, CD14, CD45, and TLR4 expression (supporting information Table 1). VE-cadherin expression was not different in mBMPCs and mECs (supporting information Fig. S2K), whereas Flk1 was greater in mBMPCs (supporting information Fig. S2L). In addition, integrins expressed in mBMPCs were cell adhesion-competent as these cells formed focal adhesions and induced tyrosine phosphorylation of Fak in response to plating onto purified matrix proteins (supporting information Fig. S3A-K). Immunofluorescence microscopy analyses suggested that both mECs and mBMPCs were positive for anti-vWF immunoreactivities (supporting information Fig. S4A-P). Accordingly, Western blotting showed that mECs expressed mature vWF polypeptide, whereas mBMPCs expressed an abundant amount of slow-moving immature vWF polypeptide variants (supporting information Fig. S4Q). Compared to mECs, mBMPCs had low eNOS expression (supporting information Fig. S4R). For protein-loading control, we used anti-tubulin antibody (supporting information Fig. S4S).
α4β1 and α5β1 Integrin-Dependent mBMPCs Migration and Branching Point Structure Formation
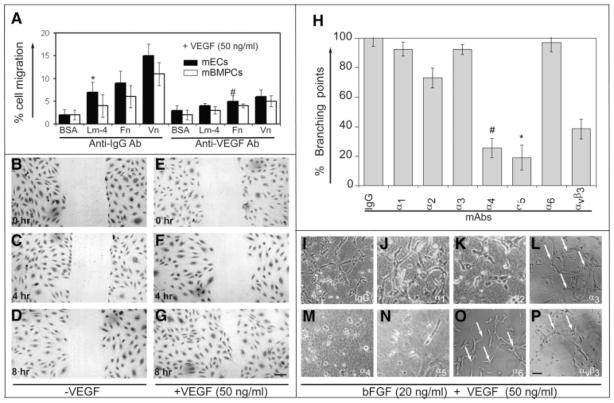
We carried out an in vitro migration assay to study haptotactic and chemotactic behavior of mBMPCs in response to extracellular matrix (ECM) proteins. Cells from the upper compartment of a Boyden chamber migrated through ECM-coated porous membrane to a lower chamber containing EBM-2 media supplemented with VEGF. mBMPCs migrated in response to VEGF (Fig. 1A) but not PDGF stimulation (data not shown). The migration of mBMPCs and mECs were comparable in both Fn and Vn substrates. Migration was inhibited by addition of anti-VEGF mAb regardless of the ECM component used (Fig. 1A). We also used the scratch assay in the absence (Fig. 1B–1D) or presence (Fig. 1E–1G) of VEGF to examine the role of mBMPCs in wound healing. mBMPCs migration induced wound closure over an 8-hour period (Fig. 1E–1G).
Figure 1.
Migration of mBMPCs requires the action of VEGF165 and mBMPCs morphogenic differentiation requires α4 and α5 integrins. (A): Haptotactic and chemotactic movement of mBMPCs in response to the chemoattractant VEGF165 (50 ng/ml) were analyzed as described in Materials and Methods. Data from three independent experiments are expressed as mean ± SEM. *, p < .05; #, p < .02. Wound closure (B–D) in the absence or (E–G) in the presence of VEGF165 was examined as described in Materials and Methods. mBMPCs were fixed and stained with eosin. Magnification 200×. Bar 200 μm. (H–P): mBMPCs were pre-incubated with control IgG or with indicated mAbs (50 μg/ml) and cultured in the presence of bFGF and human recombinant VEGF165. After 8 hours, fresh media were added containing the indicated mAbs (50 μg/ml). mBMPCs formed elongated structures and mosaics of interconnections (branching) after 16 hours. Data are representative of at least three independent experiments. Data were calculated and expressed as % branching points. n = 15; *, p < .01 versus mBMPCs plated on BSA; #, p < .02 versus mBMPCs plated on BSA. Magnification 200×. Scale bar, 150 μm. Abbreviations: bFGF, basic fibroblast growth factor; BSA, bovine serum albumin; ECs, endothelial cells; Fn, fibronectin; lm, laminin; mBMPCs, mouse bone-marrow-derived progenitor cells; VEGF, vascular endothelial growth factor.
