Abstract
Breathing disorders with recurrent apnea produce periodic decreases in arterial blood O2 or chronic intermittent hypoxia (CIH). Recurrent apnea patients and CIH-exposed rodents exhibit several co-morbidities including diabetes. However, the effects of CIH on pancreatic beta cell function are not known. In the present study, we investigated pancreatic beta cell function in C57BL6 mice exposed to 30 days of CIH. CIH-exposed mice exhibited elevated levels of fasting plasma insulin, but comparable glucose levels, and higher homeostasis model assessment (HOMA), indicating insulin resistance. Pancreatic beta cell morphology was unaltered in CIH- exposed mice. Insulin content was decreased in CIH-exposed beta cells, and this effect was associated with increased proinsulin levels. mRNA and protein levels of the enzyme pro-hormone convertase 1 (PC1) which converts proinsulin to insulin were down regulated in CIH-treated islets. More importantly, glucose-stimulated insulin secretion (GSIS) was impaired in CIH-exposed mice and in isolated islets. Mitochondrial reactive oxygen species (ROS) levels were elevated in CIH-exposed pancreatic islets. Treatment of mice with mito-tempol, a scavenger of mitochondrial ROS during CIH exposure, prevented the augmented insulin secretion and restored the proinsulin as well as HOMA values to control levels. These results demonstrate that CIH leads to pancreatic beta cell dysfunction manifested by augmented basal insulin secretion, insulin resistance, defective proinsulin processing, impaired GSIS and mitochondrial ROS mediates the effects of CIH on pancreatic beta cell function.
Keywords: Intermittent hypoxia, Beta cells, Reactive oxygen species
Introduction
Sleep disordered breathing with recurrent apneas (periodic cessations of breathing) is a major cause of morbidity and mortality affecting several million adult males and females (Marshall et al., 2008; Young et al., 2008). Patients with recurrent apneas develop several co-morbidities, including type 2 diabetes (Tasali et al., 2008; Idris et al., 2009; Laaban et al., 2009). Periodic decreases in arterial blood O2 levels or chronic intermittent hypoxia (CIH) is a hallmark manifestation of recurrent apnea patients (Nieto et al., 2000; Shahar et al., 2001). Rodents exposed to chronic CIH exhibit insulin resistance (O'Donnell, 2007). It is known that insulin resistance leads to compensatory increase in pancreatic beta cell function in order to maintain normal blood glucose levels and when beta cell function is unable to compensate for insulin resistance it would lead to progressive loss of beta cell function (Asghar et al., 2006). However, the effects of chronic CIH on beta cell function and the underlying mechanisms have not been established.
Increased generation of reactive oxygen species (ROS) and the ensuing oxidative stress have been implicated in the progression of type 2 diabetes (Fridlyand & Philipson, 2006; Lavie, 2009; Rahangdale et al., 2009). Several studies have documented that ROS generated by mitochondria impairs pancreatic beta cell function contributing to the development of type 2 diabetes (Newsholme et al., 2007; Morgan et al., 2009; Sivitz & Yorek, 2010; WI, 2010). We recently reported that chronic CIH leads to persistent increase in mitochondrial ROS, which was due to impaired electron transport chain function at the complex I (Khan et al., 2011). In the present study, we examined the effects of chronic CIH on pancreatic beta cell function and assessed the role of mitochondrial ROS. Our results demonstrate that chronic CIH increases insulin secretion both in vivo and in vitro pancreatic islets, and this effect was associated with impaired proinsulin processing and defective glucose stimulated insulin secretion (GSIS). Our results further demonstrate that chronic CIH increases mitochondrial ROS levels in pancreatic beta cells and treating CIH exposed mice with mitochondrial ROS scavenger restored basal insulin secretion and proinsulin processing.
Materials and Methods
Experimental protocols are approved by the Institutional Animal Care and Use committee of the University of Chicago and were performed on adult male C57BL6 mice weighing 20-30g.
Exposure of mice to Intermittent Hypoxia (CIH)
The protocols for exposing mice to chronic CIH were essentially same as described previously (Peng et al., 2006). Briefly, conscious mice were placed in a specialized chamber and exposed to alternating cycles of hypoxia (15sec of ∼5% O2) followed by 5 min of room air, 8h/ day. Control experiments were performed on mice exposed to alternating cycles of room air in the same chamber. The duration of the gas flows were regulated by timer controlled solenoid valves. Ambient O2 and CO2 levels in the chamber were continuously monitored and the CO2 levels were maintained ∼0.1%. In the experiments involving treatment with mitochondrial ROS scavenger, mice were treated with mito-tempol, (10mg/Kg; IP; gift from Dr. Kalyanaraman, Medical College Wisconsin), or vehicle every day before exposure to normoxia or CIH. Acute experiments were performed one hour after terminating the chronic CIH challenge.
