Abstract
To determine the genetic regulation of hair length in the domestic cat, a whole genome scan was performed in a multi-generational pedigree in which the long-haired phenotype was segregating. The two markers that demonstrated the greatest linkage to the long-haired trait (LOD≥6), flanked an estimated 10 Mb region on cat chromosome B1 containing the Fibroblast Growth Factor 5 gene (FGF5), a candidate gene implicated in regulating hair follicle growth cycle in other species. Sequence analyses of FGF5 in 26 cat breeds and two pedigrees of non-breed cats, revealed four separate mutations predicted to disrupt the biological activity of the FGF5 protein. Pedigree analyses demonstrated that different combinations of paired mutant FGF5 alleles segregated with the long-haired phenotype in an autosomal recessive manner. Association analyses of over 380 genotyped breed and non-breed cats were consistent with mutations in the FGF5 gene causing the long-haired phenotype in an autosomal recessive manner. In combination, these genomic approaches demonstrated that FGF5 is the major genetic determinant of hair length in the domestic cat.
Introduction
The hair follicle provides a uniquely mammalian model in which to study the complex genetic regulation between stem and stromal cells during self-renewal and terminal differentiation of a tissue. Genetic modulation of the hair follicle cycle can affect hair length, providing a rapid means for significant phenotypic change under either artificial or natural selection. The large variety of cat breeds with different hair textures and lengths provides a potential wealth of mammalian models with spontaneous mutations at unknown loci affecting hair follicle structure and function (Vella and Robinson 1999). Discovery and comparison of mutations in orthologous genes between mammals can provide additional understanding about the conserved domains that are required for protein function.
The initial molecular studies of the long hair locus were done in mice. Breeding experiments of spontaneously occurring long-haired Angora mice demonstrated that the go locus was the major determinant of hair length in the mouse, and that the long-haired phenotype was inherited in an autosomal recessive manner (Dickie 1963, Pennycuik and Raphael 1984). Subsequent targeted mutation of the fibroblast growth factor 5 gene (Fgf5) and cross-breeding experiments between Fgf5 knock-out (Fgf5neo) and Angora mice demonstrated that Fgf5neo and go represented null alleles of the same locus (Hébert et al. 1994).
FGF5 was originally identified as a human oncogene (Zhan et al.1987), belonging to a family of 23 related Fibroblast Growth Factor genes (For a review see Katoh 2002 and Katoh and Katoh 2005). FGF5 shares a β-trefoil superfold structure composed of five β-hairpin folds (that is required for receptor binding) and an N-terminal, signal peptide (that is required for paracrine secretion) with the prototypical members, FGF1 and 2 (Ornitz and Itoh 2001). During embryonic and fetal development of the mouse, Fgf5 is first expressed in the extra-embryonic ectoderm of the epiblast and then restricted to differentiating myotomes, skeletal muscles, and neurons (Hébert et al. 1991; Haub and Goldfarb 1991). The orthologous human FGF5 gene product is over-expressed in some mammary, prostatic and renal carcinomas and can be presented on their MHC class I receptors after intra-cellular processing, providing a potential antigenic target for cancer immunotherapy (Vigneron et al. 2004). However, experimental over-expression of FGF5 has been used to induce angiogenesis in the myocardium and to promote photoreceptor survival in animal models without inducing tumors in situ (Giordano et al. 1996 and Green et al. 2001). In the adult mouse, FGF5 is normally expressed in neurons in most regions of the brain, in pancreatic β-islet cells involved in glucose-homeostasis and in hair follicles in the skin (Haub et al. 1990; Gómez-Pinilla and Cotman 1993; Hart et al. 2000).
During the normal, cyclic hair growth of adult mice, expression of Fgf5 is restricted to cells in the lower third of the outer root sheath (ORS) and in the inner root sheath (IRS) at the base of the follicle during the anagen phase just prior to progression into the catagen phase (Hébert et al. 1994). Two major Fgf5 isoforms have been described in the mouse (Suzuki et al. 2000); the full-length Fgf5 mRNA results from transcription of all three exons, while splicing of exon 1 to an alternative acceptor site in exon 3 introduces a frame-shift producing a shorter transcript (Fgf5S) that lacks exon 2 and most of exon 3. Both the FGF5 and FGF5S gene products bind primarily to the FGF Receptors 1 and 2, Isoforms c (FGFR1c and 2c) (Ornitz et al. 1996) found on dermal papilla cells (DPC) (Clements et al. 1993; Rosenquist and Martin 1996). However, the full-length FGF5 actively inhibits DPC stimulation of ORS cell proliferation and synthesis of hair fibers during anagen (Limat et al. 1993; Suzuki et al. 2000), thereby triggering catagen. In contrast, FGF5S antagonizes the inhibitory effects of FGF5, providing a degree of autoregulatory modulation (Ota et al. 2002). The overall effect of the absence of both isoforms in Fgf5-null mice is that the anagen stage is prolonged, resulting in longer hair (Hébert et al. 1994). However even in these mice, the hair follicles do eventually proceed into the catagen phase, indicating the presence of additional signaling pathways (reviewed by Paus and Foitzik 2004). Comparative studies of Fgf5 orthologs in other mammalian species exhibiting variation in hair length may reveal novel spontaneous mutations identifying key domains that influence protein function.
The inheritance of long hair has been documented as a recessive trait in other mammalian species including rabbits (Fraser 1953), dogs (Burns and Fraser 1963) and cats (Vella and Robinson 1999). While we were pursuing our study of the involvement of the FGF5 locus in the control of hair length in cats, another group demonstrated that mutations in the canine FGF5 gene explained the long-haired phenotype observed in multiple dog breeds (Housley and Venta 2006). Similar to dogs, the foundation of cat breed groups committed to the propagation of specific phenotypic traits resulting from spontaneous mutations has provided opportunities to study the genetic basis of several traits. Coat lengths and colors can be specified as part of a breed standard allowing for mutations carried by founders to become fixed within these closed breeding populations. The large variety of breeds containing potential mutations affecting hair follicle structure and function from the hairless Sphynx to the kinked-haired Cornish, Devon and Selkirk Rexes to the long-haired breeds such as Maine Coon, Persian and Turkish Angora cats provides a wealth of animal models for understanding the complex gene regulation of the hair growth cycle. In this study we have performed a genetic survey of long and short-haired breeds, as well as genetic linkage and pedigree analyses of non-breed cats to interrogate the influence of the FGF5 locus on hair length in domestic cats.
Materials and Methods
Collection of DNA Samples and Phenotypic Data from Breed and Non-Breed Cats
Whole blood preserved with EDTA or cheek swab samples were taken from cats for DNA extraction using a salt-precipitation/column-filtration kit (Qiagen). Samples were obtained from 119 unrelated cats with the permission of private owners from 12 short-haired breeds (Abyssinian, American Shorthair, British Shorthair, Burmese, Chartreux, Cornish Rex, Devon Rex, Egyptian Mau, Havana, Ocicat, Russian Blue and Siamese), 12 long-haired breeds (Angora, Balinese, Birman, Himalayan, Maine Coon, Norwegian Forest Cat, Persian, Ragdoll, Siberian, Somali, Turkish Angora and Turkish Van), and two breeds (Manx and Scottish Fold) that currently maintain separate short and long-haired registries within the Cat Fanciers Association. Private owners reported the phenotypes of their cats as either short or long-haired in accordance with breed standards and provided photographs in most cases. All breed cats were assigned an anonymous registry (Fc) number, and phenotypic data was recorded in a database at the Laboratory of Genomic Diversity to preserve the anonymity of individual cats and their owners.
Samples were obtained from 261 related cats that were used for nutrition studies at the Nestlé-Purina PetCare Company in St. Louis, MO, and from 50 related, White Deaf cats that were used for hearing studies at Johns Hopkins University in Baltimore, MD, with the approval of their respective Internal Animal Care And Use Committees. Both colonies were established from random-bred, short and long-haired cats obtained from commercial vendors and were maintained as closed colonies through planned matings. Since these cats were no longer being randomly mated or out or crossbred, they were defined in this study as non-breed cats to distinguish them from the registered breed cats. As in breed cats the hair length was sufficiently different to qualitatively phenotype non-breed cats as either short or long-haired. Photographs were used to confirm the reported phenotypes, but direct measurements of hair length were not taken. All non-breed cats in this study were maintained in facilities inspected by the United States Department of Agriculture, under conditions established by the American Association of Laboratory Animal Care in compliance with the federal Animal Welfare Act.
Whole-genome scan
The long-haired trait segregated in the Nestlé-Purina, multi-generational pedigree of non-breed domestic cats (Eizirik et al. 2003). In conjunction with the development of a third generation, genetic linkage map we genotyped 261 cats in this pedigree for 483 autosomal and 30 X-linked (513 total) microsatellite markers, using amplification conditions and analyses as previously described (Ishida et al. 2006).
Genetic Linkage Analysis
The long-haired phenotype was modeled as a binary trait with fully penetrant, autosomal recessive inheritance, and log of the odds (LOD) scores were computed using the Superlink software package (Fishelson and Geiger 2002, 2004). For the LOD scores shown in Table 1, the allele associated with the long-haired phenotype was assumed to have a frequency of 0.25 with all other markers of equal allele frequencies. If the trait allele frequency of 0.50 was used, peak LOD scores of all the linked markers were higher by at most 0.6 LOD units, and estimates of the optimal recombination fraction (θ) differed by at most 0.01.
Table 1.
Linkage analysis of the long-haired trait in the cat to FGF5.
| Marker | LOD to Long-Haireda | θb | Cat RH Map Positionc | Cat Chr.#d | Fca Start |
|---|---|---|---|---|---|
| FCA212 | 3.0 | 0.07 | 1188.1 | B1 | 127,108,235 |
| FCA074 | 3.3 | 0.09 | 1233.1 | B1 | 133,650,025 |
| FCA1144 | 4.5 | 0.07 | ND | B1 | 135,390,194 |
| FCA612 | 4.6 | 0.04 | 1276.7 | B1 | 139,688,359 |
| FCA824 | 9.5 | 0.03 | 1356.2 | B1 | 156,292,359 |
| FGF5e | (11.6) | (0.00) | ND | B1 | 158,450,684 |
| FCA823 | 6.3 | 0.06 | 1476.2 | B1 | 166,387,690 |
LOD scores were calculated using 513 microsatellite markers typed on 135 potentially informative meioses with the allele associated with the long-haired phenotype assumed to have a frequency of 0.25. LOD scores were 0.6 LOD units higher, if an allele frequency of 0.50 was used.
Estimates of the optimal recombination fractions differed at most by 0.01, depending upon the allele frequencies assumed.
Assignment of microsatellite positions within a cat radiation hybrid (RH) map. “ND” indicates positions not determined in the cat RH map.
Assignment of microsatellite positions to cat chromosome B1 contigs in the 2x feline genome database using the Garfield Cat Genome Browser. Fca start indicates sequences corresponding to the contig start sequences.
Results of subsequent linkage analyses for the FGF5 locus are presented in parentheses, after genotyping the 3 predicted mutations in the FGF5 CDS found within this pedigree.
PCR amplification and DNA sequence analysis of FGF5
PCR primers were designed in the introns flanking the three exons of FGF5 (Figure A1, Table A1). Primers flanking exon 1 were designed from domestic cat DNA sequence that was detected in the cat 2x whole genome by cross-species MegaBLAST (Zhang et al. 2000) (http://www.ncbi.nlm.nih.gov/BLAST/tracemb.shtml). The forward primer for exon 2 was anchored in a region identical in both dog and cow, and the reverse primer was a consensus sequence between the dog and cow and contained two degenerate oligonucleotide positions. The primers for exon 3 were derived from the dog DNA sequence from a region highly conserved between dog and human. Touchdown PCR and DNA sequencing were performed as previously described (Guo et al. 2006, Ishida et al. 2006).