We observed that mBMPCs induced the formation of branching point structures, which were not blocked by the addition of control IgG or anti-α1, -α2, -α3, and -α6 mAbs (Fig. 1H–1L, 1O). In contrast, anti-α4 and -α5 mAbs effectively blocked these branching points (Fig. 1H, 1M, 1N). Inhibition of β1 integrin prevented mBMPCs adhesion to fibronection and formation of branching point structures (data not shown). A 50% reduction in branching points was seen following addition of αvβ3 integrin mAb (Fig. 1H, 1P).
mBMPCs Uptake in Lungs After LPS Injury
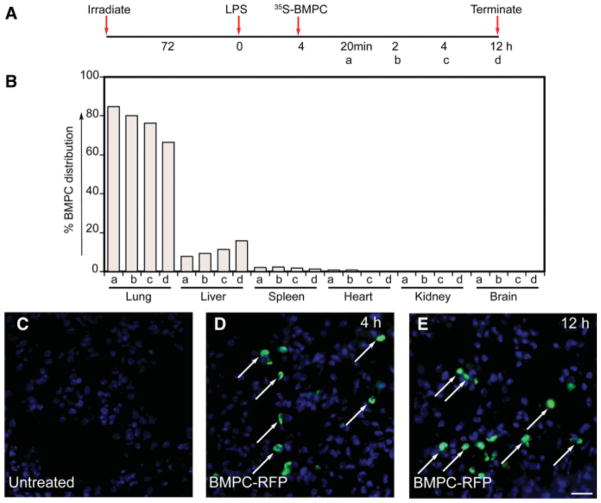
To examine whether mBMPCs can mount endothelial barrier protective effect in a LPS-induced lung injury model, we used (a) C57BL6 mice (with irradiation) and (b) nude mice (without irradiation). To test the protective effects of transplantation of CD34+ and Flk1+ mBMPCs, the experiments were carried out in irradiated mice (Materials and Methods; supporting information Fig. S5). To determine distribution of mBMPCs, CD34+ and Flk1+ mBMPCs were metabolically radiolabeled in vitro with 35S-methionine/cysteine and administered by the r.o. vein and followed for 24 hours (timeline in Fig. 2A, 2B) after the LPS challenge. At 20 minutes, more than 80% of injected mBMPCs were present in lungs, whereas less than 10% and 5% were present in the liver and spleen, respectively (Fig. 2B). This distribution remained stable during the 4 hours after mBMPCs injection. At 12 hours, 65% of mBMPCs remained in the lungs, whereas the percentage of mBMPCs in the liver steadily increased to 15% (Fig. 2B). The results of mBMPCs expressing RFP tag paralleled these results (Fig. 2D, 2E). Long-term persistence (1, 4, and 8 weeks) of RFP-expressing Flk1+ and CD34+ mBMPCs were confirmed by confocal microscopy and quantified (supporting information Fig. S6A–S6F).
Figure 2.
mBMPCs distribution in lungs and other tissues. (A): Schematic of experimental timeline. (B): 35S-Met/Cys-labeled mBMPCs were administered via the retro-orbital vein. Lung, liver, spleen, heart, kidney, and brain tissues were collected at (a) 20 minutes, (b) 2 hours, (c) 4 hours, or (d) 12 hours after LPS (7.5 mg/kg BW) treatment. Tissues were washed briefly with phosphate-buffered saline. Tissue wet weights were recorded and radioactivity (CPM per gram of tissue) was measured. Total radioactivity (4 × 105 per cell per mouse) before injection was calculated as 100% mBMPCs at 0 minutes. Data represent average percentage of mBMPCs distribution per gram of tissue obtained from three mice for each time point. The experiment was repeated two times. (C–E): Representative images of distribution of RFP-labeled mBMPCs at 4 and 12 hours were also examined by staining with rabbit anti-RFP polyclonal antibody (fluorescein isothiocyanate, green). Magnification 400×. Scale bar, 100 μm. Abbreviations: BMPCs, bone-marrow-derived progenitor cells; BW, body weight; LPS, lipopolysaccharide; RFP, red fluorescent protein.