Measurements of plasma insulin, proinsulin, glucose and glucagon levels
Mice were anesthetized with Avertin (240mg/kg body weight) and blood samples were collected by retro-orbital bleeding. Fasting insulin and proinsulin levels were determined by Mouse Insulin and Proinsulin ELISA kit respectively (Mercodia, Uppsala, Sweden). Plasma glucagon levels were measured by Glucagon Chemiluminescent ELISA kit (Millipore, Missouri). The detection level for glucagon is 3ρg/ml. Glucose levels were analyzed by blood glucose meter (One touch ultra soft, Life scan) or glucose oxidase assay kit (Pointe Scientific Inc., Michigan, USA). In the experiments involving assessment of glucose stimulated insulin secretion (GSIS), mice were fasted overnight and glucose was administered intraperitonially (2g/Kg body weight). 80-100μl of blood samples were collected from tail snip at 0, 15, 30, 60, 90 and 120 min after administration of glucose and blood glucose and plasma insulin levels were determined.
Isolation of pancreatic islets and exposure to CIH in vitro
Pancreata were harvested from anesthetized mice (Ketamine 50mg/ml/kg and Xylazine 2.5mg/m/kg, IP) and islets were isolated by collagenase digestion and Ficoll step gradient density separation as described (Jacobson et al., 2007). Islets were cultured for 24 hr in RPMI-1640 medium supplemented with 5.8mM glucose, 10% FBS, 1% Penicillin/Streptomycin. Prior to the experiment, islets were serum starved overnight in RPMI-1640 medium containing 2.8mM glucose and 0.1% BSA and then were exposed to either alternating cycles of room air (normoxia, 21% O2) or to 60 cycles of CIH (30 sec of hypoxia ∼1% O2 followed by 5 min of 21% O2 per cycle).
Morphometric analysis
Mice were anesthetized with urethane (1.2 g/kg, IP), and were perfused transcardially with ice-cold heparinized PBS followed by 4% paraformaldehyde. Pancreatic tissue was harvested, mounted in OCT compound (Tissue Tek, VWR Scientific), 8 μm sections were cut and stored at −80° C till further analysis. Sections were washed three times in PBS, and blocked with 20% normal goat serum and 0.2% Triton X-100 in PBS for 30 minutes, and were incubated with anti-insulin (1:2000, Sigma, USA) or rabbit anti-glucagon (1:2000, DiaSorin Inc, MN, USA) or anti-proinsulin (1/2000 dilution; Abcam, MA) or anti-Ki67 (1:500, Abcam, MA, USA) antibodies. Antibody binding was detected using fluorescein iso-thiocyanate-conjugated or texas red secondary antibodies (1:250, Molecular Probes, Oregon, USA). Sections were double stained with DAPI for nuclei and visualized using a fluorescent microscope (Eclipse E600, Nikon). For morphometric analysis, cells positive for insulin and glucagon were counted in each islet (4-5 islets per each section / 5 sections separated by an interval of 50 μm / animal) were divided by the total number of cells stained with DAPI and expressed as percentage of beta- and alpha- cells using J IMAGE (NCIH, Frederick, MD). Islet size was calculated by dividing the total area of the islet by the total number of DAPI stained cells.
Measurement of proinsulin and insulin in the islets
Isolated islets were homogenized in acid ethanol (0.18M HCl in 96% (vol /vol) ethanol) at 4°C. The homogenates were diluted 200 times to prevent interactions of ethanol with ELISA. Proinsulin and insulin levels were determined by Mouse Insulin and Proinsulin ELISA kit (Mercodia, Uppsala, Sweden).
Western Blot analysis
Islet extracts (20 μg) prepared in RIPA buffer (phosphate buffer, pH 7.4 containing150 mM NaCl, 1% triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM EDTA and protease inhibitor cocktail) were fractionated by polyacrylamide-SDS gel electrophoresis and blotted with monoclonal proinsulin (1/3000 dilution; Abcam, MA), polyclonal PC1, PC2 (1/3000; Abcam, MA) and monoclonal tubulin (1/3000; Sigma) antibodies to determine relative protein expression. Proinsulin protein was detected using HRP-conjugated secondary antibody (Millipore, Missouri) and ECL. PC1 and PC2 proteins were detected using secondary goat anti rabbit IR Dye 680 and anti mouse 800CW from LI-COR and blots scanned on Odyssey Fc imaging system (LI-COR). For proinsulin maturation analysis, cell lysates were run on a non-reducing 16.5 % Tris-Tricine precast gels from BioRad labs.