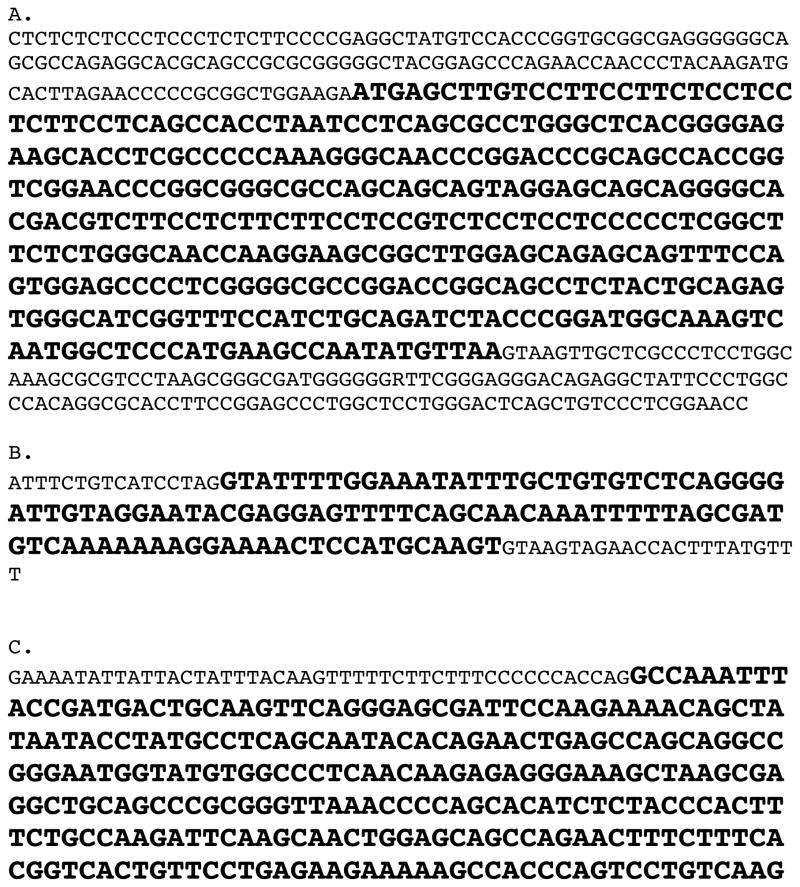
Figure A1.
FGF5 isoform 1 coding exons and flanking sequence for a short-haired Abyssinian cat (A) Coding Exon 1 and flanks, (B) Coding Exon 2 and flanks, (C) Coding Exon 3 and flanks. Coding exon sequence is in bold. Sequence 5′ of exon 1 coding sequence represents 5′UTR and sequence 3′ of exon 3 coding sequence represent 3′UTR. The remaining non-bolded text represents intronic sequence. (D) Putative feline FGF5 mRNA with coding sequence in bold.
Table A1.
PCR primers designed to amplify the 3 exons of domestic cat FGF5
| FGF5 Exon amplified | Forward Primer | Reverse Primer | PCR product size (bp) |
|---|---|---|---|
| Exon 1 | CGCCGAGATCCATTCGAG | TAGATGCACCTTCACCCAAC | 698 |
| Exon 2 | AGAGGAGTCTGTGTTTTATTTTGGG | GTAAAATCTCYRTAACACCTTTAAC | 193 |
| Exon 3 | GACCTCATTTTATTAGATGCT | AAGGCATGGTTTCTCACCAG | 448 |
PCR-RFLP Genotyping Assay Development
Three fluorescence-based assays were developed to genotype the 4 FGF5 mutations detected (Table A2). Separate assays were developed to detect Mutation 1 (c.ins356T) and 2 (c.C>T406), and a third assay was designed which could detect both of the adjacent Mutations 3 (c.del474T) and 4 (c.A>C475), as well as determine the phase of the two mutations. In all three assays a fluorescent dye was incorporated into the polymerase chain reaction (PCR) product (Boutin-Ganache et al. 2001) modified by attaching the –21M13F primer sequence (TGTAAAACGACGGCCAGT) to the 5′ end of one of the primers. PCR reactions and product detection was performed as previously described (Guo et al. 2006). For Mutation 1 (c.ins356T) PCR primers were designed flanking the mutation as the two alleles could be discriminated by their 1 bp size difference. For the other two assays PCR-RFLP approaches were developed. Oligonucleotide substitutions were made in one of the primers such that a restriction site was generated which would recognize one of the alleles at each mutation. Additionally the restriction enzyme recognition sequence and a pig-tail sequence (gtgtctt) was appended to the 5′ end of the primer adjacent to the polymorphic site to introduce a positive control for enzyme digestion (Brownstein et al. 1996). Touchdown PCR cycling conditions were used to amplify Mutation 2 (c.C>T406). For the other two assays, the PCR conditions previously described were used (Menotti-Raymond et al. 1999) with the exception that the initial 93° C denaturation cycle was extended to 10 minutes and the enzyme AmpliTaq Gold® was substituted for AmpliTaq® (Applied Biosystems, Foster City, CA). Fluorescent primer labeling, touchdown PCR amplification and analysis on an Applied Biosystems Model 3100 DNA sequencher were performed as previously described (Guo et al. 2006). Prior to electrophoresis, digested products were purified by centrifugation through Multiscreen plates (Millipore, Bedford, MA) packed with Sephadex G-50 (Amersham Biosciences, Uppsala, Sweden) according to the manufacturer’s instructions.
Table A2.
Development of a PCR-RFLP genotyping assay for (A) FGF5 mutations 1 and 2 and (B) Mutations 3 and 4 responsible for long hair in the domestic cat.
| A
| ||||||
|---|---|---|---|---|---|---|
| Mutationb | Forward Primer | Reverse Primer | Restriction Enzyme | Undigested Product (bp) | Product Size (bp) following digestiona
|
|
| Normal Allele | Mutant allele | |||||
| Mutation 1 | TGTAAAACGACGGCCAGTTCTACTGCAGAGTGGGCATC | GTGTCTTGCTTAGGACGCGCTTTGC | none | 149 | 148 | 149 |
| Mutation 2 | GTGTCTTGTACCTCAGGGGATTGTAGGAGTA | TGTAAAACGACGGCCAGTTCTCCTGTAACACCTTTAACAAACA | RsaI | 155 | 122 | 143 |
| B
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Mutationb | Forward Primer | Reverse Primer | Restriction Enzyme | Undigested Product (bp) | Product Size (bp) following digestiona
|
|||
| No Mutation | Mutation 3 | Mutation 4 | Mutations 3 and 4 | |||||
| Mutation 3/Mutation 4 | TGTAAAACGACGGCCAGTGACCTCATTTTATTAGATGCT | GTGTCTTACCGTCTCCCTGAACTTGCAGTCAACG | HpyCH4 III | 132 | 98 | 97 | 122 | 121 |
The product sizes include the 1 bp non-templated A addition expected by the addition of the pig-tail sequence to one of the primers.
Mutations are coded as described in the text. 1 - c.ins356T, 2 - c.C>T406, 3 - c.del474T, 4 – c.A>C475 and 5 – no mutation.
Results
Whole Genome Scan
We tested for genetic linkage between the long-haired trait and 513 microsatellite markers genotyped in the non-breed, Nestlé-Purina pedigree. A portion of the Nestlé-Purina pedigree included 135 potentially informative meioses from mixed litters of 62 progeny produced from F1 by F1 short-haired matings and 11 long-haired cats generated from F1 short-haired cats crossed with long-haired cats. The six microsatellites that demonstrated single-marker LOD scores at or above 3.0 were all found within contiguous sequences (contigs) of the assembled cat genome and assigned to an estimated 39.3 MB region of B1 through the Garfield Cat Genome Browser (http://lgd.abcc.ncifcrf.gov/cgi-bin/gbrowse/cat) (Dr. Joan Pontius, personal communication) (Table 1). All other markers not on B1 had peak single-marker LOD scores less than 1.75 (not shown). The two markers (FCA823 and FCA824) that demonstrated the highest LOD scores to the long-haired locus (6.3, θ=0.06 and 9.5, θ=0.03 respectively) flanked an estimated 10 Mb region of cat chromosome B1 containing the candidate gene, FGF5, (Table 1). We next sequenced this candidate gene in related, non-breed cats in the Nestlé-Purina pedigree and in an independent research colony at Johns Hopkins University, as well as in unrelated, long and short-haired breed cats.
DNA Sequence Analyses of FGF5 in 50 Short and Long-Haired Breed Cats
To detect possible mutations in the predicted coding sequence (CDS) of the feline FGF5 gene beginning at position 158,481,515 on chromosome B1 of the annotated feline genome (Dr. Joan Pontius, personal communication), primers were designed in flanking regions located in the 5′ and 3′ UTRs and in introns (Table A1, Figure A1) and used to amplify the three exons in 50 breed cats with known hair length (Table 2). The size of the assembled predicted CDS was 810 basepairs (bp) and the inferred full-length, feline FGF5 protein (Isoform 1 translated from all three exons) was 270 amino acids (aa’s) exhibiting 91% residue identity with the human FGF5 protein (Figure 1A). The potential shorter feline FGF5S protein (Isoform 2 resulting from alternative splicing of exons 1 and 3) was predicted to be 125 aa’s in length and shared 86% residue identity with the human FGF5S (Figure 1B). A recent report confirmed that the transcripts for both predicted FGF5 isoforms are expressed in the skin of domestic cats (Drögemüller et al. 2007).
Table 2.
DNA sequence analysis of FGF5 in 50 breed cats with known hair length, identifies four putative mutations associated with long hair.