EPC α4 and α5 Integrin Expression Reduces LPS-Induced Lung Edema Formation and Mortality
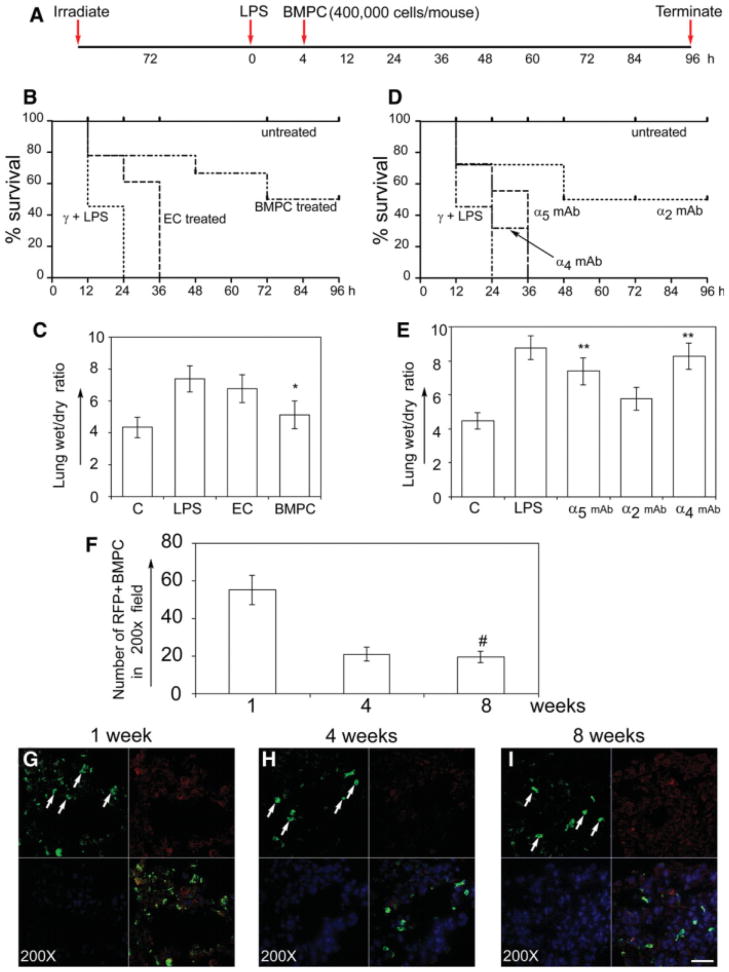
Next, to determine the role of mBMPCs-integrins, we focused on α4 and α5 integrin subunits; α2 integrin was included as control. We administered the ex vivo expanded population of mBMPCs expressing RFP, via the r.o. vein, after 4 hours of LPS injury (Fig. 3A). mECs were used as controls. Administration of mBMPCs after LPS injury reduced mortality by 50% (n = 24, p < .001), whereas mECs had no effect (Fig. 3B). To evaluate pulmonary edema, we measured the wet/dry (w/d) lung tissue weight at 12 hours after LPS challenge (i.e., 8 hours after the mEC or mBMPCs treatment). Control mice showed no edema (w/d ratio of 4.1), whereas LPS-treated mice had a ratio of 8.9 w/d (Fig. 3C), indicating severe pulmonary. Treatment of LPS-injured mice with mBMPCs significantly reduced edema formation (p < .001, n = 18; Fig. 3C). Lung sections obtained from these mice at the end of 24 hours were subjected to immunofluorescence microscopy analyses using anti-RFP antibody and the number of RFP-positive cells were quantified (supporting information Fig. S7). Next, to evaluate if administration of mBMPCs promoted survival in the absence of irradiation, we administered PBS or mBMPCs via the r.o. vein after 4 hours of LPS injury in immunodeficient athymic nude mice (supporting information Fig. S8A). At 48 hours, administration of mBMPCs after LPS injury increased survival compared with LPS control (65% vs. 45%, p < .05). At 96 hours, administration of mBMPCs after LPS injury increased survival significantly compared with control (65% vs. 15%, p < .01). To evaluate pulmonary edema, we measured the w/d lung tissue weight at 12 hours after LPS challenge (i.e., 8 hours after mEPC treatment). Control mice showed no edema (w/d ratio of 4.1), whereas LPS-treated mice had a ratio of 8.1 w/d (supporting information Fig. S8B), indicating severe pulmonary edema. The edema response in these mice as with the irradiated mice was significantly reduced following mBMPCs administration. We also examined the incorporation of RFP-expressing mEPCs within vWF+ vascular structures. The intercalation of mBMPCs with the resident ECs occurred but as a rare event (supporting information Fig. S9A–S9D). In contrast, mEPCs did not co-localize with macrophages (supporting information Fig. S9E–S9H).
