Clinical Vignette
Patient OT is a 26 year old Caucasian woman who works in the music industry. She was diagnosed with pneumonia and treated with “inhalers”. Shortly afterwards, she developed “spells of tachycardia.” Her episodes of tachycardia were primarily associated with upright posture. In addition to rapid palpitations, she complained of lightheadedness and presyncope on standing, intermittent stabbing chest pains (typically on standing), mental clouding with an inability to concentrate, severe fatigue and exercise intolerance. Orthostatic vital signs recorded a supine heart rate (HR) of 73 bpm with a blood pressure (BP) of 103/72 mmHg. After standing for 1 min, her HR increased to 106 bpm with BP of 109/80 mmHg, and after 5 min, her HR was 122 bpm with BP of 118/75 mmHg. She was diagnosed with postural tachycardia syndrome (POTS).
Upright Posture
Under normal conditions, the assumption of upright posture effects an instantaneous shift of ∼500 ml of blood from the thorax to the lower abdomen, buttocks, and legs. There is a secondary shift of plasma volume (10-25%) out of the vasculature and into the interstitial tissue, which decreases venous return to the heart (preload), resulting in a transient decline in cardiac filling and BP. This unloads the baroreceptors, and triggers a compensatory decrease in parasympathetic tone and an increase in sympathetic activation, with a resultant increase in HR and systemic vasoconstriction (countering the initial decline in BP). The net hemodynamic effect of transition to upright posture is a 10-20 bpm increase in HR, a negligible change in systolic BP, and a ∼5 mmHg increase in diastolic BP. Orthostatic dysregulation occurs when this gravitational regulatory mechanism does not respond properly. Patients can present with orthostatic hypotension (seen in autonomic nervous system failure), or with orthostatic tachycardia (seen in POTS). Patients with POTS typically maintain (or even increase) their BP on standing. The cardinal hemodynamic feature in POTS is that HR increases excessively and is associated with multiple symptoms on standing, which improve with recumbency.
Diagnostic Criteria & Common Clinical Features of POTS
POTS is defined (Table 1) as the presence of chronic symptoms of orthostatic intolerance (at least 6 months) accompanied by an increased HR ≥30 bpm within 10 minutes of assuming an upright posture and in the absence of orthostatic hypotension (a fall in BP >20/10 mmHg) 1. An example of a tilt test in a POTS patient is shown in Figure 1. In young children, a higher HR threshold (≥40 bpm) should be used since healthy younger children have a greater orthostatic tachycardia 2. There is significant diurnal variability in the magnitude of orthostatic tachycardia 3; therefore postural vital signs should be performed in the morning to optimize diagnostic sensitivity for POTS. The orthostatic tachycardia must occur in the absence of other overt causes of orthostatic tachycardia, such as prolonged bed rest, medications that impair autonomic regulation (such as vasodilators, diuretics, antidepressants or anxiolytic agents), or chronic debilitating disorders that might cause tachycardia (such as dehydration, anemia, or hyperthyroidism).
Table 1. Criteria for the Postural Tachycardia Syndrome.
|
Figure 1. Heart Rate and Blood Pressure with Upright Tilt in POTS.