Measurements of mitochondrial reactive oxygen species (ROS)
Islets (800-900 pooled from 3-4 mice) were homogenized and mitochondrial fractions were prepared by differential centrifugation as described (Lai & Clark, 1979; Yuan et al., 2004). Aconitase activity and malondialdehyde (MDA) levels were monitored as indices of ROS in mitochondrial fractions as described previously (Nanduri et al., 2009). Briefly, MDA levels were determined by measuring the relative fluorescence intensity of the homogenate in presence of thiobarbituric acid at excitation and emission wavelengths of 530 and 550 nm, respectively and values are expressed as nanomoles of MDA formed per milligram of protein. Aconitase enzyme activity was measured by monitoring the increase in absorbance at 340 nm associated with the formation of NADPH in the conversion of isocitrate to a-ketoglutarate. The rate of NADPH production is proportional to aconitase activity and expressed as nanomoles of isocitrate formed per minute per milligram of protein
Real time RT-PCR
Proinsulin, pro-convertase 1 and 2 (PC1 and PC2) mRNAs were analyzed in the islets by quantitative real-time RT-PCR with mouse-specific primers and 18s RNA as a housekeeping gene using SYBR as a fluorogenic binding dye as described previously (Nanduri et al 2009). Primer sequences for real-time PCR amplification are: Proinsulin (NM_001185084); For: TGGCTTCTACACACCCAAG; Rev:ACAATGCCACGCTTCTGCC. PC1 (NM_013628.2); For: CGCTGACCTGCCAATGACT;Rev:CAGATGCTGCATATCTCTCCAGG.PC2 (NM_013628.2); For: AAGAGGCTCGCCAAGTTGC; Rev: GGTCAAATCCTTCTTGTTGCAGCG. 18S ((NR_003278); For: CGCCGCTAGAGGTGAAATTC. Rev: CGAACCTCCGACTTTCGTTCT.
Results
Effect of intermittent hypoxia (CIH) on plasma insulin and glucose levels
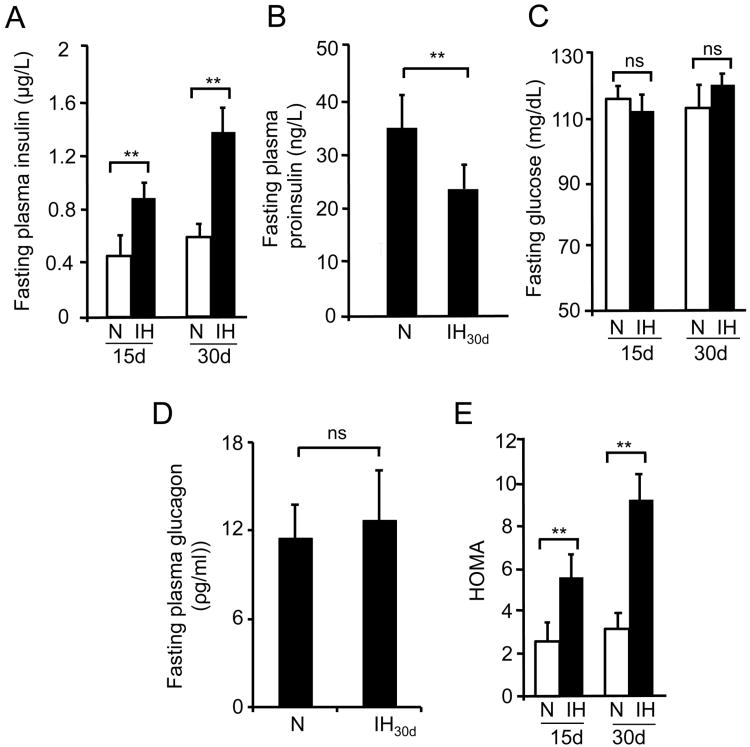
Fasting plasma insulin and blood glucose levels were measured in mice exposed to either 15 or 30 days of CIH. Plasma insulin levels increased by ∼2.5 and 4-fold in mice exposed to 15 days and 30 days of CIH, respectively compared to control mice (Fig. 1A). Higher levels of proinsulin in the plasma can contribute to increased insulin levels observed. To examine this possibility, proinsulin levels were measured by ELISA. In contrast to increased insulin levels, plasma proinsulin levels decreased ∼2 fold in CIH exposed mice compared to controls (Fig. 1B). Inspite of elevated plasma insulin levels, fasting blood glucose levels, however, were comparable between control and CIH-exposed mice (Fig. 1C). Blood sugar homeostasis is a balance between insulin and glucagon acting together. It is therefore possible that increased glucagon levels by CIH may play a role in maintaining plasma glucose. However, plasma glucagon levels measured from 30 days of CIH-exposed mice were comparable to control group (P= 0.772; Fig. 1D). Homeostasis model assessment (HOMA = Fasting insulin (μU/ml) × Fasting glucose (mg/dL) / 22.5), which is an index of insulin resistance, was significantly higher in mice treated with either 15 days or 30 days of CIH (Fig. 1E). All further experiments were performed with 30 days of CIH exposure, which produced more robust changes in insulin levels.
Figure 1.
Effect of intermittent hypoxia (IH) on fasting plasma insulin, glucose and Homeostatic Model Assessment (HOMA). C57B6J mice were exposed to either 15 or 30 days of IH or normoxia (N). Fasting plasma (A), proinsulin (B), blood glucose (C), and glucagon (D) were measured as described in methods. E) HOMA (Homeostasis model assessment) an index of insulin resistance was calculated using the formula Fasting insulin (μU/ml) × Fasting glucose (mg/dL)/22.5. Data from n=8 animals is shown as mean ± SEM. ** denote p value <0.01; n.s. = not significant p value >0.05 as determined by one way ANOVA test.