Figure 3.
mBMPCs transplantation improves survival in mouse model of ALI in an α4 and α5 integrins-dependent manner. (A): Schematic time-line for mBMPCs administration and survival assay. (B): mBMPCs improved survival (50%, n = 24), whereas mECs failed to improve survival (0%, n = 18) 24 hours after LPS injection. Irradiation and LPS induced 100% mortality with a median survival time of 12 hours. mBMPCs significantly (p < .001 vs. control 12-hour group) reduced mortality with a median survival time of 48 hours. (C): Lung wet/dry ratios (n = 12–18 per group) were determined after mECs and mBMPCs treatments. Data represent mean ± SEM (n = 18; *, p < .001 vs. control group). (D): Blocking of either α4 integrin or α5 abolished the protective effect of mBMPCs, whereas some protection persisted after blocking of α2 integrin (50%, n = 24; *, p < .001 vs. control group). (E): Lung wet/dry ratios for control (n = 18) and LPS-treated (n = 24) mice were 4.2 ± 0.9 and 9.0 + 1.1, respectively. Blocking of either mBMPCs α5 integrin or α4 integrin failed to prevent pulmonary edema (7.6 ± 1.8; n = 18; *, p < .002 vs. control group), whereas blocking α2 integrin resulted in lung water content of 5.9 ± 1.9 (n = 18). (F): RFP-positive mBMPCs were quantified at 1, 4, and 8 weeks from six sections from six different areas separated by 200 μM intervals. Five fields were selected at random from each section for quantification. Data from three independent experiments are expressed as mean ± SEM. *, p < .001 versus 1 hour. (G–I): Representative lung sections from RFP-positive mBMPCs-treated mice were examined at (G) 1, (H) 4, and (I) 8 weeks by confocal microscopy. One week after mBMPCs treatment (G), lungs contained significantly higher numbers of RFP-positive mBMPCs and a few scattered inflammatory cells, but this number declined by weeks 4 and 8. At week 1, lungs from mBMPCs-treated mice exhibited normal lung architecture and no acute inflammation or edema. Scale bar, 100 μm. For higher resolution images, please see supporting information Figure S6. Abbreviations: ECs, endothelial cells; LPS, lipopolysaccharide; mBMPCs, mouse bone-marrow-derived progenitor cells; RFP, red fluorescent protein.
To determine the role of α4 or α5 integrins in the mechanism of the protective benefit of mBMPCs, mBMPCs incubated with adhesion-blocking anti-mouse α2 integrin or anti-mouse α4 or α5 integrin mAb were administered via the r.o. vein (Fig. 3D). At 36 hours of α4 or α5 integrin mAb treatment, which blocked mBMPCs adhesion, 100% of the mice died (n = 24, p < .001; Fig. 3D). Mean lung w/d weight ratio of integrin α2 mAb-treated group was the same as that of the untreated mice (n = 24, p < .001) (Fig. 3D, 3E). Thus, inhibition of α4 or α5 integrin abolished the protective effect of mBMPCs. Quantification of long-term retention (persistence) of mEPCs is shown in Figure 3F. Representative images of retention of mBMPCs at the end of 1, 4, and 8 weeks are shown in Figure 3G–3I, indicating a sufficient number of transplanted EPCs were present to mount protective effect.