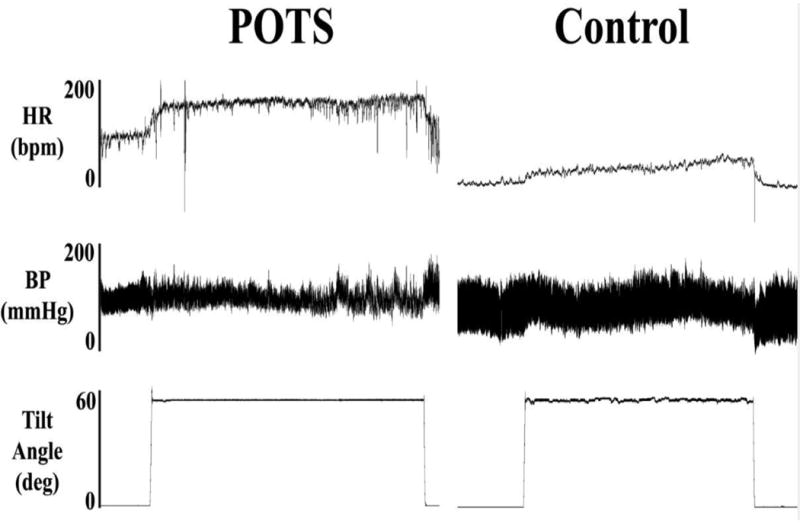
Heart rate (HR), blood pressure (BP), and tilt table angle are shown for a representative patient with the postural tachycardia syndrome (POTS; left) and for a healthy subject (right) during a 30 minute head-up tilt test. With tilt, HR immediately increases in POTS and peaks at over 170 bpm prior to the end of the tilt, while the HR of the healthy subject rises to just over 100 bpm. BP was largely unchanged in the POTS patient. Figure reprinted with permission from Raj SR et al., Indian Pacing Electrophysiol. J. 2006;6:84-99 1.
Symptoms often include both cardiac symptoms (rapid palpitations, lightheadedness, chest discomfort, and dyspnea) and non-cardiac symptoms (mental clouding [“brain fog”], headache, nausea, tremulousness, blurred or tunneled vision, poor sleep, exercise intolerance, and fatigue). Even activities of daily living, such as bathing or housework, may greatly exacerbate symptoms, with resultant fatigue. This can pose significant limitations on functional capacity. While pre-syncope and lightheadedness are common in these patients, only a minority (∼30%) actually faint. The chest pains are almost never due to coronary artery obstruction, but may be associated with electrocardiographic changes in the inferior leads, particularly when upright.
The overwhelming majority of patients with POTS are women (80-85%) of child-bearing age (13-50 years) 4.
Patients frequently report that their symptoms began following acute stressors such as pregnancy, major surgery, or a presumed viral illness, but in others cases, symptoms develop more insidiously. About 80% of female patients report an exacerbation of symptoms around menstruation 5. Many patients have been co-diagnosed with irritable bowel syndrome, some have hypermobile joints, and some have abnormal sudomotor regulation.
A striking physical feature in ∼50% of patients with POTS is a dependant acrocyanosis (Figure 2). These patients experience a dark red-blue discoloration of their legs (feet to above knees), which are cold to the touch. The reasons underlying this phenomenon are not clear, but may relate to abnormalities in nitric oxide activity in the skin of POTS patients 6.
Figure 2. Dependent Acrocyanosis in POTS.
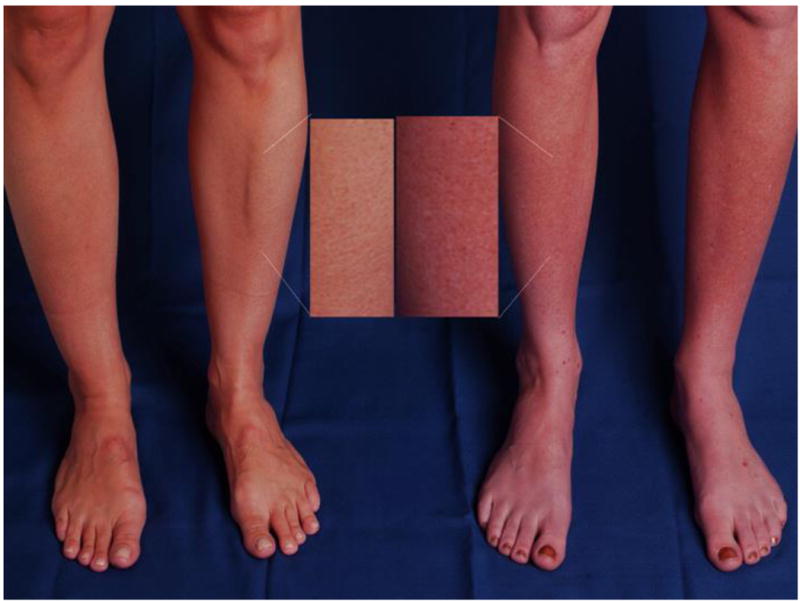
A striking physical feature in postural tachycardia syndrome (POTS) is the gross change in dependent skin color that can occur with standing. The panel shows the legs of a healthy subject (left) and a patient with POTS (right) after standing for 5 minutes. The patient with POTS (right) has significant dark red mottling of her legs extending up to the knees while standing, while the healthy subject does not have a similar discoloration. Figure reprinted with permission from Raj SR et al., Indian Pacing Electrophysiol. J. 2006;6:84-99 1.