Effect of CIH on beta cell morphology
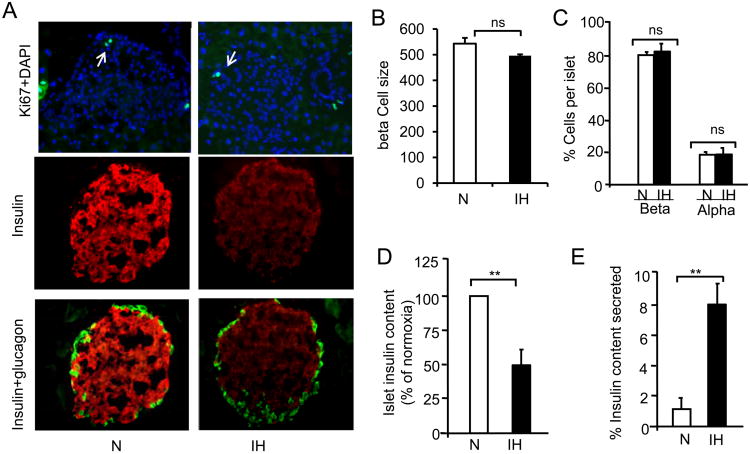
The augmented basal insulin secretion by IH could be due to increased beta cell proliferation or increased beta cell mass. This possibility was examined by staining pancreatic sections from mice exposed to 30 days of CIH with Ki-67, a marker of cell proliferation along with insulin staining. As shown in Fig 2A (left panel), Ki-67 staining was indistinguishable between control and CIH-treated samples. The total number of islets obtained from both groups were comparable (∼350±40) from each mouse (n=8). Morphometric analysis revealed no significant changes in beta cell size (Fig. 2B) or number of beta cells in CIH-exposed compared to control mice (Fig. 2C).
Figure 2.
Effect of CIH on beta cell morphology. A) Pancreatic sections from normoxia (N) and CIH-exposed mice were stained with antibodies specific for Ki67, a cell proliferation marker, insulin, glucagon and DAPI, a marker of nucleus. A) Example of an islet stained for Ki67+DAPI (left panel), insulin in beta cells (middle panel), glucagon in alpha cells (right panel). Analysis of islet size (B) and number of beta and alpha cells (C) in control and CIH-treated mice. Number of cells was determined from 100-125 islets from each mouse and 4 mice in each group. C) Quantitative measurement of insulin content in control (N) and CIH-exposed islets by ELISA. D) Percentage of insulin secreted relative to islet insulin content. Data expressed as mean ± SEM from 4-6 mice. ** denote p value <0.01; n.s. = not significant p value >0.05 analyzed by one way ANOVA test.
Effect of CIH on beta cell insulin content
The augmented basal insulin secretion is not due to increased insulin content in pancreatic islets, because CIH-exposed mice exhibited remarkable decrease in the intensity of insulin staining in pancreatic sections compared to normoxic controls as shown in Fig. 2A (middle panel). Glucagon staining, a marker of alpha cells, was restricted to periphery of the islets and there was no obvious difference in the intensity of glucagon staining between CIH-exposed and control pancreatic islets (Fig. 2A, right panel).
Quantitative analysis by ELISA revealed a ∼50% decrease in islet insulin content in CIH-exposed compared to control mice (P< 0.01; n=8; Fig. 2D). In the same animals, plasma insulin levels were measured and the ratio of plasma insulin to insulin content in pancreatic islets was determined. The ratio of plasma insulin to pancreatic insulin content was significantly higher in islets from CIH-exposed compared to control mice (CIH=8.1±1.2% vs. Control= 1.2±0.64%; P<0.01; Fig. 2E).
CIH impairs processing of proinsulin to insulin
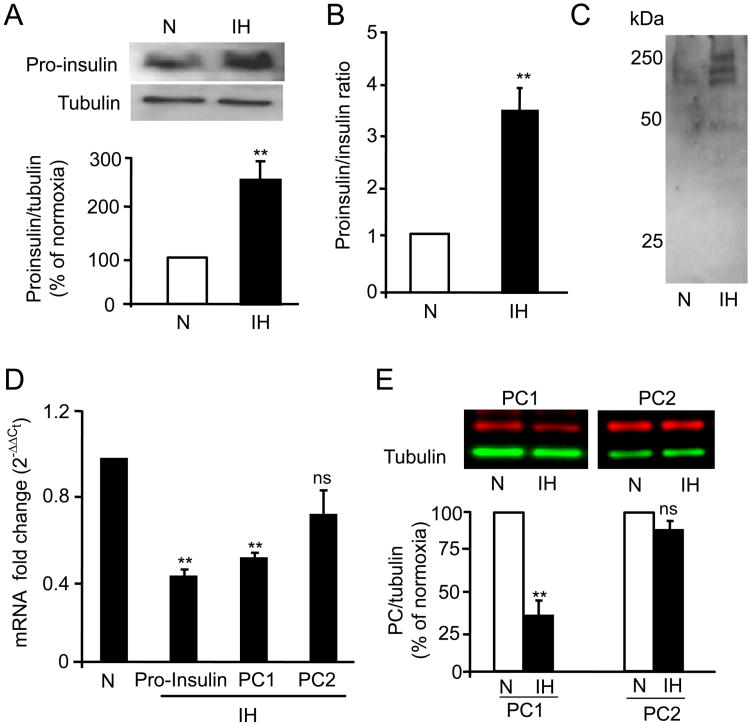
Since plasma proinsulin levels decreased by 40% by CIH, we determined whether the decrease in islet insulin content is due to alterations in proinsulin levels and/or processing of biologically active insulin from proinsulin. Western blot assay showed a ∼2.5 fold increase in proinsulin protein levels, in islets from CIH exposed mice (P<0.01; Fig. 3A). Analysis of ratio of proinsulin to insulin by ELISA revealed ∼3 fold increase in islets from mice exposed to CIH (Fig. 3B). In addition, analyses of islet lysates on non-reducing gels by immunoblots with proinsulin antibody showed accumulation of high molecular weight bands (∼250kDa) in islets from CIH-exposed mice compared to controls (Fig. 3C) suggesting proinsulin aggregates. Real time RT-PCR analysis showed a ∼2-fold decrease in proinsulin mRNA levels (Fig. 3D), further confirming that elevated proinsulin protein levels were not due to increased transcriptional changes, but due to accumulation of proinsulin protein as a consequence of impaired processing of proinsulin to insulin.