Expression of mEPC α4 and α5 Integrins Prevent Increased Lung Vascular Permeability
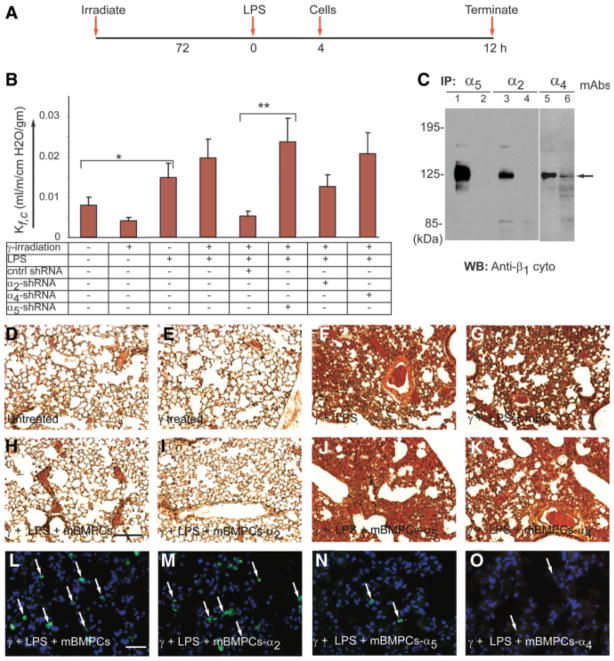
We next determined the contributions of CD34+ and Flk1+ mBMPCs α4 and α5 integrins in mediating a reduction in lung vascular permeability as a cause of the observed reduction in pulmonary edema. To do so, mBMPCs were transduced with shRNA constructs to silence the α2 and α4 or α5 integrin genes. Timeline of experimental strategy is shown in Figure 4A. In mice treated with LPS, the lung capillary filtration coefficient (Kfc) increased and peaked at 6 hours after challenge. Control mice, mice receiving mBMPCs alone, and mice receiving EPCs transduced with α2 integrin shRNA had comparable Kfc values (Fig. 4B). In contrast, mice receiving no mBMPCs or mBMPCs transduced with α4 or α5 integrin shRNA showed increased Kfc (Fig. 4B). Extent of shRNA mediated the α2 and α4 or α5 integrin knockdown was confirmed by immunoprecipitation (Fig. 4C). Hemorrhage, edema, and changes in lung architecture were not seen in control or irradiated mBMPCs-treated mice after 24 hours (n = 10; Fig. 4D), whereas mice challenged with γ-irradiation plus LPS had disrupted lung architecture, hemorrhage, and accumulation of leukocytes (Fig. 4E). Treatment with mECs showed no improvement in morphology (Fig. 4F), whereas treatment with mBMPCs normalized the lung architecture (Fig. 4G). Mice receiving mBMPCs transduced with α2 integrin shRNA (n = 10) also showed normal lung architecture (n = 10; Fig. 4H), whereas mBMPCs transduced with α4 or α5 shRNA did not differ from irradiated LPS-challenged mice (n = 10; Fig. 4I). Studies using RFP-expressing mBMPCs transduced with α2 and α5 shRNA constructs (Fig. 4J–4L) showed that mBMPCs transduced with α5 integrin shRNA abolished the protective effects of mBMPCs.
Figure 4.
Silencing of α4 or α5 integrin abolishes mBMPCs-mediated restoration of lung fluid balance. (A): Timeline used to determine capillary filtration coefficient (Kfc) following treatment with mBMPCs. (B): Kfc assay following treatment with mBMPCs, mBMPCs-α5-shRNA, or mBMPCs-α2-shRNA or mBMPCs-α4-shRNA (n = 10 per group). Experiments were performed five times. *, p < .05. **, p < .01. (C): shRNA-mediated silencing of α2, α4, and α5 integrins was determined by immunoprecipitation followed by WB with indicated antibodies. (D–H): H&E-stained lung sections: (D) normal alveolar septa with no obvious PMN infiltration; (E) normal alveolar septa with no obvious polymorphonuclear (PMN) infiltration in response to γ-irradiation; (F) septal thickening indicative of edema in response to γ-irradiation and LPS treatment; (G) no protection with mECs, uptake of tissue PMN, and edema formation; (H) normal alveolar septa with no PMN infiltration in mouse lung following mBMPC treatment; (I) silencing of α2 integrin fails to abolish effect of mBMPCs, as illustrated by normal lung architecture; and (J, K) silencing of either α4 or α5 integrin offered no therapeutic benefit to mice that were previously injured by irradiation and LPS. (L–O): Retention of mBMPCs expressing RFP was examined at the end of 48 hours by staining with an anti-RFP polyclonal antibody (green). White arrows indicate mBMPCs expressing RFP. For quantification, please see supporting information Figure S7. Data are representative of at least three independent experiments. Magnification 200×. Scale bar 150 μm. Abbreviations: ECs, endothelial cells; LPS, lipopolysaccharide; mBMPCs, mouse bone-marrow-derived progenitor cells; RFP, red fluorescent protein; WB, Western blotting.