Psychological Profile in POTS
Patients with POTS are sometimes clinically diagnosed as having anxiety disorders such as panic disorder. When assessed using a structured evaluation for Diagnostic & Statistical Manual (DSM) 4-TR criteria, POTS patients did not have a higher incidence of major depressive disorder, anxiety disorders, or substance abuse than the general population 7.
When assessed using the Beck Anxiety Inventory (BAI), patients report elevated anxiety scores (23±10 vs. 7±8; P<0.001) 7. However, the BAI scores both somatic anxiety & psychological symptoms, which is a problem since somatic symptoms may overlap with hyperadrenergic states (as is seen in POTS). When POTS patients were assessed with a psychological-based measure of anxiety (Anxiety Sensitivity Index), there was a trend toward less anxiety in the patients than the general population (15±10 vs. 19±9; P=0.063) 7. It is possible that some of the anxiety attributed to patients with POTS might be due to a misinterpretation of their physical symptoms.
Many POTS patients complain of memory problems. In formal testing with the Inattention score from the Connors Adult ADHD Rating Scale, POTS patients scored significantly worse than did healthy control subjects 7. This suggests that the problem in POTS may not be with memory per se, but with diminished attention.
Fatigue, Sleep Problems & Quality of Life in POTS
POTS patients commonly complain of fatigue, unrefreshing sleep, and daytime sleepiness. When formally assessed using a Fatigue Visual Analogue Scale, the Medical Outcomes Study Sleep Survey, and the Epworth Sleepiness Scale (respectively), POTS patients had more sleep problems (Sleep Problems Index: 58±18 vs. 20±13; P <0.0001) and excessive daytime sleepiness (10.2±5.7 vs. 6.2±3.2; P<0.0001) compared with healthy controls 8. POTS patients also had higher fatigue levels (7.5±2.0 vs. 2.8±2.5; P<0.0001). We 8 and others 9 have documented low health related quality of life in patients with POTS. Using the SF-36, Benrud-Larssen et al. 9 reported that physical and mental composite scores for POTS patients were comparable to patients with congestive heart failure. It is noteworthy that of the 8 domains specifically addressed by the SF-36, the only one in which POTS patients did not fare worse than the control group was in mental health 9.
The subjects in the aforementioned sleep study each also completed the RAND-36, a validated general health-related quality of life tool. There was a strong correlation between the RAND-36 physical health composite scores and the Sleep Problems Index (R2=0.53; P<0.0001), with over 50% of the variance in physical health explained by the variance in sleep quality 8.
Pathophysiology of POTS
POTS is a syndrome and not a disease. Many disorders with the common key clinical presentation of orthostatic tachycardia have been described. Much has been learned about specific features or sub-types within POTS, although a simple test to categorize the individual patient remains elusive. We discuss here some common POTS phenotypes.
Neuropathic POTS
Although many POTS patients have high plasma NE levels, it would seem paradoxical that an autonomic neuropathy is proposed as an underlying process. Yet some patients have a form of dysautonomia, with preferential denervation of sympathetic nerves from the lower limbs. Jacob et al. 10 showed that some patients with POTS had less norepinephrine release (less sympathetic activation) in their lower extremities.
