Abstract
Despite positive animal studies, clinical angiogenesis trials have been disappointing, possibly due to risk factors present in humans but usually unexplored in animals. We recently demonstrated aging causes impaired collateral remodeling and collateral dropout; here, we investigate potential mechanisms responsible for these findings. Four-, 10-, and 18-month-C57BL/6J mice were subjected to femoral artery ligation; flow was measured using laser Doppler perfusion imaging. Endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS were measured in calf muscle. Apoptosis was assessed in endothelial (EC) and smooth muscle (SMC) cells isolated from young and old mice. Angiogenesis was measured using a Matrigel plug assay. Lethally irradiated young and old mice received bone marrow cells (BMC) from either young or old donors and were subjected to femoral artery ligation (FAL). BMC mobilization and homing were assessed. Flow recovery was impaired and less eNOS and phosphorylated eNOS was present in older vs. young mice (p<0.001 and p=0.015, respectively). ECs and SMCs from older mice were more sensitive to an apoptotic stimulus, but were rescued by NO-enhancing drugs. In older mice, angiogenesis (Matrigel plug assay) was impaired, as was mobilization and homing of BM progenitor cells following FAL. Although both mobilization and homing improved when older mice received BMC transplantation from young donors, flow recovery failed to improve. Aging impairs BMC mobilization and homing, collateral responsiveness to angiogenic stimuli, and increases EC and SMC susceptibility to apoptosis via dysfunctional eNOS signaling. The latter could contribute to impaired remodeling and collateral dropout. These finding identify potential obstacles to therapeutic interventions in elderly patients.
Keywords: Aging, Progenitor cell, Angiogenesis, Collateral, eNOS, Apoptosis
Background
The natural capacity of collaterals to compensate for the reduced flow occurring after obstruction of a major artery rarely restores maximal flow capacity. The resulting clinical problems are enormous, as in the USA alone 300,000 to 900,000 patients have persistent angina despite medical management [1, 2]. Similar issues pertain to patients with peripheral vascular disease [3].
Despite animal studies demonstrating that multiple interventions enhance collateral flow recovery following acute arterial ligation, the results of clinical angiogenesis trials have been disappointing [4, 5]. A key contributor to these disparities may be the impact of risk factors present in patients but often not in experimental animals [4]. One such risk factor is age. Thus, after hindlimb ischemia blood flow recovery is lower and ischemic injury is greater in aged vs. young mice [6]. We have recently definitively documented by direct measurement of collaterals that two of the mechanisms contributing to these findings are aging-related collateral rarefaction (decreased collateral number) and impaired collateral remodeling, both related at least in part to a dysfunctional endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signaling pathway (Faber and Epstein, unpublished data).
Collateral remodeling and maintenance of native (i.e., pre-existing) collaterals undoubtedly are affected by multiple mechanisms that may be compromised by aging. For example, increased shear stress induced by arterial obstruction is a critically important factor in remodeling, followed by homing of progenitor cells to the expanding collaterals which, through the secretion of cytokines, further enhance the remodeling process. To gain additional insights into how aging contributes to collateral dysfunction, we therefore examined the impact of aging on bone marrow (BM) progenitor cell mobilization and their homing to regions of developing collaterals, and the actual responsiveness of preexisting collaterals of old mice to the potential therapeutic benefit of administering BM cells derived from young mice. In addition, aging leads to increased oxidative stress [7–9], a stimulus that may predispose endothelial cell (ECs) and smooth muscle cell (SMCs) to undergo apoptosis. Because eNOS/NO signaling exerts anti-apoptotic effects [10], we examined the possibility that aging-induced eNOS/NO dysfunction predisposes ECs and SMCs to undergo apoptosis, a mechanism that could contribute both to impaired collateral remodeling and to collateral dropout.
Methods
Animal Model
Animal procedures were approved by the Institutional Animal Care and Use Committee (MedStar Research Institute). Thirty-eight 4-month-old, five 10-month-old, and thirty-six 18-month-old female C57Bl/6 mice, sedated with ketamine and xylazine, underwent surgery to remove the right femoral artery [11] B6.129S7-Gt(ROSA)26Sor/J mice (4 and 18 months) were used as donors in BM transplantation studies. Animals were maintained on a normal chow diet.