Discussion
Here we showed the role of mBMPCs (cultured for 21–22 days) in promoting lung vascular barrier function and preventing edema formation in a mouse model of LPS-induced lung injury. FACS analysis showed that the cells used (which were all passage 3) expressed a subset of β1 integrins (α1β1, α2β1, α3β1, α4β1, α5β1, and α6β1) and αvβ3 integrins, formed characteristic branching point structures in three-dimensional type I collagen gel, and remained sequestered in lung tissue for up to 8 weeks after intravenous injection. The mBMPCs were nearly 100% positive for CD34 and Flk1 antigens but were negative for β2 integrin, CD14, CD45, and TLR4 antigens, indicative of their BMPC phenotype [15–18, 21]. Our inability to detect CD14 and CD45 on the surface of mBMPCs could be attributed to protease-mediated shedding. We have also found no evidence that mature endothelial cells, CD45+ leukocytes, blood leukocytes, or blood monocytes provide any therapeutic benefit in LPS-induced acute lung injury [50]. Nevertheless, we have focused on the role of α4β1 and α5β1 integrins as they are known to be key determinants of angiogenesis demonstrated by their ability to induce endothelial cell elongation and branching point structures [37–40]. We observed that α4β1 and α5β1 integrins were indeed expressed abundantly in cultured mBMPCs. Expression of integrins such as α4β1 and α5β1 strongly correlate with their ability to mediate cell adhesion and de-adhesion including homing events [24, 42, 43]. Although α4β1 and α5β1 integrins are both capable of binding extracellular matrix proteins, α4β1 has been shown to mediate homing of immune and metastatic cells [24, 25, 37], whereas α5β1 integrin mediates stable cell adhesion and regulates anchorage-dependent cell phenotype [38, 43, 46]. Thus, in transfection experiments expression of α4β1 into CHO cell provided velocity to cell migration, whereas α5β1 integrin did not [37, 38]. Accordingly, the quantification of functional branching point structures demonstrated that incubation of mBMPCs with anti-α4 or -α5 mAb inhibited the formation of these structures. Upon activation, the mBMPCs migrate to sites of injury, attach to the provisional matrix, and adhere to matrix fibronectin, laminin-4, and collagen IV [5–10, 23, 24]. Integrins promote the binding of progenitor cells to ECM protein and their proliferation and differentiation [24, 25, 37–39]. Cells expressing α4β1 or α5β1 are known to induce binding to a variety of matrix proteins—fibronectin, fibrinogen, von Willebrand factor (vWF), osteopontin, collagen, and laminin [39–45]. Binding of α5β1 integrin to fibronectin not only mediates cell adhesion but also controls cell cycle progression [46].
It is not known which matrix components are recognized by mBMPCs. During inflammation, vessel wall injury results in release and exposure of fibrin, fibrinogen, fibronectin, osteopontin, and vWF, which serve as sites of adhesion for fibroblasts and endothelial cells [42, 43]. We were unable to detect fibronectin or collagen ECM molecules in LPS-injured mouse lung microvessels (data not shown), suggesting lack of detectable fibrosis in our model system. Recent studies have implicated the crucial role of osteopontin in homing of EPC to the region of vascular injury [44, 45]. Thus, release and deposition of ECM molecules by the injured endothelium may promote the recruitment of mBMPCs to repair the injured endothelium. In the present study, expression of α4 or α5 integrins in EPCs, which partner with the β1 subunit to form α4β1 or α5β1 heterodimers, would therefore allow the recognition of these matrix proteins. Our data showed that mBMPCs were in fact capable of sensing matrix proteins and mediating signaling through focal adhesion kinase (Fak). We observed tyrosine phosphorylation of Fak and activation of Erk1/2, indicating that mEPC α4β1 or α5β1 integrins were functional. Importantly, the activation of Fak and Erk1/2 in this system suggests the ability of mBMPCs to migrate and survive.