Central Hyperadrenergic POTS
Many patients with POTS have elevated levels of plasma NE, suggestive of a hyperadrenergic state. This is most commonly secondary to a partial dysautonomia or hypovolemia. There is a small subgroup of patients in whom the primary underlying problem seems to be excessive sympathetic discharge. These patients often have extremely high levels of upright plasma norepinephrine (>1000 pg/ml and occasionally >2000 pg/ml, with an upper limit of normal of 475 pg/ml in our clinical laboratory). Plasma metanephrines will exclude a pheochromocytoma. This subgroup of patients sometimes has large increases in blood pressure on standing, indicating that baroreflex buffering is somehow impaired. Therapy in these cases targets a decrease in sympathetic tone both centrally and peripherally. Central sympatholytics such as methyldopa or clonidine may be used. Peripheral beta-adrenergic blockade may be better tolerated by these patients than by those with primary hypovolemia.
Norepinephrine Transporter Deficiency & Blockers
A specific genetic abnormality has been identified in a kindred with hyperadrenergic POTS 11. These individuals have a single point mutation causing loss of function in the norepinephrine transporter (NET). The resultant diminished norepinephrine clearance leads to a hyperadrenergic state in response sympathetic nerve activation.
Although functional NET mutations might be infrequent, many antidepressant and attention deficit medications work at least in part through inhibition of NET. This includes traditional drugs such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine, venlafaxine, or milnacipran), or purer NET inhibitors (e.g., atomoxetine or reboxetine). Pharmacological NET inhibition can recreate an orthostatic tachycardia phenotype in susceptible healthy volunteer subjects 12. POTS patients might have less “tachycardia reserve” and be more susceptible to exaggerated tachycardia with these medications.
Mast Cell Activation
Some patients with POTS present with episodic flushing associated with surges in tachycardia, and have co-existent mast cell activation. They may have abnormal increases in urine methylhistamine (the primary urinary metabolite of histamine) 13, which should ideally be measured from a 4 hour sample at the time of a flushing episode (not a 24 hour sample). This can be associated with dyspnea, headache, lightheadedness, excessive diuresis, and gastrointestinal symptoms such as diarrhea, nausea, and vomiting. These patients often have a hyperadrenergic response to posture, with both orthostatic tachycardia and hypertension. There are many triggers for the flushing, including prolonged standing, exercise, premenstrual cycle, meals, and sexual intercourse.
Centrally acting agents to decrease the sympathetic nervous system discharge (e.g., methyldopa or clonidine) may prove effective, although beta-blockers may actually trigger mast-cell degranulation and worsen symptoms. Treatment can also target mast cell mediators, with a combination of antihistamines (H1- and H2-antagonists) and possible use of non-steroidal agents in refractory cases.
Hypovolemia & Blood Volume Regulation
Many, but not all, patients with POTS have low blood volumes 14. Using the 131-I labeled human serum albumin method, we found that POTS patients had a plasma volume deficit of almost 13% 14.
The renin-angiotensin-aldosterone system plays a key role in the neurohormonal regulation of plasma volume in humans. Plasma renin activity and angiotensin II would be expected to increase in response to hypovolemia in order to promote blood volume expansion. Angiotensin II promotes sodium and water retention indirectly by stimulating aldosterone secretion.
Patients with orthostatic tachycardia who were also hypovolemic have inappropriately low levels of standing plasma renin activity and aldosterone compared to normovolemic subjects 14. One would have expected a compensatory increase in both plasma renin activity and aldosterone resulting from the hypovolemia in these patients, and these low levels are a paradox that remains unexplained.
More recently, we 15 have reported high levels of angiotensin II levels circulating in POTS patients, without a commensurate increase in angiotensin-(1-7), suggesting that POTS patients might have decreased angiotensin II metabolism. The aldosterone level is lower per unit angiotensin II in POTS patients 15. These data suggest that abnormalities in the renin-angiotensin-aldosterone axis might have a role in the pathophysiology of POTS by contributing to hypovolemia and impaired sodium retention.