| Cat Breed | Cat # | Polymorphisma | R51S | V61D | P65H | Mutation 1
|
Mutation 2
|
Mutations 3,4d
|
N195N | Q223Q | L265L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ins356T | R136X | del474T, T159P | ||||||||||
|
| ||||||||||||
| Nuc Positionb | 153 | 182 | 194 | 356 | 406 | 474,475 | 585 | 669 | 795 | |||
|
| ||||||||||||
| Allelesc | G:T | T:A | C:A | −:+ | C:T | T: del474T, A:C | C:T | G:A | G:C | Genotypee | ||
| Hair Length | ||||||||||||
| Abyssinian | Fc640 | Short | G | T | C | − | C | A | C | A | G | N,N |
| Abyssinian | Fc653 | Short | G | T | C | − | C | A | C | A | G | N,N |
| American Shorthair | Fc330 | Short | G | TA | C | − | C | A | C | GA | CG | N,N |
| American Shorthair | Fc2387 | Short | G | T | CA | − | C | A | C | A | C | N,N |
| British Shorthair | Fc2588 | Short | G | A | C | − | C | A | C | A | G | N,N |
| British Shorthair | Fc2593 | Short | G | T | A | − | C | CA | CT | G | G | N,4 |
| British Shorthair | Fc2596 | Short | G | TA | CA | − | C | A | CT | GA | G | N,N |
| Burmese | Fc305 | Short | G | T | A | − | C | A | C | G | G | N,N |
| Burmese | Fc488 | Short | G | T | A | − | C | A | C | G | G | N,N |
| Chartreux | Fc2505 | Short | G | T | C | − | C | A | C | G | C | N,N |
| Chartreux | Fc2698 | Short | G | T | A | − | C | A | C | GA | CG | N,N |
| Cornish rex | Fc2478 | Short | G | T | C | − | C | A | C | G | C | N,N |
| Devon Rex | Fc1455 | Short | G | T | CA | − | C | CA | C | G | C | N,4 |
| Egyptian Mau | Fc523 | Short | G | TA | C | − | C | A | C | GA | G | N,N |
| Egyptian Mau | Fc1671 | Short | G | T | C | − | C | A | C | G | CG | N,N |
| Havana | Fc733 | Short | T | T | C | − | C | A | C | G | C | N,N |
| Havana | Fc2591 | Short | G | T | CA | − | C | A | C | GA | CG | N,N |
| Manx | Fc2813 | Short | G | T | CA | − | C | CA | C | G | C | N,4 |
| Ocicat | Fc2386 | Short | G | TA | CA | − | C | A | C | GA | G | N,N |
| Ocicat | Fc2919 | Short | G | T | A | − | C | A | C | G | G | N,N |
| Russian Blue | Fc1143 | Short | G | A | C | − | C | A | C | A | G | N,N |
| Russian Blue | Fc2659 | Short | G | TA | CA | − | C | A | CT | G | CG | N,N |
| Scottish Fold | Fc2536 | Short | G | T | A | − | C | CA | C | GA | CG | N,4 |
| Scottish Fold | Fc2843 | Short | G | TA | C | − | C | CA | C | GA | CG | N,4 |
| Siamese | Fc2735 | Short | G | T | C | − | C | A | C | GA | G | N,N |
|
| ||||||||||||
| Birman | Fc1916 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Birman | Fc2349 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Birman | Fc2569 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Maine Coon | Fc1231 | Long | G | T | A | − | C | del474T/C | CT | G | CG | 3,4 |
| Maine Coon | Fc2325 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Maine Coon | Fc2586 | Long | G | T | A | − | C | del474T/C | CT | G | CG | 3,4 |
| Maine Coon | Fc2608 | Long | G | T | A | − | C | del474T | T | G | G | 3,3 |
| Norwegian Forest Cat | Fc1914 | Long | G | A | C | − | T | A | C | A | G | 2,2 |
| Norwegian Forest Cat | Fc2086 | Long | G | TA | CA | − | CT | CA | C | GA | CG | 2,4 |
| Norwegian Forest Cat | Fc2587 | Long | G | A | C | − | T | A | C | A | G | 2,2 |
| Norwegian Forest Cat | Fc2598 | Long | G | A | C | − | T | A | C | A | G | 2,2 |
| Persian | Fc1166 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Persian | Fc1931 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Persian | Fc2064 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Persian | Fc2276 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Ragdoll | Fc582 | Long | G | T | C | + | C | A | CT | G | C | 1,1 |
| Ragdoll | Fc1621 | Long | G | T | A | − | C | del474T/C | C | G | CG | 3,4 |
| Ragdoll | Fc2271 | Long | G | T | CA | −+ | C | CA | C | G | C | 1,4 |
| Ragdoll | Fc4013 | Long | G | T | CA | −+ | C | CA | C | G | C | 1,4 |
| Turkish Angora | Fc2521 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Turkish Angora | Fc2707 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Turkish Angora | Fc2742 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Turkish Van | Fc569 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Turkish Van | Fc2517 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
| Turkish Van | Fc2737 | Long | G | T | A | − | C | C | C | G | C | 4,4 |
Mutation 1 (ins356T) and Mutation 3 (del474T) result in the predicted truncation of FGF5 Isoform 1.
Nucleotide position in coding sequence of predicted mRNA
Putative mutations are marked in bold. When only 1 base is shown, the cat is homozygous at that position. For ins356T “+” and “−” refer to insertion and no insertion, respectively.
The haplotypes of the 2 adjacent mutations 3 and 4 were inferred from the DNA sequence. Haplotypes are coded as follows: nucleotide positions 474,475 on allele 1/positions 474,475 on allele 2:
| del474T | del474T,A/del474T,A |
| A | No Del 474T,A/No Del 474T,A |
| C | No Del 474T,C/No Del 474T,C |
| del474T/C | No Del474T,C/Del474T,A |
| CA | No Del 474T,C/No Del 474T,A |
Genotypes of both alleles are coded as follows:
N=normal, 1–4=Mutations 1–4.
Figure 1.
Figure 1A. Alignment of FGF5 Isoform 1 (full length form translated from 3 exons) for human, short-haired (SH) cat and long-haired (LH) cats with 4 putative recessive mutations. Residue changes after putative mutations are marked in bold.
Figure 1B. Alignment of FGF5 Isoform 2 (translated from exon 1 spliced to exon 3) for human, short-haired cat and long-haired cats with 4 putative recessive mutations.
In our study, comparison of the feline FGF5 CDS in 50 unrelated breed cats identified 10 single nucleotide polymorphisms (SNPs) (Table 2). By examining the observed allele distributions between short and long-haired cats (Table 2), three SNPs in exon 1 of FGF5 producing non-synonymous substitutions in both FGF5 isoforms, and three SNPs encoding synonymous substitutions in exon 3 were all excluded as possible causative mutations for the long-haired phenotype. The four remaining independent changes detected in exons 1, and 3 appeared to be functionally significant (described below as Mutations 1–4). All 25 long-haired breed cats were either homozygous for one or compound heterozygous for two of these four predicted mutations, while none of the 25 short-haired cats carried two predicted mutant FGF5 alleles.
Mutation 1
In three unrelated, long-haired Ragdoll cats (Fc582, Fc2271 and Fc4013) an insertion of a thymine base was detected 356 bp (c.ins356T) downstream of the predicted translation initiation start site of FGF5 CDS (Table 2). This frame-shift mutation was predicted to introduce three non-synonymous substitutions at the end of exon 1, 31 substitutions in exon 2, and a stop codon in exon 3, truncating the Mutation 1 FGF5 protein prematurely at 160 aa’s. In comparison the size of the full-length, wildtype feline FGF5 protein was predicted to be 270 aa’s in length (Figure 1A). The result of the c.ins356T mutation in the short FGF5 mRNA (FGF5S), if spliced and translated correctly, would be to extend the predicted protein to 226 aa’s, compared to 125 aa’s in the wildtype FGF5S (Figure 1B). One long-haired Ragdoll cat (Fc582) was found to be homozygous for the c.ins356T genotype, supporting the conclusion that the substantial changes caused by this frame-shift mutation are likely to completely disrupt the biological activity of both Mutation 1 FGF5 isoforms. The other two Ragdoll cats (Fc2271 and Fc4013) were compound heterozygous for Mutation 1 and a second mutant allele (Mutation 4 discussed below). None of the 25 short-haired cats were found to carry the Mutation 1 allele in this survey.
Mutation 2
Among all of the sequenced breed cats, only Norwegian Forest Cats possessed a potential nonsense mutation introduced in exon 2 by the substitution of a cytosine to a thymine base at nucleotide position 406 (c.C>T406). While the Mutation 2 FGF5S mRNA lacking exon 2 should be translated normally, the full-length Mutation 2 FGF5 protein was predicted to contain a change at aa position 135 from an arginine to a premature stop codon (p.R136X) (Figure 1A). Three long-haired Norwegian Forest Cats (Fc1914, Fc2587 and Fc2598) were homozygous for Mutation 2 in the FGF5 gene, suggesting a loss of biological activity. One Norwegian Forest Cat (Fc2086) was compound heterozygous for Mutations 2 and 4 (discussed below)(Table 2).
Mutation 3
A deletion of a thymine base at nucleotide position 474 (c.del474T) was found to introduce a frame-shift mutation near the beginning of exon 3 in one Ragdoll (Fc1621) and three Maine Coon cats (Fc1231, Fc2586, Fc2608) (Table 2). The predicted Mutation 3 FGF5 protein should contain extensive non-synonymous substitutions starting at aa position 158 and truncate prematurely at 260 residues (Figure 1A). Whereas, the predicted Mutation 3 FGF5S should have extensive changes starting at aa position 144 and be extended to 235 aa’s in comparison to 125 aa’s for the wildtype FGF5S (Figure 1B). As a result of Mutation 3, both isoforms were unlikely to maintain their normal biological activity. One Maine Coon cat (Fc2608) was found to be homozygous for Mutation 3, while the other three long-haired cats were compound heterozygous for Mutations 3 and 4. Recombination spanning the single nucleotide position between Mutations 3 and 4 was not detected by phase haplotype analyses in any long-haired cats (Table 2). None of the 25 short-haired breed cats genotyped carried the Mutation 3 allele.
Mutation 4
A change from an adenine to a cytosine base was detected at nucleotide position 475 (c.A>C475) in exon 3 in at least one individual from all of the long-haired cat breeds sequenced (Table 2). This change was predicted to result in the missense substitution of a single threonine with a proline residue at aa position 159 (p.T159P) in FGF5 (Figure 1A), and the replacement of a tyrosine with a serine residue at the penultimate position in the potential FGF5S isoform (Figure 1B). The majority of long-haired cats sequenced in this initial survey were homozygous for Mutation 4 (Table 2). While five breed cats from short-haired registries [British Shorthair (Fc2593), Devon Rex (Fc1455), Manx (Fc2813) and Scottish Fold cats (Fc2536 and FC2843)] were heterozygous carriers of this allele, no short-haired cats were found to be homozygous for Mutation 4 in the FGF5 gene.
DNA Sequence Analyses of FGF5 in Additional Short and Long-Haired Breed Cats
To assess the frequency of normal and mutant FGF5 alleles in cat populations of 26 breeds, we genotyped 66 additional breed cats (29 short and 37 long-haired) using RFLP assays designed to detect Mutations 1–4 (Table A2), and then sequenced the entire FGF5 CDS to detect any other potential mutations. Their genotypes were combined with those of the original 50 cats (Table 2) to estimate the allele frequencies in the 21 original breeds and 5 additional long-haired breeds (Angora, Balinese, Himalayan, Siberian and Somali) (Table 3). Mutations 1 and 2 remained unique to the Ragdoll and Norwegian Forest Cat breeds respectively, while Mutation 3 continued to be detected only in Maine Coon and Ragdoll cats. Mutation 4 was the most prevalent mutant FGF5 allele in this study. It was detected in all 14 long-haired breeds sampled, and was the only mutation detected in 11 of these breeds (Table 3). No additional mutations were detected in the FGF5 CDS in these additional cats. Most importantly, no short-haired breed cats (n=54) were homozygous for any of the four FGF5 mutant alleles, while all long-haired breed cats (n=62) were either homozygous or compound heterozygous for two separate mutant alleles. Only three of the six possible compound heterozygous allelic combinations (1/4, 3/4, 2/4) were observed in breed cats in this study (Table 3). The results of this extended breed analysis spurred us to genotype and test whether the inheritance of the four mutations detected in the FGF5 gene segregated in pedigrees of non-breed cats with the long-haired phenotype in an autosomal recessive manner as previously reported (Vella and Robinson 1999).
Table 3.