Monitoring Blood Flow Recovery
Laser Doppler perfusion imaging (LDPI; Moor Instruments) was utilized to record serial blood flow measurements. Imaging was performed after limb hair was removed and mice had been placed on a heating blanket at 37°C. Perfusion is expressed as a ratio of the ischemic to normal limb [12]. The laser Doppler instrument used had a penetration of 1 mm. Since the region of interest that was measured was the foot, the penetration depth should not be a confounding factor, as the foot thickness is not much greater than 1 mm.
Tissue Collection
At killing, mice were anesthetized using ketamine and xylazine; peripheral blood was collected and stored in RNALater (Ambion). Gastrocnemius muscle was harvested and snap frozen in liquid nitrogen. In a subset of mice, the gastrocnemius muscle was harvested and fixed in OCT for histological assessment. BM was collected from the femur and tibia, filtered, and pelleted by centrifugation (400×g), and frozen in Trizol (Invitrogen).
RNA Isolation and Gene Expression Analysis
Total RNA was isolated from tissue using Trizol reagent (Life Technologies) and from the peripheral blood using the RiboPure Kit (Ambion). Samples were reverse transcribed using the High Capacity cDNA Archive kit (Applied Biosystems, Inc.), according to manufacturers' instructions. Real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR system, using commercially available primer/probe sets (Applied Biosystems, Inc.).
Isolation of Aortic Endothelial Cells
Twelve-week-old and 24-month-old mice were killed, and the aortas were removed. The vessel was cleaned of periadventitial fat and connective tissue, cut into rings, and incubated in 0.25% Trypsin for 30 min. Rings were transferred to a conical tube and pipetted up and down in 5% fetal bovine serum (FBS) in Dulbecco's modified Eagle's medium (DMEM). Cells were collected by centrifugation at 250×g for 10 min, resuspended by EGM-2 (LONZA, cc-3156), and incubated in 100 mm dishes at 37°C for 24 h.
Isolation of Aortic Smooth Muscle Cells
Thoracic aortas were flushed and excised, and the adventitia and surrounding connective tissue were removed. Vessels were opened by a longitudinal cut, and the intima and a thin portion of the subjacent media were removed. The aortas were trimmed and cut into four pieces, and each piece was placed into a separate well of a 12-well plate containing DMEM (high glucose) with 20% FBS and antibiotics. The vascular smooth muscle cells migrated from the tissue and were subcultured in DMEM containing 20% FBS and 5% CO2, as previously described. [13] SMC phenotype was verified by measuring expression of α-actin mRNA by RT-PCR. Cells were used prior to passage P12 for all in vitro assays.
Western Blotting Analysis
Gastrocnemius muscle was harvested and snap frozen in liquid nitrogen, or aortic endothelial cells were used. Total protein lysate (80 μg) was separated on 4∼20% SDS-polyacrylamide gels and transferred to nitrocellulous membranes. The following primary antibodies were used: α-eNOS (1:700, BD) and α-phospho-eNOS (Ser 1177; 1:500, Cell Signaling Technology). Westerns were developed using chemiluminescent reagents (Supersignal, Pierce), and bands were quantified with densitometry.
Apoptosis Assay
Caspase-3 Assay for TNF-α-Induced EC Apoptosis EC apoptosis was detected using a caspase-3 activity colorimetric kit (R&D, BF3100). Aortic ECs isolated from young and old mice (passage 5) were seeded at 1×106 cells/dish in 100 mm culture dishes in triplicate. Cells were grown for 1 day in EGM-2 medium and then were starved for 6 hours. Cells were incubated with 30 ng/ml tumor necrosis factor (TNF)-α for 18 h to induce apoptosis. For rescue experiments, sodium nitroprusside (10 μM) was added with the TNF-α. Cells were collected in a conical tube by centrifugation at 250×g for 10 min, lysed on ice for 10 min, and centrifuged at 10,000×g for 1 min. Total protein concentration was measured using a BCA assay kit (Pierce 23225). Equal amounts (100 μg in 50 μl) of total protein were used for each reaction, 5 μl DEVD-pNA substrate was added. The optical density was measured at 405 nm wavelength, according to the manufacturer's instructions.