Investigation of POTS
The evaluation of a patient with POTS starts with a detailed history and physical examination. POTS can be confused with pheochromocytoma because of the paroxysms of hyperadrenergic symptoms (e.g., palpitations and lightheadedness). Patients with pheochromocytoma are more likely to have these symptoms while lying down than POTS patients. The diagnosis of pheochromocytoma is made by assessment of plasma or urinary metanephrines. We order a CBC and an electrolyte panel to exclude severe anemia or gross electrolyte disturbances. Some physicians specializing in POTS will also assess Vitamin B12 levels, iron indices, and serological markers for celiac disease, although there are insufficient data supporting the routine use of these tests.
The tachycardia in POTS patients should originate from the sinus node. An electrocardiogram should be routinely performed to exclude the presence of an accessory bypass tract or other abnormalities of cardiac conduction. A Holter monitor might prove useful to exclude a reentrant tachyarrhythmia, especially if the tachycardia is paroxysmal with a sudden onset and offset. The physician must determine that cardiac left ventricular function is normal, with an echocardiogram if needed. Peripartum cardiomyopathy, for example, can present in a manner similar to POTS.
We often measure plasma norepinephrine levels in both a supine and standing position (at least 10 minutes in each position prior to blood sampling). The supine norepinephrine is often within the normal range in POTS patients, while the upright norepinephrine is frequently elevated (>600 pg/ml), reflecting the exaggerated neural sympathetic tone that is present in these patients while upright.
Autonomic reflexes are usually intact on formal tests of autonomic nervous system function. POTS patients often have preserved vagal function (as reflected by their sinus arrhythmia ratio in response to deep breathing) and a vigorous pressor response to the Valsalva maneuver, with an exaggerated blood pressure recovery and overshoot both before and after release 13. Given the complaints of exercise intolerance, formal cardiopulmonary exercise testing can be useful for objective documentation of exercise capacity, and this can also be used serially to quantify functional capacity over time.
The blood volume is low in many patients with POTS 14. This can be objectively assessed with nuclear medicine tests to directly measure either the plasma volume or the red cell volume. Some patients with POTS have co-existent complaints of episodic flushing, and a minority of these cases result from an associated mast cell activation disorder 13. This can be diagnosed by measuring methylhistamine levels from a 4 hour urine sample immediately following a severe flushing spell.
Non-Pharmacological Treatment of POTS
No therapy is uniformly successful, and combinations of approaches are often needed. Efforts should initially focus on treating any reversible causes. If a patient has had a bout of prolonged bedrest, their symptoms should gradually improve as they recondition themselves to upright posture. Treatment should be optimized for any chronic disease that is present. Radiofrequency ablation may be needed to treat reentrant supraventricular tachyarrhythmia, but radiofrequency sinus node modification for the sinus tachycardia of POTS is not recommended, as this often makes the patient's symptoms worse (and occasionally pacemaker dependent). Specific therapies are summarized in Table 2.
Table 2. Treatments for the Postural Tachycardia Syndrome.
| Therapy | Comments |
|---|---|
| Exercise Program |
|
|
| |
| Augment Blood Volume/Venous Return | |
| Increase water intake |
|
| Increase NaCl intake – diet or tablets |
|
| IV Saline (acute effect) |
|
| “Pantyhose style” compression stockings Withdraw OCP with drosperinone |
|
| Fludrocortisone Desmopressin (DDAVP) |
|
|
| |
| Hemodynamic Agents | |
| Withdraw drugs that block the norepinephrine transporter (NET) |
|
| Propranolol |
|
| Pyridostigmine |
|
| Midodrine |
|
| Other | |
| Modafinil |
|
NaCl – Table salt; IV – intravenous; PO – by mouth; OCP – oral contraceptive pill; ADHD – attention deficit hyperactivity disorder; SNRI – serotonin-norepinephrine reuptake inhibitor; BID – twice daily; TID – three times daily; QID – four times daily;
Patient education is important. Patients with POTS should avoid aggravating factors such as dehydration, and extreme heat. To ensure adequate hydration, we ask our patients to consume 8-10 cups of water daily and to increase their sodium intake to up to 8-10 gm/day. If this cannot be accomplished with dietary modification, supplemental NaCl tablets (with meals) can be used. Elastic support hose can help to minimize the degree of peripheral venous pooling and enhance venous return. We recommend panty-hose (waist high) style stockings with 30-40 mm Hg of pressure.