FGF5 Allele Frequencies Among 116 Cats From 26 Registered Breeds
| CFA Registered Breeds Long-haired: |
# of Cats | Mut. # 1 | 2 | 3 | 4 | None | Coat Length |
|---|---|---|---|---|---|---|---|
| Angora | 2 | 1.00 | Long | ||||
| Balinese | 2 | 1.00 | Long | ||||
| Birman | 6 | 1.00 | Long | ||||
| Himalayan | 4 | 1.00 | Long | ||||
| Maine Coon | 4 | 0.50 | 0.50 | Long | |||
| Norwegian Forest Cat | 4 | 0.88 | 0.12 | Long | |||
| Persian | 7 | 1.00 | Long | ||||
| Ragdoll | 13 | 0.27 | 0.23 | 0.50 | Long | ||
| Siberian | 3 | 1.00 | Long | ||||
| Somali | 6 | 1.00 | Long | ||||
| Turkish Angora | 4 | 1.00 | Long | ||||
| Turkish Van | 5 | 1.00 | Long | ||||
| Long or Short-haired: | |||||||
| Manxc | 1 | 1.00 | 0.00 | Long | |||
| 2 | 0.50d | 0.50 | Short | ||||
| Scottish Foldb,c | 1 | 1.00 | 0.00 | Long | |||
| 3 | 0.33d | 0.66 | Short | ||||
| Short-haired: | |||||||
| Abyssinian | 8 | 1.00 | Short | ||||
| American Shorthair | 7 | 1.00 | Short | ||||
| British Shorthaira | 5 | 0.20d | 0.80 | Short | |||
| Burmese | 2 | 1.00 | Short | ||||
| Chartreux | 2 | 1.00 | Short | ||||
| Cornish Rex | 1 | 1.00 | Short | ||||
| Devon Rexa,b | 3 | 0.33d | 0.67 | Short | |||
| Egyptian Mau | 10 | 1.00 | Short | ||||
| Havana | 2 | 1.00 | Short | ||||
| Ocicat | 2 | 1.00 | Short | ||||
| Russian Blue | 3 | 1.00 | Short | ||||
| Siamese | 4 | 1.00 | Short |
Breeds with histories of out-crossing with Persian cats
Breeds that the CFA allows out-crossing with British Shorthair cats
Breeds with separate short and long-haired CFA registries
Mutation 4 alleles were only present in the heterozygous state in short-haired cats
FGF5 Sequence and Linkage analyses in Non-Breed Cats
To test for Mendelian segregation of the four mutations identified in FGF5 with the long-haired trait, all three exons were successfully sequenced in 225 of the 261 cats in the Nestlé-Purina pedigree and 40 of 50 cats in a second, non-breed pedigree from Johns Hopkins University. Of the 211 short-haired cats genotyped in the Nestlé-Purina pedigree, all were either homozygous for the wildtype FGF5 allele, or heterozygous for Mutations 2, 3 or 4 (Table A3). Whereas, all of the 14 long-haired cats were either homozygous for Mutations 2 or 4 or compound heterozygous for paired combinations of Mutations 2, 3 or 4. When the genotypes of the 225 individuals in the Nestlé-Purina pedigree FGF5 were coded for the one wildtype and four mutant alleles (Table A3), and included in additional linkage analyses, linkage between the long-haired phenotype and FGF5 locus was established with peak LOD scores of 11.6 at a θ=0.00 (Table 1). Similarly, all nine genotyped long-haired cats in the Johns Hopkins University pedigree were either homozygous for Mutation 4 or compound heterozygous for Mutations 3 and 4, while all 31 short-haired cats were either heterozygous carriers of Mutations 3 or 4 or were homozygous for the wildtype FGF5 allele (Table A3). As in the breed survey, the concordant phenotype and genotype data for the total of 242 short-haired and 23 long-haired, non-breed cats were consistent with mutations in the FGF5 gene segregating with the long-haired phenotype in an autosomal recessive manner.
Table A3.
Coded FGF5 Genotypes and Phenotypes for 2 Non-breed Pedigrees
| Pedigree | Animala | Sirea | Dama | Sexb | Lengthc | FGF5d | FGF5d |
|---|---|---|---|---|---|---|---|
| Purina | 41253 | 0 | 0 | 1 | 1 | 5 | 5 |
| Purina | 41314 | 0 | 0 | 1 | 1 | 5 | 5 |
| Purina | 41360 | 0 | 0 | 1 | 1 | 4 | 5 |
| Purina | 921755 | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | 922033 | 0 | 0 | 1 | 1 | 2 | 5 |
| Purina | 932132 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | 932149 | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | 932174 | 0 | 0 | 2 | 1 | 2 | 5 |
| Purina | 932244 | 0 | 0 | 2 | 1 | 5 | 5 |
| Purina | 163D | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | 172B | 0 | 0 | 2 | 1 | 4 | 5 |
| Purina | 173C | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | 186A | 0 | 0 | 1 | 0 | 0 | 0 |
| Purina | C4-296 | 0 | 0 | 2 | 1 | 3 | 5 |
| Purina | 164C | 0 | 0 | 1 | 0 | 0 | 0 |
| Purina | C5-145 | 0 | 0 | 1 | 1 | 4 | 5 |
| Purina | C7-123 | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | C7-142 | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | C8-110 | 0 | 0 | 1 | 0 | 0 | 0 |
| Purina | CB-308 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | CB-39 | C5-145 | C7-123 | 2 | 1 | 0 | 0 |
| Purina | CB-46 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | CB-460 | 0 | 0 | 2 | 1 | 4 | 5 |
| Purina | CB-461 | 0 | 0 | 2 | 1 | 5 | 5 |
| Purina | CB-473 | C5-145 | 173C | 2 | 1 | 2 | 5 |
| Purina | CB-5 | 0 | 0 | 2 | 1 | 0 | 0 |
| Purina | CB-92 | C5-145 | E7-95 | 2 | 1 | 5 | 5 |
| Purina | CC-159 | 186A | E6-100 | 2 | 1 | 5 | 5 |
| Purina | CC-160 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | CD-41 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | CE-104 | 186A | CB-461 | 2 | 1 | 5 | 5 |
| Purina | CE-115 | 186A | CB-92 | 1 | 1 | 4 | 5 |
| Purina | CE-125 | 186A | CB-5 | 2 | 1 | 5 | 5 |
| Purina | CE-127 | 186A | CB-5 | 2 | 1 | 0 | 0 |
| Purina | CE-145 | 186A | 163D | 2 | 1 | 0 | 0 |
| Purina | CE-147 | 186A | E7-78 | 2 | 1 | 5 | 5 |
| Purina | CE-45 | C5-145 | C7-123 | 1 | 1 | 4 | 5 |
| Purina | CE-46 | C5-145 | C7-123 | 1 | 1 | 4 | 5 |
| Purina | CE-95 | 186A | CB-473 | 2 | 1 | 2 | 5 |
| Purina | CF-101 | 0 | 0 | 2 | 1 | 5 | 5 |
| Purina | CF-107 | 41253 | E8-97 | 1 | 1 | 4 | 5 |
| Purina | CF-113 | 41253 | CB-46 | 1 | 1 | 0 | 0 |
| Purina | CF-115 | 41253 | CB-46 | 1 | 1 | 5 | 5 |
| Purina | CF-24 | 41253 | CB-39 | 2 | 1 | 0 | 0 |
| Purina | CF-267 | 41253 | E8-99 | 1 | 1 | 3 | 5 |
| Purina | CF-268 | 41253 | E8-99 | 1 | 1 | 0 | 0 |
| Purina | CF-269 | 41253 | E8-99 | 1 | 1 | 4 | 5 |
| Purina | CF-271 | 41253 | E8-99 | 2 | 1 | 4 | 5 |
| Purina | CF-272 | 41253 | E8-99 | 2 | 1 | 3 | 5 |
| Purina | CF-280 | 41253 | CC-159 | 1 | 1 | 5 | 5 |
| Purina | CF-283 | CE-115 | 921755 | 1 | 1 | 5 | 5 |
| Purina | CF-29 | 41253 | CB-461 | 2 | 1 | 5 | 5 |
| Purina | CF-294 | CE-115 | 932132 | 1 | 1 | 5 | 5 |
| Purina | CF-303 | 41253 | CB-92 | 1 | 1 | 5 | 5 |
| Purina | CF-304 | 41253 | CB-92 | 1 | 1 | 5 | 5 |
| Purina | CF-306 | 41253 | CB-92 | 2 | 1 | 5 | 5 |
| Purina | CF-307 | 41253 | CB-92 | 2 | 1 | 5 | 5 |
| Purina | CF-316 | CE-46 | 932174 | 2 | 1 | 5 | 5 |
| Purina | CF-318 | CE-46 | 932174 | 2 | 1 | 2 | 5 |
| Purina | CF-33 | 41314 | CC-160 | 2 | 1 | 5 | 5 |
| Purina | CF-45 | 41314 | C7-123 | 2 | 1 | 5 | 5 |
| Purina | CF-47 | C8-110 | CB-308 | 2 | 1 | 5 | 5 |
| Purina | CF-48 | C8-110 | CB-308 | 2 | 1 | 5 | 5 |
| Purina | CF-53 | 41314 | CB-460 | 2 | 1 | 4 | 5 |
| Purina | CF-64 | 41253 | C7-142 | 2 | 1 | 4 | 5 |
| Purina | CF-83 | 41253 | 172B | 2 | 1 | 4 | 5 |
| Purina | CG-10 | 41314 | CD-41 | 2 | 1 | 5 | 5 |
| Purina | CG-115 | CE-115 | CF-101 | 1 | 1 | 5 | 5 |
| Purina | CG-116 | CE-115 | CF-101 | 1 | 1 | 4 | 5 |
| Purina | CG-14 | CE-115 | CB-460 | 2 | 1 | 4 | 5 |
| Purina | CG-15 | CE-115 | CB-460 | 2 | 2 | 4 | 4 |
| Purina | CG-151 | CE-46 | 932149 | 2 | 1 | 0 | 0 |
| Purina | CG-152 | CE-46 | 932149 | 2 | 1 | 0 | 0 |
| Purina | CG-158 | 41253 | CE-147 | 1 | 1 | 5 | 5 |
| Purina | CG-159 | 41253 | CE-147 | 2 | 1 | 5 | 5 |