DAPI Assay for H2O2-Induced SMC Apoptosis SMC isolated from young and old mice (passage 5) were cultured in 24-well plates at 50,000/well. After 24 h, 50 μm H2O2 was added and the cells were incubated overnight. Cells were washed three times with phosphate-buffered saline (PBS) and 4% PFA was added for 5 min, followed by 2 μg/ml of DAPI. Cells were observed in microscope, and those with an intact nucleus were counted as live cells.
Matrigel Plug Assay
Angiogenesis was assessed through a Matrigel plug assay. Young (3 months; n=5 per group) and old (24 months; n=5 per group) female C57 Bl/6 mice were subcutaneously injected with low growth factor containing Matrigel infused with either bFGF (1 μg/ml or 5 μg/ml), or PBS (control). After 5 days, the plugs were harvested, paraffin embedded, and stained using H&E. Angiogenesis was quantified by measuring cell invasion and red blood cell-containing vessels per section. CD31 staining was used to verify endothelial cell phenotype.
Flow Cytometry
BM cells were harvested; 5×105 cells were incubated with antiCD26-FITC antibody (e-Bioscience, 10 μg/ml) at 4°C for 40 min. The CD26-positive cells were sorted on a FACSAria (Becton Dickinson Immunocytometry Systems, San Jose, CA).
BM Transplantation
BM cells were flushed from femurs and tibias of donor B6.129S7-Gt(ROSA)26Sor/J mice. Following lethal irradiation with 9.5 Gy, 1×107 BM cells were transplanted into recipient mice through retro-orbital injection. Recipient mice were maintained in sterile cages for 6 weeks. Chimeric mice were confirmed by detecting Lac Z protein (β-galactosidase staining of peripheral blood).
Statistical Analysis
Statistical analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC). Continuous variables were expressed as mean±SD. Blood recovery data were compared among different age groups by analysis of variance with repeated measure. A t test was used for Western blotting quantification and caspase-3 assay. A p value of <0.05 was considered statistically significant.
Results
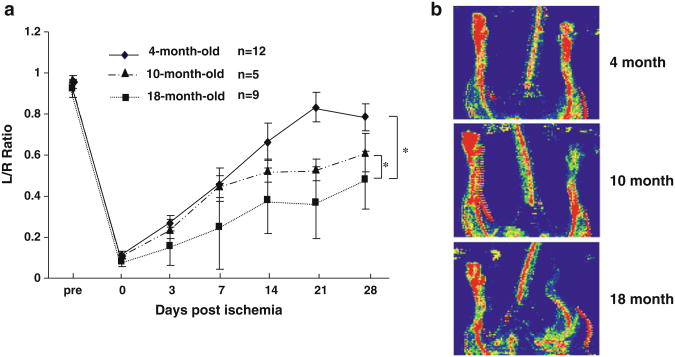
Collateral Flow Recovery Collateral flow recovery was inversely related to age of the mice (Fig. 1). Perfusion was impaired in 10- and 18-month-old mice compared with 4-month-old animals (p<0.001).
Fig. 1. a Blood flow recovery in a 4-, 10- and 18-month-old C57BL/6J mice, as measured using LDPI. Flow recovery is impaired in 10 and 18 vs. 4 month-old mice (*p<0.001, by RMANOVA for the entire curve). b LDPI images from day 21 post-ligation.
Western Blotting Analysis Multiple studies have demonstrated the important role of upregulation of eNOS and its activity in the remodeling that occurs following occlusion of the major conduit artery they accompany [14]. However, aging has been shown to decrease eNOS expression [15, 16] and reduce shear stress-induced release of NO in rats [17]. In our model, we found that aging decreased eNOS protein in calf muscle at baseline in old vs. young mice (Fig. 2a, b). It also decreased eNOS phosphorylation (ser-1177). Phosphorylated eNOS/total eNOS ratio was significantly lower in older mice (p=0.015).
Fig. 2.