Acute blood volume expansion will over the short-term improve symptoms and control the heart rate. Jacob et al. 16 found that normal saline 1L infused intravenously over 1 hour normalized the orthostatic tachycardia (PRE: 33±5 bpm; POST:15±3 bpm). Acutely, this treatment is more effective at heart rate control than other medications. While this can be a very effective “emergency” therapy, it is not a practical “day to day” approach.
Exercise
Exercise has routinely been recommended as a part of the treatment regimen for many years. Unfortunately, POTS patients report feeling debilitated for days post-exertion, limiting compliance. Anecdotally, patients who exercise seem to have a better long-term prognosis, but it is not certain if this is due to the exercise or because of their ability to exercise. Fu et al. 17 recently administered a structured 3 month exercise program to 19 patients with POTS. This relatively short intervention reduced orthostatic tachycardia and improved quality of life. Physiological parameters such as blood volume, stroke volume, and LV mass all improved over the 3 months. This study elegantly showed that exercise is important in this population, and not just the ability to exercise.
Pharmacological Treatment of POTS
The Food and Drug Administration has not approved any medications for the treatment of POTS. Therefore, all agents used for this disorder are “off label”. Furthermore, all trials have been acute or of a short duration, with none tested in a long-term properly powered randomized clinical trial.
The initial pharmacological approach is to withdraw medications that might be predisposing to tachycardia (such as diuretics, vasodilators, and norepinephrine transporter blockers). Given their demographic, many POTS patients take oral contraceptives. Some agents (e.g., Yaz™ or Yasmin™) include drosperinone as the progestin, which is a spironolactone analogue. Given that inappropriately low aldosterone and low blood volume has been identified as a problem in some POTS patients, we recommend that this be switched to an oral contraceptive that uses a different progestin.
In patients in whom the presence of hypovolemia is either known or strongly suspected, fludrocortisone (aldosterone analogue) is often used. Through enhanced sodium retention, it should expand the plasma volume. Adverse effects can include hypokalemia, worsening headaches, acne, and fluid retention with edema. Another volume expanding agent that may be helpful for short-term use is desmopressin (DDAVP) 18. This agent causes the kidney to retain free water, but not sodium. Potential side effects include hyponatremia, edema, and headache. We have only allowed patients to use this less than once per week in a “pill in the pocket” manner for special events. Although DDAVP is safely used in children for enuresis, we have been concerned about the risk of hyponatremia in patients told to drink free water copiously.
Midodrine is a peripheral alpha-1 agonist that serves as a vasoconstrictor. It might be most useful in patients with “neuropathic POTS”, which can be associated with a failure of vascular resistance. Jacob et al. have documented that midodrine reduces orthostatic tachycardia, but this effect is more modest than that of IV saline 16. Midodrine can cause goosebumps, scalp tingling, or headaches, which can limit its tolerability.
Beta-adrenergic blockers are commonly used in cardiology clinics to control tachycardia. Many patients with POTS, however, will complain of excessive fatigue or intolerance to beta-blockers. Reducing the HR in POTS would be counter-productive if the increase in HR were purely compensatory for another physiological shortfall (e.g., low stroke volume), but could be useful if the tachycardia was “over-compensation” for the physiological stimuli. We have found low dose short-acting propranolol (10-20 mg PO) to be very effective at lowering standing HR and improving symptoms in POTS patients 19. More complete beta-blockade, however, is less well tolerated 19.