Irradiation was used to mitigate partially the contribution of endogenous stem cells to endothelial repair process induced by transplantation of mBMPCs. Although we used a relatively low dose of 5-Gy radiation, which may still have direct effects on the lungs [47, 48], we observed no appreciable difference in the lung uptake of PMNs in response to LPS in the irradiated versus control lungs; thus, it does not appear that this level of irradiation was sufficient to damage lungs or induce inflammatory injury. LPS treatment in control mice increased lung microvessel permeability that peaked within 6 hours. We noted severe pulmonary edema secondary to vascular injury and 100% mortality of these mice. We also observed widespread PMN and monocyte/macrophage sequestration and disruption of the lung architecture. The intravenous administration of radioactively labeled BMPCs showed that 80%–95% of injected cells were trapped in lung tissue within 20 minutes, and a significant population of these cells remained in the lungs during the first 12 hours. The number of cells retained in the lungs declined to 40%–50% by the end of 24 hours. Examination of RFP-labeled Flk1+ and CD34+ EPCs showed that a relatively large number of cells remained sequestered in lungs even for up to 8 weeks. At the end of 8 weeks, there was no evidence of lung injury in surviving mice. Although intercalation of mBMPCs with a resident endothelial cell monolayer was seen, it was a rare event (not quantifiable). Most of the mBMPCs were sequestered in the lung parenchyma. We observed that administration of mBMPCs provided 50% survival benefit at 48–96 hours, whereas control mECs were not sequestered in the lungs and also had no protective effect. In the mBMPCs-transplanted group, we also observed reduced lung vascular injury and edema compared with controls and normal lung morphology in contrast to the EC-administered mice.
Because the irradiation dose of 5 Gy used is much lower than the lethal dose of 10–12 Gy [47, 48], it is possible that BM cell number may not have been markedly reduced. It is likely that sublethally irradiated mice will still have the “mBMPCs” because the sublethal dose we used would not be completely myeloablative. The dose of 10–12 Gy is lethal, but not sublethal. This higher dose would have caused a BM suppression that would have killed the mice in 10–12 days. Thus, the protection may be ascribed to some extent to mobilization of residual BM-derived progenitor cells. To test this possibility, studies were also made using the immune-deficient athymic nude mice (data not shown). Transplantation of mBMPCs in these recipient nude mice also reduced mortality induced by the LPS treatment, suggesting that the protection was largely the result of administration of exogenous (donor) mBMPCs.
The present studies demonstrate that α4β1 andα5β1 expression in mBMPCs was responsible for the endothelial barrier protective effect of mBMPCs. In the mouse studies, the prevention of the increase in lung vascular permeability was abrogated by blocking or silencing the function of α4 or α5 in the mBMPCs. These data show that α4β1- and α5β1-mediated mBMPCs adhesion event is required for endothelial barrier protection. As cell surface receptors including integrins are subject to protease-mediated ectodomain shedding from the plasma membrane [49], it is likely that we underestimated the endothelial barrier protective effect of mBMPCs since a sub-population of mBMPCs integrins could be the target of protease-induced shedding.
How do mBMPCs mediate endothelial barrier protection remains unclear. The present studies support the role of mBMPCs sequestration in lungs as a mechanism of lung endothelial barrier protection; however, we have also shown that a humoral mechanism involving secretion by mBMPCs of paracrine factors such as sphingosine-1-phosphate that contribute to endothelial barrier normalization [50]. In summary, we have demonstrated that a donor population of mBMPCs expressing adhesion-competent α4β1 or α5β1 integrins when transplanted in lungs of mice prevents lung vascular injury and edema formation and improves survival following LPS injury. The results indicate the importance of α4β1 or α5β1 integrin expression in mBMPCs as a means of ensuring proper adhesion to matrix proteins and tissue sequestration of the cells in order for BMPC transplantation to be efficacious.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants to K.K.W. (R01HL079356) and A.B.M. (R01HL090152). Authors thank Craig Mock, Tom Ruginis, and Myla Petterson for excellent technical assistance.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: K.K.W.: conception and design, data collection, analysis, and data interpretation, financial support, manuscript writing, and final approval of manuscript; S.M.V.: data analysis and interpretation; S.G.: animal experiments and data collection; Y.D.Z.: provision of study material; A.B.M.: financial support and manuscript writing.