Western blotting was carried out in the calf muscle of older mice at baseine (prior to surgery), and compared with younger mice, and quantified using densitometry. a Western blots. b The ratio of phosphorylated/total eNOS protein levels was significantly lower in old vs. young mice (*p=0.015). eNOS/G=eNOS/GAPDH, p-eNOS/G=p-eNOS/GAPDH
Apoptosis We recently demonstrated that aging leads to collateral vessel rarefaction in multiple vascular beds (Faber, unpublished data). Since eNOS levels are decreased in the hindlimb of older mice, we hypothesized that lower levels of eNOS increase the sensitivity of the cells lining the collaterals to undergo apoptosis, perhaps leading to collateral rarefaction. Our study results were compatible with such a hypothesis. ECs isolated from old mice were more sensitive to TNF-α-induced apoptosis (Fig. 3a). Preincubation of the ECs with the NO donor sodium nitroprusside (SNP) afforded protection from apoptosis (Fig. 3b) such that caspase activity in the old cells was no different than in the young cells. Rescue of the ECs by a NO donor suggests deficient NO contributes to the increased sensitivity to apoptosis.
Fig. 3.
Cells derived from young vs. old mice are more sensitive to an apoptotic stimulus. When endothelial cells are exposed to TNF-α, the “old” cells are more sensitive to this apoptotic stimulus, as reflected by a greater increase in caspase levels (a). Exposure of cells to sodium nitroprusside (SNP), an NO donor, attenuates the TNF-α-induced apoptotic response (b). SMCs derived from old mice are also more sensitive to an apoptotic stimulus. In this case the apoptotic stimulus is H2O2(c). Exposure of cells to tadalafil, which inhibits degradation of cyclic GMP (a critical downstream product of eNOS signaling), the apoptotic response to H2O2 is attenuated (d). SMCs isolated from old mice have more PDE-5 mRNA expression that young SMCs (e)
SMCs derived from old mice were also more sensitive to another apoptotic stimulus H2O2 (p<0.05; Fig. 3c). When the cells were exposed to the phosphodiesterase-5 (PDE-5) inhibitor, tadalafil, the apoptotic response was attenuated (p<0.05; Fig. 3d). PDE-5 induces the breakdown of cyclic GMP, an important downstream product of NO signaling, promoting vasodilatation and arteriogenesis while preventing apoptosis and decreasing oxidative stress. Thus, the addition of a PDE-5 inhibitor would be expected to prevent apoptosis, as it leads to an increase in cyclic GMP.
PDE-5 Expression Interestingly, SMCs from old mice have higher PDE-5 expression (p=0.005; Fig. 3e), suggesting that cyclic GMP is reduced in these cells, possibly contributing to an increased sensitivity to apoptosis, increased oxidative stress, and decreased collateral remodeling.
Matrigel Studies To further assess aging-related impairments in vascular cell function, we performed a Matrigel assay to quantify endothelial cell responsiveness to an angiogenic stimuli in vivo. Cellular infiltration to bFGF-impregnated Matrigel plugs was significantly less in older mice (p=0.02) (Fig. 4a, b) as was the number of red blood cell-containing vessels (p=0.03) (Fig. 4a, c) and vessels lined by CD31-positive cells (Fig. 4a).
Fig. 4.