Pyridostigmine is a peripheral acetylcholinesterase inhibitor that can increase the levels of synaptic acetylcholine at both the autonomic ganglia and the peripheral muscarinic parasympathetic receptors. Pyridostigmine 30-60 mg PO TID has been reported to result in chronic symptom improvement in ∼50% of POTS patients 20. Pyridostigmine can enhance bowel motility, and thus gastrointestinal adverse events are the most common reason for discontinuation of the drug 20.
Central sympatholytic agents can be useful in patients with the central hyperadrenergic form of POTS, but may not be as well tolerated in neuropathic POTS. Clonidine is an alpha-2 agonist that acts centrally to decrease sympathetic nervous system outflow. Clonidine 0.1-0.2 mg PO BID-TID (eventually switched to a long-acting patch) can stabilize heart rate and blood pressure in patients with high sympathetic nervous system activity. Methyldopa 125-250 mg PO BID is a false neurotransmitter that is sometimes better tolerated due to its longer half-life. Unfortunately, both drugs can cause drowsiness, fatigue, and worsen the mental clouding of some patients.
Many patients are also greatly troubled by mental clouding or troubles concentrating. Modafinil, a stimulant whose mechanism is not yet clear, can improve alertness in some patients with POTS. Caution is advised, however, as modafinil may aggravate the orthostatic tachycardia.
Conclusions
POTS is a disorder of the autonomic nervous system that can produce substantial disability among previously healthy people. Patients with POTS demonstrate a HR increase of ≥30 bpm within 10 min of standing (or higher in children), are often hyperadrenergic, and tend to have a low blood volume. Therapies targeting the hypovolemia and the excess sympathetic nervous system activation may help relieve symptoms.
Epilogue
Patient OT was intolerant to both midodrine (“felt like I had bugs in my hair”) and fludrocortisone (marked bloating). She was not able to increase her dietary salt intake, so she took NaCl tablets 1 gm PO TID. She used waist high compression stockings and reported significant improvement in her symptoms with propranolol 20 mg PO TID. Her Vitamin B12 level was low, and this was supplemented. She discontinued her oral birth control containing drosperinone and switched to an agent with a different progestin. Finally, she uses DDAVP 0.2 mg PO occasionally for improved orthostatic tolerance for special events. This combination of therapies did help to improve her orthostatic tolerance, but she was not able to continue to work due to her “brain fog”.
Acknowledgments
The author thanks David Robertson, MD for his thoughtful review of this manuscript.
Funding Sources: This work was supported in part by NIH grants P01 HL56693, R01 HL102387 and UL1 TR000445.
Footnotes
Conflict of Interest Disclosures: None.
Reference List
- 1.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural Tachycardia in Children and Adolescents: What is Abnormal? J Pediatr. 2012;160:746–752. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewster JA, Garland EM, Biaggioni I, Black BK, Ling JF, Shibao CA, Robertson D, Raj SR. Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Sci (Lond) 2012;122:25–31. doi: 10.1042/CS20110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet. 2012;118:242–246. doi: 10.1016/j.ijgo.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H2161–H2167. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, Robertson D, Shelton RC. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009;80:339–344. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, Robertson D, Raj SR. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204–210. [PMC free article] [PubMed] [Google Scholar]
- 9.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 10.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 11.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder C, Tank J, Boschmann M, Diedrich A, Sharma AM, Biaggioni I, Luft FC, Jordan J. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. doi: 10.1161/hc0302.102597. [DOI] [PubMed] [Google Scholar]
- 13.Shibao C, Arzubiaga C, Roberts LJ, Raj S, Black B, Harris P, Biaggioni I. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385–390. doi: 10.1161/01.HYP.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- 14.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 15.Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011;8:422–428. doi: 10.1016/j.hrthm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, Dupont WD, Robertson D, Raj SR. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 2012;9:1484–1490. doi: 10.1016/j.hrthm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanjwal K, Karabin B, Sheikh M, Elmer L, Kanjwal Y, Saeed B, Grubb BP. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol. 2011;34:750–755. doi: 10.1111/j.1540-8159.2011.03047.x. [DOI] [PubMed] [Google Scholar]