| Purina | CG-161 | 41253 | CE-147 | 2 | 1 | 5 | 5 |
| Purina | CG-163 | 41253 | CE-95 | 2 | 1 | 2 | 5 |
| Purina | CG-164 | 41253 | CE-95 | 2 | 1 | 2 | 5 |
| Purina | CG-166 | 41253 | CE-95 | 2 | 1 | 5 | 5 |
| Purina | CG-23 | 41253 | CE-104 | 2 | 1 | 4 | 5 |
| Purina | CG-24 | 41253 | CE-104 | 2 | 1 | 4 | 5 |
| Purina | CG-4 | 41253 | CE-127 | 2 | 1 | 0 | 0 |
| Purina | CG-45 | 41253 | 172B | 1 | 1 | 4 | 5 |
| Purina | CG-5 | 41253 | CE-127 | 2 | 1 | 0 | 0 |
| Purina | CG-9 | 41314 | CD-41 | 2 | 1 | 0 | 0 |
| Purina | CG-97 | CE-46 | CF-53 | 1 | 1 | 0 | 0 |
| Purina | CH-13 | CE-46 | 932174 | 1 | 1 | 5 | 5 |
| Purina | CH-14 | CE-46 | 932174 | 1 | 1 | 0 | 0 |
| Purina | CH-24 | 41253 | CB-460 | 1 | 1 | 5 | 5 |
| Purina | CH-27 | 41253 | CB-460 | 2 | 1 | 5 | 5 |
| Purina | CH-3 | CE-46 | 932244 | 2 | 1 | 0 | 0 |
| Purina | CH-31 | 41253 | CE-145 | 1 | 1 | 0 | 0 |
| Purina | CH-4 | CE-46 | 932244 | 2 | 1 | 0 | 0 |
| Purina | CH-45 | CE-46 | CF-33 | 1 | 1 | 0 | 0 |
| Purina | CH-5 | CE-46 | 932244 | 2 | 1 | 0 | 0 |
| Purina | CH-78 | 41360 | CB-460 | 1 | 1 | 4 | 5 |
| Purina | CH-81 | CE-46 | 932132 | 1 | 2 | 0 | 0 |
| Purina | CH-82 | CE-46 | 932132 | 1 | 1 | 0 | 0 |
| Purina | CH-85 | CE-46 | 932132 | 2 | 2 | 0 | 0 |
| Purina | CH-86 | CE-46 | 932132 | 2 | 1 | 0 | 0 |
| Purina | CI-13 | 41253 | CF-48 | 2 | 1 | 5 | 5 |
| Purina | CI-14 | 41253 | CF-48 | 2 | 1 | 0 | 0 |
| Purina | CI-29 | CE-46 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CI-30 | CE-46 | CF-33 | 1 | 1 | 4 | 5 |
| Purina | CI-34 | 41253 | CE-95 | 1 | 1 | 2 | 5 |
| Purina | CI-35 | 41253 | CE-95 | 1 | 1 | 5 | 5 |
| Purina | CI-38 | CE-46 | 932174 | 2 | 1 | 5 | 5 |
| Purina | CI-39 | CE-46 | 932174 | 2 | 2 | 2 | 4 |
| Purina | CI-42 | 41253 | CC-159 | 2 | 1 | 4 | 5 |
| Purina | CI-43 | 41253 | CC-159 | 2 | 1 | 5 | 5 |
| Purina | CI-45 | 41253 | CE-125 | 1 | 1 | 5 | 5 |
| Purina | CI-53 | 41253 | CB-92 | 1 | 1 | 0 | 0 |
| Purina | CI-67 | CE-46 | CF-29 | 2 | 1 | 0 | 0 |
| Purina | CI-68 | 41253 | CB-460 | 1 | 1 | 0 | 0 |
| Purina | CI-74 | CE-46 | 932244 | 1 | 1 | 5 | 5 |
| Purina | CI-76 | CE-46 | 932244 | 2 | 1 | 0 | 0 |
| Purina | CI-77 | CE-46 | 932244 | 2 | 1 | 4 | 5 |
| Purina | CI-78 | CE-46 | 932244 | 2 | 1 | 5 | 5 |
| Purina | CI-79 | CE-46 | 932244 | 2 | 1 | 4 | 5 |
| Purina | CI-80 | CH-78 | 932174 | 1 | 1 | 5 | 5 |
| Purina | CJ-1 | CI-35 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-10 | CE-115 | CF-29 | 2 | 1 | 4 | 5 |
| Purina | CJ-110 | CI-34 | CH-4 | 1 | 2 | 2 | 4 |
| Purina | CJ-112 | CI-34 | CH-4 | 2 | 1 | 4 | 5 |
| Purina | CJ-113 | CI-34 | CH-4 | 2 | 2 | 2 | 4 |
| Purina | CJ-115 | CH-78 | CG-166 | 1 | 1 | 4 | 5 |
| Purina | CJ-116 | CH-78 | CG-166 | 1 | 1 | 5 | 5 |
| Purina | CJ-117 | CH-78 | CG-166 | 1 | 1 | 0 | 0 |
| Purina | CJ-118 | CH-78 | CG-166 | 1 | 2 | 0 | 0 |
| Purina | CJ-119 | CH-78 | CG-166 | 2 | 1 | 0 | 0 |
| Purina | CJ-125 | CI-34 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-126 | CI-34 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-127 | CI-34 | CF-33 | 1 | 1 | 2 | 5 |
| Purina | CJ-128 | CI-34 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-129 | CI-34 | CF-33 | 1 | 1 | 0 | 0 |
| Purina | CJ-131 | CI-34 | CF-33 | 2 | 1 | 0 | 0 |
| Purina | CJ-132* | CI-34 | CF-33 | 2 | 1 | 5 | 5 |
| Purina | CJ-133 | CI-34 | CH-3 | 1 | 1 | 2 | 5 |
| Purina | CJ-134 | CI-34 | CH-3 | 1 | 1 | 2 | 5 |
| Purina | CJ-142 | CG-158 | CF-48 | 2 | 1 | 5 | 5 |
| Purina | CJ-143 | CE-115 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CJ-144 | CE-115 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CJ-145 | CE-115 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CJ-146 | CE-115 | CH-27 | 2 | 1 | 4 | 5 |
| Purina | CJ-147 | CE-115 | CH-27 | 2 | 1 | 4 | 5 |
| Purina | CJ-153 | CE-115 | CF-29 | 1 | 1 | 5 | 5 |
| Purina | CJ-154 | CE-115 | CF-29 | 1 | 1 | 0 | 0 |
| Purina | CJ-155 | CE-115 | CF-29 | 1 | 1 | 4 | 5 |
| Purina | CJ-157 | CE-115 | CF-29 | 2 | 1 | 4 | 5 |
| Purina | CJ-158 | CE-115 | CF-29 | 2 | 1 | 4 | 5 |
| Purina | CJ-166 | CI-34 | 932174 | 1 | 1 | 0 | 0 |
| Purina | CJ-167 | CI-34 | 932174 | 1 | 2 | 0 | 0 |
| Purina | CJ-168 | CI-34 | 932174 | 1 | 2 | 0 | 0 |
| Purina | CJ-169 | CI-34 | 932174 | 2 | 2 | 2 | 2 |
| Purina | CJ-170 | CI-34 | 932174 | 2 | 2 | 2 | 2 |
| Purina | CJ-171 | CI-34 | 932174 | 2 | 1 | 5 | 5 |
| Purina | CJ-172 | CE-115 | CG-159 | 1 | 1 | 0 | 0 |
| Purina | CJ-173 | CE-115 | CG-159 | 1 | 1 | 0 | 0 |
| Purina | CJ-175 | CE-115 | CG-159 | 2 | 1 | 4 | 5 |
| Purina | CJ-19 | CG-158 | CH-3 | 2 | 1 | 5 | 5 |
| Purina | CJ-2 | CI-35 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-20 | CG-158 | CH-3 | 2 | 1 | 5 | 5 |
| Purina | CJ-23 | CE-115 | CG-166 | 2 | 1 | 0 | 0 |
| Purina | CJ-24 | CE-115 | CG-166 | 2 | 1 | 0 | 0 |
| Purina | CJ-25 | CH-78 | CF-83 | 2 | 1 | 5 | 5 |
| Purina | CJ-26 | CE-115 | 932244 | 1 | 1 | 5 | 5 |
| Purina | CJ-27 | CE-115 | 932244 | 1 | 1 | 0 | 0 |
| Purina | CJ-3 | CI-35 | CF-33 | 1 | 1 | 5 | 5 |
| Purina | CJ-32 | CE-115 | CH-4 | 1 | 1 | 4 | 5 |
| Purina | CJ-33 | CE-115 | CH-4 | 1 | 1 | 4 | 5 |
| Purina | CJ-34 | CE-115 | CH-4 | 1 | 2 | 4 | 4 |
| Purina | CJ-36 | CE-115 | CH-4 | 1 | 1 | 5 | 5 |
| Purina | CJ-51 | CE-115 | CH-27 | 2 | 1 | 0 | 0 |
| Purina | CJ-52 | CE-115 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CJ-64 | CH-78 | CI-39 | 1 | 1 | 0 | 0 |
| Purina | CJ-66 | CH-78 | CI-39 | 1 | 2 | 4 | 4 |
| Purina | CJ-68 | CH-78 | CI-39 | 2 | 1 | 5 | 5 |
| Purina | CJ-7 | CE-115 | CF-29 | 1 | 1 | 4 | 5 |
| Purina | CJ-74 | CE-115 | 932174 | 1 | 2 | 2 | 4 |
| Purina | CJ-75 | CE-115 | 932174 | 1 | 1 | 2 | 5 |
| Purina | CJ-76 | CE-115 | 932174 | 1 | 2 | 2 | 4 |
| Purina | CJ-77 | CE-115 | 932174 | 1 | 1 | 4 | 5 |
| Purina | CJ-78 | CE-115 | 932174 | 2 | 2 | 2 | 4 |
| Purina | CJ-79 | CE-115 | 932174 | 2 | 1 | 4 | 5 |
| Purina | CJ-8 | CE-115 | CF-29 | 1 | 1 | 4 | 5 |
| Purina | CJ-83 | CE-115 | 932174 | 2 | 1 | 4 | 5 |
| Purina | CJ-86 | CG-158 | CH-5 | 1 | 1 | 5 | 5 |
| Purina | CJ-87 | CG-158 | CH-5 | 1 | 1 | 5 | 5 |
| Purina | CJ-88 | CG-158 | CH-5 | 1 | 1 | 0 | 0 |
| Purina | CJ-89 | CG-158 | CH-5 | 2 | 1 | 4 | 5 |
| Purina | CJ-90 | CG-158 | CH-5 | 2 | 1 | 4 | 5 |
| Purina | CJ-93 | CH-78 | CF-64 | 1 | 1 | 5 | 5 |
| Purina | CJ-94 | CH-78 | CF-64 | 1 | 1 | 4 | 5 |
| Purina | CJ-95 | CH-78 | CF-64 | 1 | 2 | 4 | 4 |
| Purina | CJ-97 | CH-78 | CF-64 | 2 | 1 | 4 | 5 |
| Purina | CJ-98 | CH-78 | CF-64 | 2 | 1 | 5 | 5 |
| Purina | CK-06 | CE-115 | CI-13 | 1 | 1 | 5 | 5 |
| Purina | CK-07 | CE-115 | CI-13 | 2 | 1 | 4 | 5 |
| Purina | CK-08 | CE-115 | CI-13 | 2 | 1 | 4 | 5 |
| Purina | CK-09 | CF-303 | CH-3 | 1 | 1 | 5 | 5 |
| Purina | CK-10 | CF-303 | CH-3 | 1 | 1 | 5 | 5 |
| Purina | CK-11 | CF-303 | CH-3 | 1 | 1 | 5 | 5 |
| Purina | CK-114 | CF-303 | CG-166 | 1 | 1 | 5 | 5 |
| Purina | CK-115 | CF-303 | CG-166 | 2 | 1 | 5 | 5 |
| Purina | CK-116 | CF-303 | CG-166 | 2 | 1 | 5 | 5 |
| Purina | CK-117 | CF-303 | CG-166 | 2 | 1 | 5 | 5 |
| Purina | CK-12 | CF-303 | CH-3 | 2 | 1 | 5 | 5 |
| Purina | CK-120 | CG-158 | 932174 | 1 | 1 | 2 | 5 |
| Purina | CK-121 | CG-158 | 932174 | 1 | 1 | 5 | 5 |
| Purina | CK-122 | CG-158 | 932174 | 2 | 1 | 5 | 5 |
| Purina | CK-123 | CG-158 | 932174 | 2 | 1 | 5 | 5 |
| Purina | CK-124 | CG-158 | 932174 | 2 | 1 | 2 | 5 |