Representative sections from the Matrigel plug assay. In (a), top left, young, 1 μg/ml bFGF; lower left, old, 1 μg/ml bFGF. Both are stained with H&E. Middle panels are stained for CD31, and right panels are negative controls. Angiogenesis was markedly impaired with aging, as fewer infiltrating cells and RBC containing vessels were present in Matrigel plugs at each concentration of bFGF (b, c)
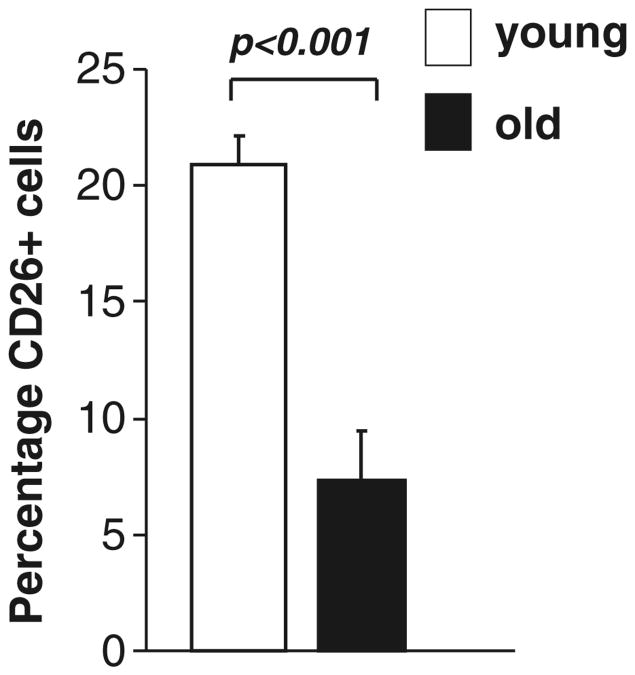
Mobilization of BM-Derived Cells CD26 (dipeptidylpeptidase IV (DPPIV)) is critically involved in release of BM cells from their stromal BM niche. CD26 is expressed on many hematopoietic cells, including stem and progenitor cells [18]. One of its substrates is SDF-1 (which is present on mesenchymal stromal cells), and when this is cleaved by CD26 the chemoattractant properties of SDF-1 for CXCR4 are abolished, thereby releasing the CXCR4-expressing hematopoietic and progenitor cells from their niche into the circulation. Christopherson et al. demonstrated that CD26 is critical for G-CSF mobilization of progenitor cells in mice [19]. When mice were treated with CD26 inhibitors during G-CSF-induced mobilization, treatment significantly reduces the number of progenitor cells in the periphery compared with G-CSF mobilization alone [19]. We, therefore, hypothesized that older mice would have reduced levels of CD26 present in their bone marrow, which would lead to an impairment in mobilization of bone marrow-derived cells. We found that CD26-positive cell number in BM was greater in young mice (p<0.05, Fig. 5), that CD26 mRNA was consistently higher in BM from young mice at baseline and following ischemia, and that more white blood cells were present in the peripheral blood of young animals 2 h post-ischemia (p<0.05, data not shown).
Fig. 5. There were more CD26-positive BM cells in young vs. old mice (p<0.05).
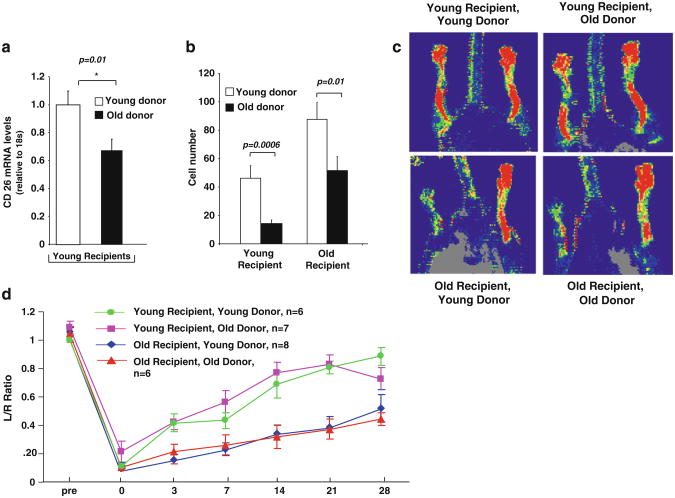
Bone Marrow Transplantation We tested whether we could salvage the impaired blood flow recovery in old mice by reconstituting them with BM from young animals. Mice reconstituted with BM from young donors had significantly more CD26-positive cells in the bone marrow (Fig. 6a) and more BM-derived cells (as identified by LacZ staining) in the ischemic tissue compared with mice reconstituted with BM from old donors (p<0.05, Fig. 6b). These findings indicate that transplanting the marrow from young donors into older mice did indeed lead to higher levels of CD26 mRNA and to higher numbers of CD26-positive cells in recipient BM, presumably contributing to the more robust mobilization and better homing of BM cells to the ischemic tissue even in old recipients. Interestingly, however, recipient age, rather than BM age, determined flow recovery outcome. Older mice had poor blood flow recovery regardless of age of the BM donor while young mice had superior blood flow recovery independent of the BM donor age (Fig. 6c). In other words, the age of the donor does not matter (p= 0.340)—rather, it is the age of the recipient alone that determines blood flow recovery following induction of hindlimb ischemia (p<0.001).