Figure 3.
Effect of CIH on proinsulin processing: A) Top panel: Representative immunoblot of proinsulin protein in islets isolated from control (N) and CIH-exposed mice with tubulin as loading control. Bottom panel: Densitometric analysis (mean ± SEM; n=4 mice). B) Proinsulin and insulin levels were measured by ELISA and the ratio of proinsulin to insulin normalized to controls is represented as mean ± SEM from n=8 mice. C) Analysis of proinsulin expression under non-reducing conditions. D) mRNA levels of proinsulin, PC1 and PC2 measured in islets by real time RT-PCR and expressed as fold change after normalizing to 18s mRNA. E) Top panel: Representative immunoblots showing PC1 and PC2 protein expression in islets isolated from normoxia (N) and CIH-exposed rats. Bottom panel: Quantitative analysis of relative fluorescence units (mean ± SEM) normalized to controls (n=8 mice). ** denote p value <0.01; n.s. = not significant p value >0.05 by one way ANOVA test.
The enzymes, pro-hormone convertase 1 and 2 (PC1 and PC2) are critical for processing biologically active insulin from proinsulin. PC1 and PC2 mRNAs and protein levels were determined in pancreatic islets from control and CIH-exposed mice. PC1 mRNA and protein levels were significantly down regulated in islets from CIH-exposed mice (P<0.01); whereas PC2 mRNA and protein levels were unaltered (P>0.05; Fig. 3D-E).
Mitochondrial ROS mediates CIH augmented insulin secretion
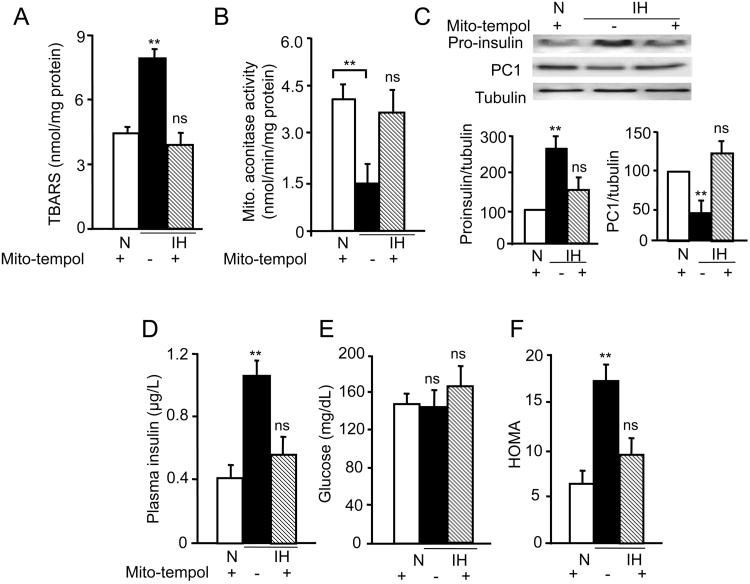
Previous studies showed increased ROS levels mediate many of the systemic and cellular responses to CIH and identified mitochondria as a major source of ROS generation by CIH (Prabhakar et al., 2007). Beta cells are particularly susceptible to mitochondrial ROS (Sivitz & Yorek, 2010; WI, 2010). ROS levels were determined by measuring: a) the levels of malondialdehyde (MDA), which represents oxidized lipids (Devasagayam et al., 2003), and b) the activity of the aconitase, a redox sensitive enzyme in the mitochondrial fractions of pancreatic islets. ROS levels were significantly elevated in mitochondrial fractions of pancreatic islets from CIH exposed mice as evidenced by increased MDA levels and decreased aconitase activity, and these effects were completely reversed by treating CIH exposed mice with mito-tempol (10 mg/Kg; I.P per day), a selective scavenger of mitochondrial ROS ((Trnka et al., 2009), every day prior to exposing them to CIH (Fig. 4A&B). Mito-tempol treatment also prevented the increase in proinsulin and the decrease in PC1 protein levels in islets from CIH-exposed mice (Fig. 4C). Mito-tempol treatment blocked the CIH-induced increase in fasting plasma insulin, but had no significant effect on fasting blood glucose levels and as a consequence, HOMA values in CIH-exposed mice treated with mito-tempol were comparable to control mice (Fig. 4D-F).