References
- 1.Fleischman RA, Custer RP, Mintz B. Totipotent hematopoietic stem cells: Normal self-renewal and differentiation after transplantation between mouse fetuses. Cell. 1982;30:351–359. doi: 10.1016/0092-8674(82)90233-1. [DOI] [PubMed] [Google Scholar]
- 2.Hall PA, Watt FM. Stem cells: The generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Scadden DT. Circadian rhythms: Stem cells traffic in time. Nature. 2008;452:416–417. doi: 10.1038/452416a. [DOI] [PubMed] [Google Scholar]
- 5.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 6.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Kajstura J, Leri A, Bolli R, et al. Endothelial progenitor cells: Neovascularization or more? J Mol Cell Cardiol. 2006;40:1–8. doi: 10.1016/j.yjmcc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 10.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 12.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 15.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 16.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prater DN, Case J, Ingram DA, et al. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 18.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss DJ, Kolls JK, Ortiz LA, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavakis E, Aicher A, Heeschen C. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin G, Ii M, Silver M, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin H, Aiyer A, Su J, et al. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–662. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humtsoe JO, Feng S, Thakker GD, et al. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 2003;22:1539–1554. doi: 10.1093/emboj/cdg165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wary KK, Humtsoe JO. Anti-lipid phosphate phosphohydrolase-3 (LPP3) antibody inhibits bFGF- and VEGF-induced capillary morphogenesis of endothelial cells. Cell Commun Signal. 2005;3:9. doi: 10.1186/1478-811X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humtsoe JO, Kim JK, Xu Y, et al. A streptococcal collagen-like protein interacts with the alpha2beta1 integrin and induces intracellular signaling. J Biol Chem. 2005;280:13848–13857. doi: 10.1074/jbc.M410605200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao YY, Gao XP, Zhao YD, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmaier K, Toya S, Gao X, et al. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med. 2007;13:920–926. doi: 10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 31.Garrean S, Gao XP, Brovkovych V, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 32.Zhao YD, Courtman DW, Deng Y, et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: Efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Frey RS, Rahman A, et al. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem. 2002;277:3404–3411. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 34.Gao X, Kouklis P, Xu N, et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1218–L1225. doi: 10.1152/ajplung.2000.279.6.L1218. [DOI] [PubMed] [Google Scholar]
- 35.Ong ES, Gao XP, Xu N, et al. E. coli pneumonia induces CD18-independent airway neutrophil migration in the absence of increased lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L879–L888. doi: 10.1152/ajplung.00134.2003. [DOI] [PubMed] [Google Scholar]
- 36.Brovkovych V, Gao XP, Ong E, et al. Augmented iNOS expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–L103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 38.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez AM, Gonzales M, Herron GS, et al. Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci U S A. 2002;99:16075–16080. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Bell K, Mousa SA, et al. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto S, Katz BZ, Lafrenie RM, et al. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann N Y Acad Sci. 1998;857:119–129. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- 42.Grinnell F, Billingham RE, Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981;76:181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- 43.Ruoslahti E, Engvall E. Integrins and vascular extracellular matrix assembly. J Clin Invest. 1997;99:1149–1152. doi: 10.1172/JCI119269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scatena M, Liaw L, Giachelli CM. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 45.Leen LL, Filipe C, Billon A, et al. Estrogen-stimulated endothelial repair requires osteopontin. Arterioscler Thromb Vasc Biol. 2008;28:2131–2136. doi: 10.1161/ATVBAHA.108.167965. [DOI] [PubMed] [Google Scholar]
- 46.Wary KK, Mainiero F, Isakoff SJ, et al. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 47.Johnston CJ, Williams JP, Elder A, et al. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30:369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R, Ghosh SN, Zhu D, et al. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: Injury and recovery. Int J Radiat Biol. 2008;84:487–497. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldsmith EC, Carver W, McFadden A, et al. Integrin shedding as a mechanism of cellular adaptation during cardiac growth. Am J Physiol Heart Circ Physiol. 2003;284:H2227–H2234. doi: 10.1152/ajpheart.00920.2002. [DOI] [PubMed] [Google Scholar]
- 50.Zhao YD, Ohkawara H, Rehman J, et al. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.