| Purina | CK-13 | CF-303 | CH-3 | 2 | 1 | 5 | 5 |
| Purina | CK-130 | 922033 | CH-27 | 1 | 1 | 2 | 5 |
| Purina | CK-132 | 922033 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CK-133 | 922033 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CK-134 | 922033 | CH-27 | 2 | 1 | 5 | 5 |
| Purina | CK-135 | 922033 | CH-27 | 2 | 1 | 5 | 5 |
| Purina | CK-136 | 922033 | CH-27 | 2 | 1 | 2 | 5 |
| Purina | CK-14 | CI-34 | CI-39 | 1 | 2 | 0 | 0 |
| Purina | CK-144 | 922033 | CF-29 | 1 | 1 | 2 | 5 |
| Purina | CK-145 | 922033 | CF-29 | 1 | 1 | 5 | 5 |
| Purina | CK-146 | 922033 | CF-29 | 1 | 1 | 2 | 5 |
| Purina | CK-147 | 922033 | CF-29 | 2 | 1 | 0 | 0 |
| Purina | CK-148 | 922033 | CF-29 | 2 | 1 | 2 | 5 |
| Purina | CK-15 | CI-34 | CI-39 | 1 | 1 | 4 | 5 |
| Purina | CK-16 | CI-34 | CI-39 | 2 | 2 | 2 | 2 |
| Purina | CK-17 | CI-34 | CI-39 | 2 | 2 | 0 | 0 |
| Purina | CK-18 | CI-34 | CI-39 | 2 | 1 | 5 | 5 |
| Purina | CK-19 | CI-34 | CI-39 | 2 | 1 | 0 | 0 |
| Purina | CK-20 | CI-34 | CI-39 | 2 | 1 | 2 | 5 |
| Purina | CK-21 | CI-34 | CI-39 | 2 | 1 | 4 | 5 |
| Purina | CK-22 | CE-115 | CF-48 | 1 | 1 | 4 | 5 |
| Purina | CK-23 | CE-115 | CF-48 | 1 | 1 | 5 | 5 |
| Purina | CK-24 | CE-115 | CF-48 | 1 | 1 | 5 | 5 |
| Purina | CK-25 | CE-115 | CF-48 | 2 | 1 | 4 | 5 |
| Purina | CK-27 | CE-115 | 932174 | 1 | 1 | 2 | 5 |
| Purina | CK-28 | CE-115 | 932174 | 1 | 1 | 2 | 5 |
| Purina | CK-29 | CE-115 | 932174 | 1 | 1 | 5 | 5 |
| Purina | CK-32 | CE-115 | 932174 | 2 | 1 | 2 | 5 |
| Purina | CK-34 | CG-158 | CH-5 | 2 | 1 | 0 | 0 |
| Purina | CK-35 | CG-158 | CH-5 | 2 | 1 | 5 | 5 |
| Purina | CK-36 | CG-158 | CH-5 | 2 | 1 | 0 | 0 |
| Purina | CK-38 | CI-34 | CI-38 | 1 | 1 | 0 | 0 |
| Purina | CK-39 | CI-34 | CI-38 | 1 | 1 | 5 | 5 |
| Purina | CK-40 | CI-34 | CI-38 | 1 | 1 | 5 | 5 |
| Purina | CK-43 | CI-34 | CI-38 | 2 | 1 | 5 | 5 |
| Purina | CK-44 | CI-34 | CI-38 | 2 | 1 | 5 | 5 |
| Purina | CK-46 | 922033 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CK-47 | 922033 | CH-27 | 1 | 1 | 4 | 5 |
| Purina | CK-48 | 922033 | CH-27 | 1 | 1 | 5 | 5 |
| Purina | CK-49 | 922033 | CH-27 | 2 | 1 | 5 | 5 |
| Purina | CK-50 | CG-158 | 932244 | 1 | 1 | 0 | 0 |
| Purina | CK-52 | CG-158 | 932244 | 2 | 1 | 5 | 5 |
| Purina | CK-55 | 922033 | CF-29 | 1 | 1 | 5 | 5 |
| Purina | CK-56 | 922033 | CF-29 | 1 | 1 | 4 | 5 |
| Purina | CK-57 | 922033 | CF-29 | 1 | 1 | 2 | 5 |
| Purina | CK-58 | 922033 | CF-29 | 2 | 1 | 5 | 5 |
| Purina | CK-59 | 922033 | CF-29 | 2 | 1 | 5 | 5 |
| Purina | CK-60 | 922033 | CF-29 | 2 | 1 | 5 | 5 |
| Purina | CK-61 | CH-78 | CG-166 | 1 | 1 | 4 | 5 |
| Purina | CK-62 | CH-78 | CG-166 | 1 | 1 | 5 | 5 |
| Purina | CK-63 | CH-78 | CG-166 | 2 | 1 | 4 | 5 |
| Purina | CK-64 | CH-78 | CG-166 | 2 | 1 | 5 | 5 |
| Purina | CK-65 | CH-78 | CG-166 | 2 | 1 | 4 | 5 |
| Purina | CK-66 | CG-158 | CH-4 | 1 | 1 | 4 | 5 |
| Purina | CK-67 | CG-158 | CH-4 | 1 | 1 | 5 | 5 |
| Purina | CK-68 | CG-158 | CH-4 | 1 | 1 | 4 | 5 |
| Purina | CK-69 | CG-158 | CH-4 | 1 | 1 | 5 | 5 |
| Purina | CK-70 | CG-158 | CH-4 | 2 | 1 | 4 | 5 |
| Purina | CK-72 | CI-34 | CF-33 | 1 | 1 | 0 | 0 |
| Purina | CK-73 | CI-34 | CF-33 | 2 | 1 | 5 | 5 |
| Purina | CK-74 | CI-34 | CF-33 | 2 | 1 | 2 | 5 |
| Purina | CK-75 | CI-34 | CF-33 | 2 | 1 | 2 | 5 |
| Purina | CK-79 | CH-78 | CE-147 | 1 | 1 | 4 | 5 |
| Purina | CK-80 | CH-78 | CE-147 | 2 | 1 | 5 | 5 |
| Purina | CK-82 | 922033 | CG-159 | 1 | 1 | 5 | 5 |
| Purina | CK-83 | 922033 | CG-159 | 1 | 1 | 5 | 5 |
| Purina | CK-84 | 922033 | CG-159 | 1 | 1 | 2 | 5 |
| Purina | CK-85 | 922033 | CG-159 | 2 | 1 | 2 | 5 |
| Purina | CK-86 | 922033 | CG-159 | 2 | 1 | 2 | 5 |
| Purina | CK-87 | 922033 | CG-159 | 2 | 1 | 0 | 0 |
| Purina | E6-100 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | E7-78 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | E7-95 | 0 | 0 | 2 | 0 | 0 | 0 |
| Purina | E8-97 | 164C | C4-296 | 2 | 1 | 4 | 5 |
| Purina | E8-99 | 164C | C4-296 | 2 | 2 | 3 | 4 |
| JHU | 02-093 | 96-260 | 94-549 | 1 | 1 | 5 | 5 |
| JHU | 02-104 | 98-436 | 95-452 | 2 | 2 | 4 | 4 |
| JHU | 02-149 | 98-147 | 96-131 | 1 | 1 | 5 | 3 |
| JHU | 02-150 | 98-147 | 96-131 | 2 | 1 | 5 | 4 |
| JHU | 03-020 | 0 | 0 | 2 | 1 | 5 | 5 |
| JHU | 03-111 | 96-260 | 98-437 | 1 | 1 | 5 | 4 |
| JHU | 03-112 | 96-260 | 98-437 | 2 | 1 | 5 | 4 |
| JHU | 04-008 | 98-436 | 02-150 | 1 | 1 | 5 | 5 |
| JHU | 04-009 | 98-436 | 02-150 | 1 | 1 | 5 | 4 |
| JHU | 04-010 | 98-436 | 02-150 | 1 | 1 | 5 | 5 |
| JHU | 04-015 | 96-260 | 03-026 | 2 | 1 | 5 | 5 |
| JHU | 04-016 | 96-260 | 03-026 | 2 | 1 | 5 | 3 |
| JHU | 04-062 | 98-436 | 02-150 | 1 | 1 | 5 | 5 |
| JHU | 04-063 | 98-436 | 02-150 | 1 | 1 | 5 | 4 |
| JHU | 04-064 | 98-436 | 02-150 | 2 | 1 | 5 | 5 |
| JHU | 04-065 | 98-436 | 02-150 | 2 | 2 | 4 | 4 |
| JHU | 04-066 | 98-436 | 02-150 | 2 | 2 | 4 | 4 |
| JHU | 04-073 | 98-147 | 03-026 | 1 | 1 | 5 | 4 |
| JHU | 04-108 | 96-260 | 98-437 | 1 | 1 | 5 | 5 |
| JHU | 04-109 | 96-260 | 98-437 | 2 | 1 | 5 | 5 |
| JHU | 04-110 | 96-260 | 98-437 | 1 | 2 | 4 | 4 |
| JHU | 04-112 | 02-093 | 02-104 | 2 | 1 | 5 | 4 |
| JHU | 05-053 | 98-436 | 02-150 | 1 | 1 | 5 | 4 |
| JHU | 05-054 | 98-436 | 02-150 | 1 | 2 | 4 | 4 |
| JHU | 05-056 | 98-436 | 02-150 | 2 | 2 | 4 | 4 |
| JHU | 06-038 | 0 | 0 | 2 | 1 | 5 | 4 |
| JHU | 06-040 | 0 | 0 | 1 | 1 | 5 | 5 |
| JHU | 06-041 | 0 | 0 | 1 | 1 | 5 | 4 |
| JHU | 06-042 | 0 | 0 | 1 | 1 | 5 | 4 |
| JHU | 93-706 | WT | 94-449 | 1 | 1 | 5 | 4 |
| JHU | 94-449 | 0 | 0 | 2 | 1 | 5 | 4 |
| JHU | 94-549 | 93-707 | 93-710 | 2 | 1 | 5 | 5 |
| JHU | 95-216 | 0 | 0 | 2 | 2 | 4 | 4 |
| JHU | 95-452 | 0 | 0 | 2 | 1 | 5 | 4 |
| JHU | 96-131 | 0 | 0 | 2 | 2 | 3 | 4 |
| JHU | 96-260 | 0 | 96-184 | 1 | 1 | 5 | 4 |
| JHU | 98-354 | 96-260 | 95-216 | 2 | 1 | 5 | 4 |
| JHU | 98-436 | 93-707 | 94-449 | 1 | 2 | 4 | 4 |
| JHU | 98-437 | 93-707 | 94-449 | 2 | 1 | 5 | 4 |
Identification numbers of sire and dam. 0=unknown.
Sex: 1=male, 2=female.
Hair Length: 1=short, 2=long
Mutations are coded: 0= not genotyped, 2 = Mutation 2 (c.C>T406), 3=Mutation 3 (c.del474T), 4 = Mutation 4 (c.A>C475) and 5 = no mutation.
Inheritance of the Long-haired Phenotype Segregates with Mutations in FGF5
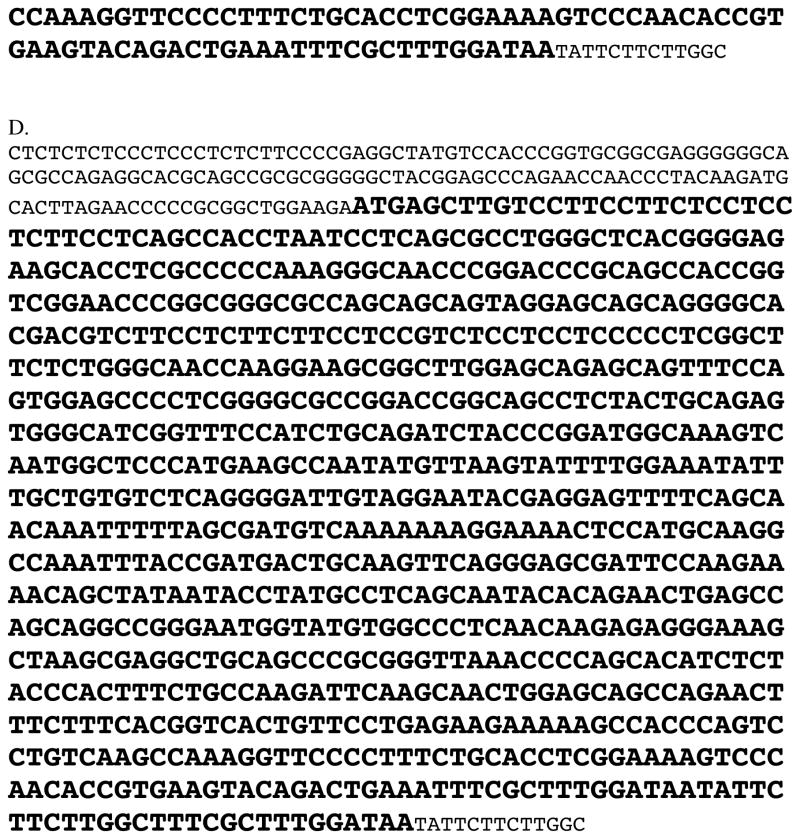
An informative portion of the Nestlé-Purina pedigree clearly demonstrated autosomal recessive inheritance of both Mutations 2 and 4 in the FGF5 locus segregating with the long-haired phenotype (Figure 2A): Multiple crosses of short-haired cats that were heterozygous for either FGF5 Mutations 2 or 4, produced mixed litters with all of the long-haired kittens possessing either 2/2, 2/4 or 4/4 genotypes. Likewise, an informative portion of the Johns Hopkins University pedigree also demonstrated Mendelian inheritance of Mutation 4 segregating with the long-haired phenotype in an autosomal recessive manner (Figure 2B). While not exclusive of autosomal dominance, a 3-generation pedigree of nine long-haired Ragdoll cats produced long-haired offspring with all three possible combinations of Mutations 1 and 4 consistent with autosomal recessive inheritance (Figure 2C). In combination, the pedigree analyses all support an autosomal recessive mode of inheritance of multiple mutant alleles in the FGF5 locus segregating with the long-haired phenotype in domestic cats.