Figure 4.
Effects of systemic administration of mito-tempol, a mitochondrial reactive species (ROS) scavenger, on CIH-induced changes in insulin signaling. Mice exposed to 30days of CIH or normoxia were treated daily with mito-tempol (10mg/kg/day/I.P). A-B) Effect of mito-tempol on mitochondrial ROS levels measured by determining malondialdehyde levels (TBARS), A) and aconitase activity (B) levels in the mitochondrial fractions of pancreatic islets. C) Top panel: Representative immunoblot showing proinsulin, PC1 and tubulin protein levels. Bottom panel: Quantitative densitometric analysis of the data shown as mean ± SEM from 8 mice. D-F) Fasting plasma insulin and glucose values were determined and HOMA values calculated as described in the methods. The data represent mean ± SEM (n =8). ** denote p value <0.01. n.s. = not significant p value >0.05 by one way ANOVA test.
Effect of CIH on glucose stimulated insulin secretion (GSIS)
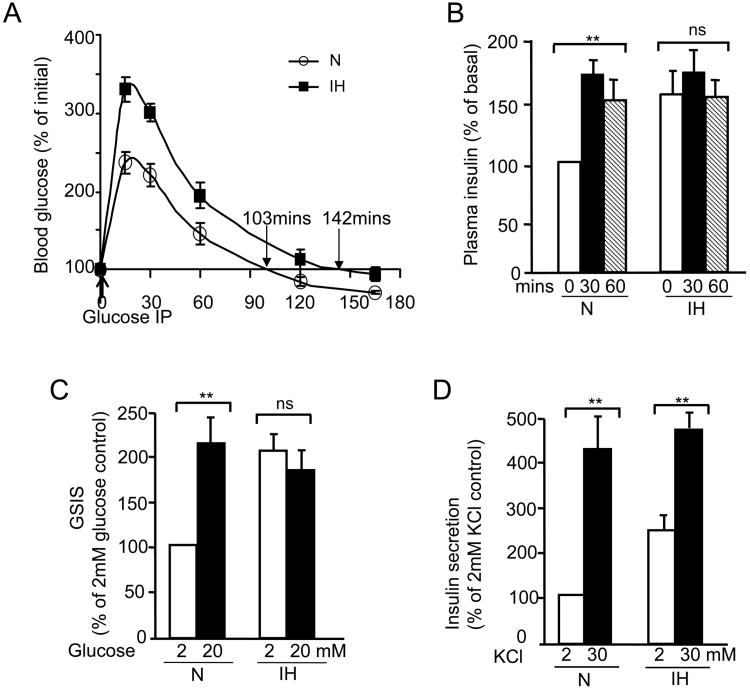
To examine the effects of CIH on GSIS, glucose was administered intra-peritonially (2gm/kg body weight) into control and CIH-exposed mice. Blood glucose and plasma insulin levels were measured at 15 min intervals for two hours. Both control and CIH-treated mice responded with increased blood glucose levels following glucose load. However, the magnitude of increase in blood glucose was significantly greater in CIH-treated mice during the first 60 minutes of post-glucose administration than controls (Fig. 5A). Blood glucose levels returned to basal levels after 145 minutes in CIH-treated mice as opposed to 103 minutes in control mice (Fig. 5A). Following glucose load, control mice responded with an increase in plasma insulin levels, which remained elevated during 30 and 60 min after glucose administration. In CIH-treated mice, basal insulin levels were higher than control mice, and glucose did not further increase plasma insulin levels (Fig. 5B).
Fig 5.
Effect of CIH on glucose stimulated insulin secretion (GSIS). Mice exposed to normoxia (N) or 30days of CIH were administered glucose (2g/Kg; I.P). Plasma insulin (A) and blood glucose (B) levels were measured at indicated times (n=8 mice). C-D) Pancreatic islets harvested from normoxic mice were exposed to normoxia (N) or IH in vitro as described in methods and then insulin release was monitored in response to 20 mM glucose (C) or 30 mM KCl (D) as described in methods. Data were expressed as mean ± SEM from n=5 individual experiments. ** denote p value <0.01. n.s. = not significant p value >0.05 by two way ANOVA test.
To determine whether CIH selectively affected insulin response to glucose, experiments were performed on pancreatic islets harvested from control mice reared under room air. Islets were exposed to IH in vitro (1.5% O2 balanced 10% CO2 for 30 sec followed by 5 minutes of 20% O2 balanced 10% CO2). Control experiments were performed on cells exposed to alternating cycles of room air (20% O2 balanced 10% CO2). Our previous studies showed that in vitro IH exposure decreases medium pO2 near cells by 25-30 mmHg during each episode of hypoxia and produces robust cellular responses (Yuan et al., 2004; Nanduri et al., 2009). Control islets (i.e., exposed to normoxia) responded to 20mM glucose with increased insulin secretion (a 2 fold increase). IH-exposed islets exhibited significantly higher basal insulin secretion, similar to that seen in CIH-exposed mice, and 20mM glucose had no further stimulatory effect on insulin secretion (Fig. 5C). In striking contrast, 30mM KCl, a non-selective secretagogue, significantly stimulated insulin secretion in both control as well as IH-exposed islets (Fig. 5D).
Discussion
The present study examined the effect of CIH on pancreatic beta cell function and assessed the underlying mechanisms. CIH-treated mice exhibited elevated fasting plasma insulin levels without significant changes in glucose levels which were not due to increased proinsulin or glucagon levels. These results suggest that CIH leads to insulin resistance, a finding consistent with an earlier study (O'Donnell, 2007). The augmented basal insulin secretion in CIH-exposed mice might be due to increased beta cell proliferation. However, we did not find evidence for beta cell proliferation in mice exposed to 30 days of CIH. Furthermore, no significant differences in beta cell mass were found suggesting that cell hypertrophy has not occurred. A previous study reported beta cell proliferation in rats exposed to 4 days of CIH (alternating cycles of 5.7% O2 and 21% O2 every 180s; (Xu et al., 2009)). The discrepancy between our findings and that of Xu et al (2009) might be due to differences in CIH paradigms. Indeed such a possibility is supported by a recent study (Raghuraman et al., 2009) which compared the CIH paradigm used by Xu et al (2009) with the paradigm used in our study and found that cellular responses critically depend on the CIH paradigm. Thus, our results indicate that changes in beta cell morphology do not seem to account for the enhanced basal insulin secretion under our experimental conditions.
CIH-induced increase in basal insulin secretion was associated with significant reduction in insulin and increased proinsulin content in pancreatic beta cells, demonstrating that CIH leads to enhanced basal insulin secretion despite the decreased insulin content. These findings are reminiscent of the study by (Bollheimer et al., 1998), who reported that free fatty acids stimulate insulin secretion with concomitant reduction in pancreatic insulin content. How might CIH leads to decreased insulin content? Pancreatic islets from CIH exposed mice showed down regulation of proinsulin mRNA, increased proinsulin protein and down regulation of pro-convertase 1 (PC1) mRNA and protein. Recent studies have shown that proinsulin homeostasis involves a balance of natively and non-natively folded states due to inherent aggregation-prone nature and low relative folding rate and is susceptible to environmental influences like oxidative stress (Wang et al., 2011; Wang & Osei, 2011). The high molecular weight proinsulin bands detected on non-reducing gels in our experiments could be disulfide bonded misfolded proinsulin aggregates, a possibility that requires further study. These findings suggest that proinsulin synthesis and processing are severely compromised by CIH. Thus, the CIH-induced decreased insulin content of islets is due to combination of increased basal secretion and decreased proinsulin synthesis and processing.
Our results further showed that the increased basal insulin secretion by CIH was associated with severely impaired GSIS both in in vivo and in vitro. The finding that GSIS in controls is of the same magnitude as CIH-induced basal insulin secretion suggest that CIH imposes an excessive functional demand on pancreatic beta cells which may lead to saturation or exhaustion of the beta cell secretory capacity, resulting in impaired GSIS over time. In striking contrast insulin secretion in response to KCl, a non-selective secretagogue was unaffected by CIH, suggesting that CIH selectively affects insulin response to glucose.
What signaling mechanism(s) mediate the effects of CIH on beta cells? Beta cells express relatively low levels of antioxidant enzymes including superoxide dismutase, glutathione-peroxidase, catalase and thioredoxin (Lenzen et al., 1996; Tiedge et al., 1997), rendering them susceptible to oxidative stress. Furthermore, increased mitochondrial ROS as well as ROS derived from NADPH oxidases (Nox) cause beta cell dysfunction (Newsholme et al., 2007; Morgan et al., 2009). We previously reported that ROS and ensuing oxidative stress play a critical role in mediating cellular response to CIH (Prabhakar et al., 2007). Our recent study showed that CIH initially activates Nox2 and the resulting ROS inhibits mitochondrial electron transport chain at the complex I leading to increased mitochondrial ROS (Khan et al., 2011). The following findings demonstrate that mitochondrial ROS mediate the effects of CIH on beta cell function. First, ROS levels were elevated in mitochondrial fractions from CIH exposed islets as evidenced by decreased aconitase and elevated MDA levels, two indices of ROS. Second, Mito-tempol, a selective mitochondrial ROS scavenger, prevented CIH-induced increase in mitochondrial ROS, abolished the enhanced basal insulin secretion, restored proinsulin processing and prevented the increased HOMA value. Whether mitochondrial ROS directly affect the maturation and conversion of proinsulin and/or function of PC1 enzymes needs further investigation.