Figure 2.
Three independent pedigrees demonstrating autosomal recessive inheritance of mutations 1, 2 and 4 in FGF5 with the long-haired trait.
A= Two-generation portion of the non-breed Nestlé-Purina pedigree.
B= Two-generation portion of the non-breed John Hopkins University pedigree.
C= Three-generation pedigree of long-haired Ragdoll cats.
Square = male, Circle = female. Open symbol = short-haired, filled = long-haired.
The identification number of cats is written over top of individual symbols. Coded genotypes are listed in parentheses: N= no mutation, 2 = Mutation 2 (c.C>T406), 3=Mutation 3 (c.del474T), 4 = Mutation 4 (c.A>C475)
Discussion
A genomic approach was effective at testing the hypothesis that the FGF5 locus is the major determinant of hair length in the cat. A genome wide scan of the Nestlé-Purina pedigree identified a region on cat chromosome B1, containing a likely candidate gene, FGF5, controlling the long-haired trait in the domestic cat. When individuals were genotyped for changes within the CDS, and the FGF5 “marker” was included in subsequent linkage analyses, a peak LOD score of 11.6 (θ of 0.00) was obtained. Sequence analyses of the feline FGF5 gene in our survey of 12 short-haired, 12 long-haired and two breeds with separate short and long-haired registries revealed four separate mutations that were predicted to disrupt the biological activity of the FGF5 protein. Association analyses of all breed and non-breed cats demonstrated uniformly that a combined total of 85 genotyped, long-haired cats were either homozygous for a single or compound heterozygous for two of four FGF5 mutant alleles, while all 296 short-haired cats were either heterozygous or homozygous for the wildtype allele (Tables 3, A3). Pedigree analyses of 2 independent, non-breed colonies and 1 family of Ragdoll cats demonstrated that multiple combinations of mutations in the FGF5 gene segregated with the long-haired phenotype in an autosomal recessive manner (Figure 2A–C). All of these genetic analyses support our conclusion that the FGF5 gene is the major determinant of hair length in the domestic cat.
While this study was under review, another group published their interpretation of SNPs within the feline FGF5 gene associated with hair length (Drögemüller et al. 2007). They concluded that the c.194C>A (p.P65H) SNP in exon 1 was a predicted mutation for the long-haired trait, while it was clearly in linkage disequilibrium in their pedigree analyses with the c.475A>C SNP in exon 3 (Mutation 4 in this study). They discounted this change as a causative mutation, due to the detection of 1 out of 50 crossbred short-haired cats that was homozygous for c.475A>C. Re-evaluating the genotype and phenotype of this animal is clearly critical in light of our results. None of the 296 short-haired cats that we genotyped in our study were homozygous for the c.475 A>C change (the most common mutation found in most long-haired cats in our study), while 6 short-haired cats were homozygous for the c.194C>A SNP (Table 2). Similarly, a second SNP in exon 1 c.182T>A was detected within five Norwegian Forest Cats and concluded to be a causative mutation in their study, but it was likely in linkage disequilibrium with the c.406C>T nonsense mutation in exon 2 (Mutation 2 found in Norwegian Forest Cats in our study). The authors did not detect this c.406>T change, because they did not sequence exon 2 in the feline FGF5 gene. However, we detected two unrelated, short-haired, registered breed cats (Fc1143 and Fc2588) that were homozygous for the c.182T>A SNP (Table 2), and ruled out this change as a causative mutation for long-hair. In addition, the other study did not detect the rare c.ins356T frame-shift mutation in exon 1 (Mutation 1 found exclusively in Ragdoll cats in this study). Importantly, all 85 of the long-haired cats in our study were either homozygous or compound heterozygous for two of four independent, predicted mutations, while none of the 296 short-haired cats were homozygous for any of these four predicted mutations in the FGF5 gene.
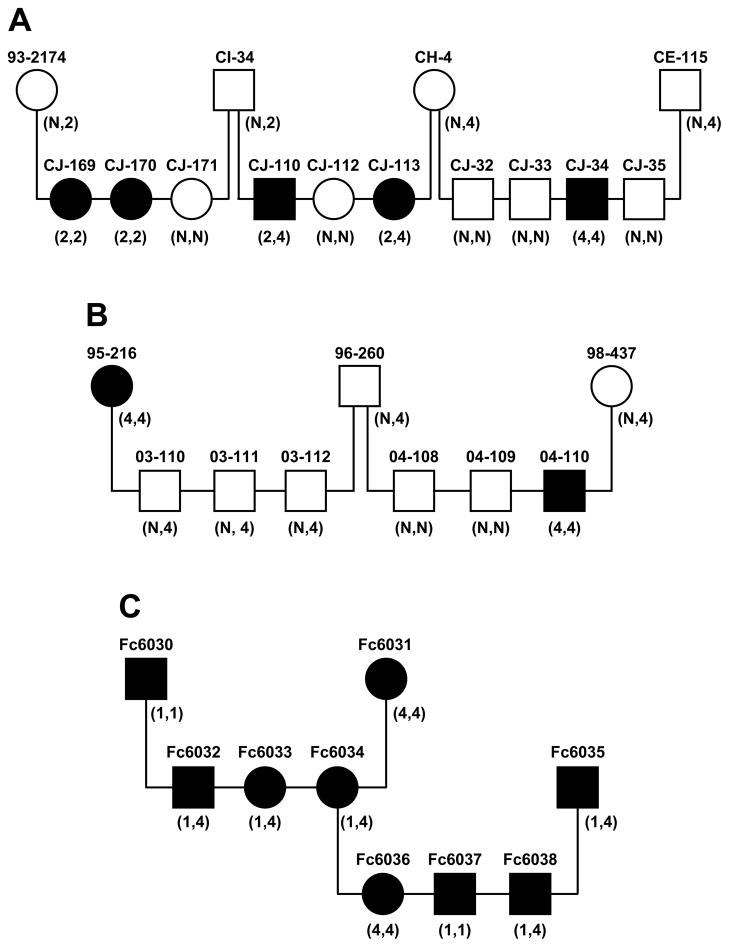
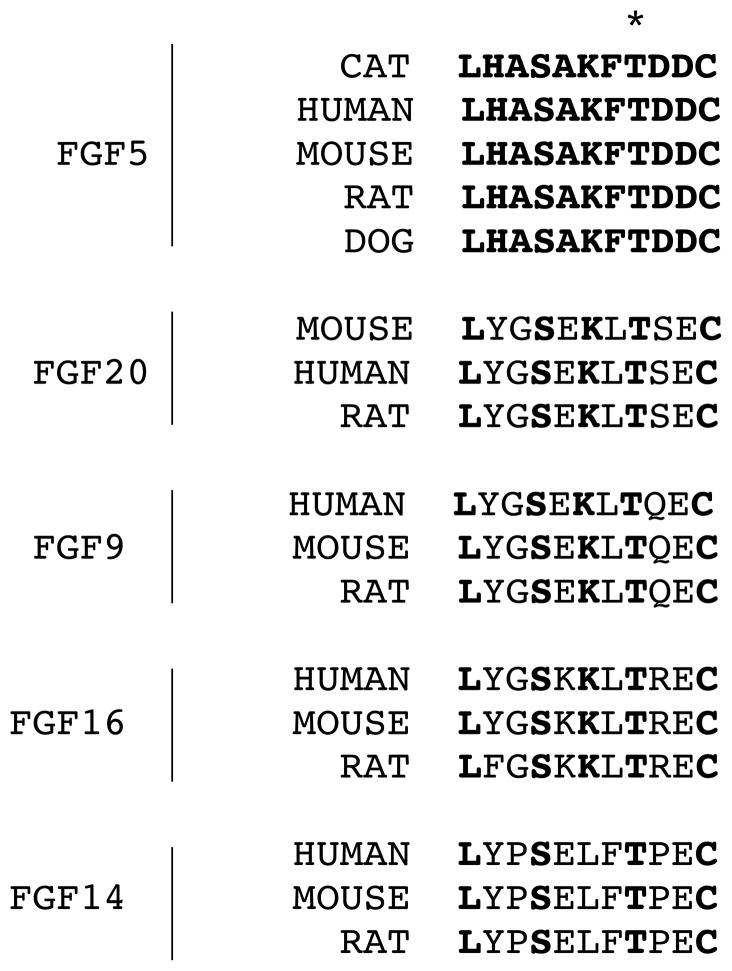
Discovery of spontaneous mutations in FGF5 orthologs of other domestic and wild species may implicate key functional protein domains. As in the Angora mice that lack all transcription of the Fgf5 gene, long-haired dogs with a single FGF5 mutation and long-haired cats with multiple combinations of FGF5 mutations show no additional gross phenotypic changes. A single SNP detected in exon 1 of the canine FGF5 gene in long-haired dogs (Housley and Venta 2006), was predicted to substitute a phenylalanine for a conserved cysteine residue at aa position 95 within the β-1 loop required for the formation of hydrogen bonds with FGFRs (Yeh et al. 2003). While the profound predicted changes caused by Mutations 1, 2 and 3 in the feline FGF5 protein preclude further functional interpretation, the feline Mutation 4 (T159P) FGF5 protein implicates an additional key domain that is likely to be required for FGF5 inhibitory activity. The threonine residue at aa position 159 falls within β-chain 7 of FGF5 (Katoh and Katoh 2002) and is part of a conserved motif found in FGF9, 14 and 20 orthologs from multiple mammalian species (Figure A2). Substitution with a proline residue may change the orientation of the following β-chains 8 and 9 and interfere with FGF5’s ability to bind to its cognate receptors (Mohammadi et al. 2005). While we have not directly assessed the biological activity of the feline wildtype and four mutant FGF5 proteins, singly or in combination, the observation of multiple long-haired cats that were homozygous for each of the four FGF5 mutant genotypes supports the conclusion that each allele is dysfunctional. By analogy to FGF5 function in the mouse, all four feline mutant FGF5 proteins likely lose their inhibitory activity on DPC proliferation, prolonging the anagen stage and extending hair growth in long-haired domestic cats.
Figure A2.
Conservation of sequence motif in region of Mutation 4 (T159P). Amino acids in bold are identical to cat FGF5. The asterisk indicates the position of Mutation 4 (T159 P).
The four mutations detected in the feline FGF5 locus may have arisen independently in geographically isolated, free ranging populations of domestic cats and increased in frequency through natural selection in cold climates where the long-haired trait might be advantageous. Subsequent artificial selection for this recessive phenotype by breeders may have fixed the alleles carried by founders within specific long-haired breeds. While reconstructing the origins of the FGF5 mutations within the context of breeds is largely conjecture due to their limited historical documentation, Mutation 4 may represent the oldest mutant allele. It was present in all 14 and was the only mutant allele in 11 long-haired breed registries sampled in this survey, including the Turkish Angora with a documented history older than 400 years (Fogle 2001).
In contrast Mutations 1, 2 and 3 were limited to three breeds with documented histories less than 200 years old (Vella and Robinson 1999). In this study, Mutations 1 and 2 were found to be unique to the Ragdoll and Norwegian Forest Cat breeds respectively. While Mutation 3 was found in both Maine Coon and Ragdoll cats, the more recently established Ragdoll breed (1960) may have obtained this allele by interbreeding with Maine Coon cats (first shown in 1860) (Fogle 2001). The inadvertent introduction of heritable congenital cardiomyopathy reported in Maine Coon cats (Kittleson et al. 1999) into some lines of Ragdolls supports these reports of prior cross-breeding with Maine Coon cats (Dr. A. Traas, personal communication). The distribution of mutant alleles detected in the breeds sampled in this study support the hypothesis that Mutations 1, 2 and 3 arose independently in non-breed cats that were used as founders of Ragdoll, Norwegian Forest Cats and Maine Coon cats respectively, while Mutation 4 was most likely also present in these early founders or subsequently introduced through crosses with other long-haired breeds such as Persian cats.
The persistence of heterozygous carriers of FGF5 Mutation 4 among four short-haired breed registries (Table 2) may also derive from this practice of out-crossing, or from the original founders of these breeds originally established in the British Isles. While long hair is now a disqualification for registry as a British Shorthair cat with the Cat Fanciers Association (CFA), British Shorthair cats were occasionally crossed with Persian cats beginning after World War II, up until 1978 (Fogle 2001). Despite selection against the long-haired phenotype by breeders, the Mutation 4 allele was still present in the American population of British Shorthair cats sampled in this study. The CFA currently permits both Devon Rex and Scottish Fold cats to be out-crossed with British Shorthair cats, a possible source of Mutation 4 in these breeds. In addition, the founders of the Scottish Fold and Manx breeds that maintain separate registries based on hair length, included long-haired individuals (Fogle 2001; Stephens 2001). In support of this historical reconstruction, the long-haired Manx and Scottish Fold cats that we sequenced were homozygous for Mutation 4 (Table 3).
While not detected in our study, it would not be unexpected to find carriers of Mutation 4 in extended populations of short-haired breeds such as the Siamese from which the long-haired Balinese breed was derived and recognized by the CFA during the 1960’s (Vella and Robinson 1999). While this study included 62 unrelated individuals from 14 long-haired breed registries and 23 long-haired non-breed cats from the JHU and Nestle-Purina pedigrees, it is possible that additional mutations in the feline FGF5 gene may be present in un-sampled long-haired breed and non-breed cats. In addition, the reported quantitative and qualitative differences between the coats of long-haired cat breeds indicates that other independent loci may modify the major influence of FGF5 on hair length in the domestic cat (Vella and Robinson 1999). Finally, this study raises the question as to whether wild felid species with long hair possess FGF5 mutations, providing an avenue to test whether free-ranging populations have undergone natural selection for genes that could affect coat length and their current phylogeographic distribution.
Acknowledgments
The authors would like to thank, Bethany Buzzell and David Wells for technical assistance, and Dr. Guo Kei Pei and Lisa Maslan for operating the automated DNA sequencers. We thank the many cat breeders who have contributed in the past to our population genetic database of cat breeds at the LGD through cooperation with the Cat Fanciers’ Association and The International Cat Association. We would also like to thank Dr. Yasuko Ishida, Dr. Solveig Pflueger and Dr. Anne Traas for their useful information about cat breeds, and Dr. Joan Pontius about the annotation of the cat genome. James Kehler was supported by the NIH/NCRR KO1/SERCA award RR019677-01. David Ryugo was supported in part by the NIH/NIDCD award DC00232. This research was supported in part by intramural research programs at the NIH, NCI and NLM. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. government.
References
- Boutin-Ganache I, Raposo M, Raymond M, Deschepper CF. M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechniques. 2001;31:24–26. 28. [PubMed] [Google Scholar]
- Brownstein MJ, Carpten JD, Smith JR. Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20:1004–1006. 1008–1010. doi: 10.2144/96206st01. [DOI] [PubMed] [Google Scholar]
- Burns M, Fraser MN. Genetics of the dog: the basis of successful breeding. Edinburgh, Scotland: Oliver and Boyd; 1963. [Google Scholar]
- Clements DA, Wang J-K, Dionne CA, Goldfarb M. Activation of fibroblast growth factor (FGF) receptors by recombinant human FGF5. Oncogene. 1993;8:1311–1316. [PubMed] [Google Scholar]
- Dickie MN. New mutations. I. Angora. Mouse News Lett. 1963;29:39. [Google Scholar]
- Drögemüller C, Rüfenacht S, Wichert B, Leeb T. Mutations within the FGF5 gene are associated with hair length in catts. Animal Genetics. 2007;38:218–221. doi: 10.1111/j.1365-2052.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- Eizirik E, Yuhki N, Johnson WJ, Menotti-Raymond M, Hannah SS, O’Brien SJ. Molecular genetics and evolution of melanism in the cat family. Curr Biol. 2003;13:448–453. doi: 10.1016/s0960-9822(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Fishelson M, Geiger D. Exact genetic linkage computations for general pedigrees. Bioinformatics. 2002;18(Suppl 1):S189–S198. doi: 10.1093/bioinformatics/18.suppl_1.s189. [DOI] [PubMed] [Google Scholar]
- Fishelson M, Geiger D. Optimizing exact genetic linkage computations. J Comput Biol. 2004;11:263–275. doi: 10.1089/1066527041410409. [DOI] [PubMed] [Google Scholar]
- Fogle B. The new encyclopedia of cats. New York: DK Publishing Inc; 2001. [Google Scholar]
- Fraser AS. A note on the growth of rex and angora coats. J Genet. 1953;51:237–242. [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, Mathieu-Costello O, Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nature Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Cotman CW. Distribution of fibroblast growth factor 5 mRNA in the rat brain: an in situ hybridization study. Brain Res. 1993;606:79–86. doi: 10.1016/0006-8993(93)91572-a. [DOI] [PubMed] [Google Scholar]
- Green ES, Rendahl KG, Zhou S, Ladner M, Coyne M, Srivastava R, Manning WC, Flannery JG. Two animal models of retinal degeneration are rescued by recombinant adeno-associated virus-mediated production of FGF-5 and FGF-18. Mol Ther. 2001;3: 507–515. doi: 10.1006/mthe.2001.0289. [DOI] [PubMed] [Google Scholar]
- Guo XC, Scott K, Liu Y, Dean M, David V, Nelson GW, Johnson RC, Dilks HH, Lautenburger J, Kessing B, Martenson J, Guan L, Sun S, Deng H, Zheng Y, de The G, Liao J, Zeng Y, O’Brien SJ, Winkler CA. General factors leading to chronic Epstein-Barr virus infection and nasopharyngeal carcinoma in South East China: Study design, methods and feasibility. Hum Genomics. 2006;2:365–375. doi: 10.1186/1479-7364-2-6-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AW, Baeza N, Apelquist Å, Edlund H. Attenuation of FGF signalling in mouse β-cells leads to diabetes. Nature. 2000;408: 864–868. doi: 10.1038/35048589. [DOI] [PubMed] [Google Scholar]
- Haub O, Drucker B, Goldfarb M. Expression of the murine fibroblast growth factor 5 gene in the adult central nervous system. Proc Natl Acad Sci USA. 1990;87:8022–8026. doi: 10.1073/pnas.87.20.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haub O, Goldfarb M. Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development. 1991;112:397–406. doi: 10.1242/dev.112.2.397. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Boyle M, Martin GR. mRNA localization studies suggest that murine FGF-5 plays a role in gastrulation. Development. 1991;112:407–415. doi: 10.1242/dev.112.2.407. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Rosenquist T, Götz J, Martin GR. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Housley DJE, Venta PJ. The long and the short of it: evidence that FGF5 is a major determinant of canine ‘hair’-itability. Anim Genet. 2006;37:309–315. doi: 10.1111/j.1365-2052.2006.01448.x. [DOI] [PubMed] [Google Scholar]
- Ishida Y, David VA, Eizirik E, Schäffer AA, Neelam BA, Roelke ME, O’Brien SJ, Menotti-Raymond M. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics. 2006;88:698–705. doi: 10.1016/j.ygeno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Katoh M. WNT and FGF gene clusters. Int J Oncol. 2002;21:1269–1273. [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Comparative genomics on FGF16 orthologs. Int J Mol Med. 2005;16:959–963. [PubMed] [Google Scholar]
- Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu S-K, Pion PD, Towbin JA. Familial hypertrophic cardiomyopathy in Maine Coon cats: An animal model of human disease. Circulation. 1999;99:3172–3180. doi: 10.1161/01.cir.99.24.3172. [DOI] [PubMed] [Google Scholar]
- Limat A, Hunziker T, Waelti ER, Inaebnit SP, Wiesmann U, Braathen LR. Soluble factors from human hair papilla cells and dermal fibroblasts dramatically increase the clonal growth of outer root sheath cells. Arch Dermatol Res. 1993;285:205–210. doi: 10.1007/BF00372010. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Lyons LA, Schäffer AA, Tomlin JF, Hutton MK, O’Brien SJ. A genetic linkage map of microsatellites in the domestic cat (Felis catus) Genomics. 1999;57:9–23. doi: 10.1006/geno.1999.5743. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Reviews. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Davis B, David VA, Agarwala R, Schäffer AA, Pearks-Wilkerson AJ, Neelam BA, O’Brien SJ, Menotti-Raymond M. A 1.5 megabase resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics. 2007;89:189–196. doi: 10.1016/j.ygeno.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271: 15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol 2 Reviews. 2001:3005.1–3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 2002;290:169–176. doi: 10.1006/bbrc.2001.6140. [DOI] [PubMed] [Google Scholar]
- Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72: 489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- Pennycuik PR, Raphael KA. The angora (go) locus in the mouse: hair morphology, duration of growth cycle and site of action. Genet Res. 1984;44:283–291. doi: 10.1017/s0016672300026525. [DOI] [PubMed] [Google Scholar]
- Rosenquist TA, Martin GR. Fibroblast growth factor signaling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Stephens G. Legacy of the cat. San Francisco: Chronicle Books; 2001. [Google Scholar]
- Suzuki S, Ota Y, Ozawa K, Imamura T. Dual-mode regulation of hair growth cycle by two Fgf-5 gene products. J Invest Dermatol. 2000;114:456–463. doi: 10.1046/j.1523-1747.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- Vella CM, Robinson R. Robinson’s genetics for cat breeders and veterinarians. Boston: Butterworth-Heinemann; 1999. [Google Scholar]
- Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, Morel S, van der Bruggen P, Boon T, Van den Eynde BJ. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- Yeh BK, Igarashi M, Eliseenkova AV, Plotnikov AN, Sher I, Ron D, Aaronson SA, Mohammadi M. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc Natl Acad Sci USA. 2003;100:2266–2271. doi: 10.1073/pnas.0436500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Culpepper A, Reddy M, Loveless J, Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1:369–376. [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]