We identified mitochondrial ROS as a major signaling mechanism mediating beta cell dysfunction by CIH manifested with augmented insulin secretion and defective proinsulin processing. Despite a large body of clinical evidence linking sleep-disordered breathing with apnea to type 2 diabetes, very little is known about the underlying mechanisms (Pallayova et al., 2011). The results of the current study provide evidence for direct effects of CIH, a hallmark manifestation of recurrent apnea on beta cell function.
New Findings.
What is the central question of this study?
Periodic decreases in arterial blood O2 or chronic intermittent hypoxia (CIH) is a hall mark feature of sleep apnea patients. Despite a large body of clinical evidence linking sleep-disordered breathing with apneas to diabetes, the causal relationships between CIH and beta cell function and the underlying molecular mechanisms have not been established.
What is the main finding and its importance?
In a rodent model, we show that CIH generated mitochondrial oxidative stress leads to pancreatic beta cell dysfunction manifested by augmented basal insulin secretion, insulin resistance, defective proinsulin processing, impaired glucose stimulated insulin secretion. The results of the current study provide evidence for direct effects of CIH on beta cell function which may be an underlying molecular mechanism contributing to the development of type 2 diabetes among sleep apnea patients.
Acknowledgments
This work was supported by P&F grant from Chicago Diabetes Research and Training Center (P60 –DK-020595) to J.N and grants from National Institute for Health HL-90554, HL-76537.
The authors thank Jonathan Johnson and Brian Kinsman for technical assistance with initial beta cell morphology experiments.
Footnotes
Author contributions: J.N. designed experiments, analyzed the researched data, and wrote the manuscript. N.W and S.K. performed the experiments and analyzed the data. N.R.P originated the initial research and provided research tools for in vivo and in vitro IH.
The authors declare no conflict of interest.
References
- Asghar Z, Yau D, Chan F, Leroith D, Chan CB, Wheeler MB. Insulin resistance causes increased beta cell mass but defective glucose-stimulated insulin secretion in a murine model of type 2 diabetes. Diabetologia. 2006;49:90–99. doi: 10.1007/s00125-005-0045-y. [DOI] [PubMed] [Google Scholar]
- Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasagayam TP, Boloor KK, Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH. Reactive species, cellular repair and risk factors in the onset of type 2 diabetes mellitus: review and hypothesis. Curr Diabetes Rev. 2006;2:241–259. doi: 10.2174/157339906776818541. [DOI] [PubMed] [Google Scholar]
- Idris I, Hall AP, O'Reilly J, Barnett A, Allen M, Andrews R, Grunstein P, Lewis K, Goenka N, Wilding JP. Obstructive sleep apnoea in patients with type 2 diabetes: aetiology and implications for clinical care. Diabetes Obes Metab. 2009;11:733–741. doi: 10.1111/j.1463-1326.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007;6:229–235. doi: 10.1016/j.cmet.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal. 2011;14:533–542. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaban JP, Daenen S, Léger D, Pascal S, Bayon V, Slama G, Elgrably F. Prevalence and predictive factors of sleep apnoea syndrome in type 2 diabetic patients. Diabetes Metab. 2009;35:372–377. doi: 10.1016/j.diabet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lai JC, Clark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Methods Enzymol. 1979;55:51–60. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
- Lavie L. Oxidative stress--a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta cells. Endocrinology. 2009;150:2197–2201. doi: 10.1210/en.2008-1149. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP. Metabolic consequences of intermittent hypoxia. Adv Exp Med Biol. 2007;618:41–49. doi: 10.1007/978-0-387-75434-5_4. [DOI] [PubMed] [Google Scholar]
- Pallayova M, Lazurova I, Donic V. Hypoxic damage to pancreatic beta cells--the hidden link between sleep apnea and diabetes. Med Hypotheses. 2011;77:930–934. doi: 10.1016/j.mehy.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- Raghuraman G, Rai V, Peng YJ, Prabhakar NR, Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal. 2009;11:1777–1789. doi: 10.1089/ars.2008.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahangdale S, Yeh SY, Malhotra A, Veves A. Therapeutic interventions and oxidative stress in diabetes. Front Biosci. 2009;14:192–209. doi: 10.2741/3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Trnka J, Blaikie FH, Logan A, Smith RA, Murphy MP. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic Res. 2009;43:4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen Y, Yuan Q, Tang W, Zhang X, Osei K. Control of precursor maturation and disposal is an early regulative mechanism in the normal insulin production of pancreatic β-cells. PLoS One. 2011;6:e19446. doi: 10.1371/journal.pone.0019446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Osei K. Proinsulin maturation disorder is a contributor to the defect of subsequent conversion to insulin in β-cells. Biochem Biophys Res Commun. 2011;411:150–155. doi: 10.1016/j.bbrc.2011.06.119. [DOI] [PubMed] [Google Scholar]
- WI S. Mitochondrail dysfunction in diabtes: from molcular mechnaism to functional significance and therapeutic opportunities. In: Ma Y, editor. Antioxid Redox Signal. Vol. 12. 2010. p. 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Long YS, Gozal D, Epstein PN. Beta cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46:783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]