Fig. 6.
a Mice reconstituted with BM from young donors had more CD26 mRNA present in BM (p=0.01) and b a greater number of LacZ-positive cells present in the ischemic tissue at 28 days post-surgery than mice reconstituted with BM from old donors. This was observed for both young and old recipients (p=0.006 and p=0.01, respectively). c, d BM transplantation studies showed that young recipients have better flow recovery regardless of the age of the donor marrow (young recipients, young donors (green)), (young recipients, old donors (pink)) while old recipients had poor flow recovery regardless of the age of the donor marrow (old recipient, young donor (red)), (old recipient, old donor (blue)). Specifically, the age of the donor does not matter (p=0.340), rather it is the age of the recipient (p <0.001). LDPI images (c) are from day 21 post-ligation
Discussion
Collaterals constitute a major compensatory means by which the body enhances flow to ischemic tissue supplied by blocked arteries, a mechanism seriously compromised by aging and other risk factors [6, 20, 21]. We have, therefore, initiated a series of studies investigating the mechanisms by which aging leads to dysfunction of the collateral circulation.
The present investigation builds on our recent findings— namely, that aging compromises remodeling of collaterals following acute arterial obstruction (a critical mechanism by which collateral function improves following arterial occlusion [22, 23]) and leads to actual collateral rarefaction (Faber et al., unpublished data). Because aging undoubtedly impairs multiple pathways that could contribute to defective collateral remodeling and collateral dropout, including impaired eNOS/NO signaling, in this investigation, we therefore examined the impact of aging on several potential mechanisms that could impair collateral function. Thus, we examined the effect of aging on BM progenitor cell mobilization and the homing of these progenitor cells to regions of developing collaterals (it is believed that these progenitor cells, through the secretion of cytokines, contribute to enhanced collateral function). We also examined the actual responsiveness of pre-existing collaterals of old mice to a collaterogenic stimulus—i.e., to the potential therapeutic benefit of administering BM cells derived from young mice. In addition, because eNOS/NO signaling exerts anti-apoptotic effects, we determined whether aging-induced eNOS/NO dysfunction predisposes endothelial and smooth muscle cells to undergo apoptosis, a mechanism that could contribute both to impaired collateral remodeling and to collateral dropout. Finally, we identified a novel mechanism by which aging could further contribute to compromise of the eNOS/NO system—an increase in the expression of phosphodiesterase-5, an enzyme that degrades a key downstream product of eNOS/NO signaling cGMP.
Aging-Induced eNOS Dysfunction
To confirm that aging leads to deficient eNOS/NO activity/bioavailability [17, 24-27] in the model used in this investigation, we first examined the effects of aging on eNOS protein levels and whether aging compromises eNOS activation and thereby its downstream signaling. The latter requires phosphorylation of eNOS at ser-1177 [28-30]. We found eNOS protein levels and phosphorylation of eNOS at ser-1177 are both decreased in older vs. young mice under baseline, non-ischemic conditions (Fig. 2a, b); the latter could not solely be accounted for by decreased eNOS protein levels, as ser-1177 phosphorylation was diminished even when normalized for eNOS protein levels (Fig. 2b). Thus, aging not only decreases eNOS protein expression, but also decreases eNOS signaling. Multiple changes induced by aging probably contribute to the dysfunction of eNOS signaling as well as to the deleterious alterations in the collateral circulation (Faber et al., unpublished data). For example, aging increases oxidative stress [7-9] and oxidative stress causes eNOS uncoupling [9]. When uncoupled, activation of eNOS results in superoxide rather than nitric oxide production [31], transforming eNOS from a vascular-protective to a vascular-disruptive enzyme. Although we showed less activation of the eNOS pathway in old mice (decreased phosphorylation of eNOS; Fig. 2b), the eNOS signaling that exists in these mice may be causing superoxide production.
Aging-Induced Increased Sensitivity of Endothelial and SMCs CELLS to Apoptosis, an Effect Attenuated by Molecules Increasing NO Activity
Our recent demonstration that aging leads to collateral “dropout” prompted us to consider possible mechanisms through which this might occur. The mechanism we examined in this study was derived from our reasoning that vessel dropout may occur when the cells comprising these collaterals (endothelial and/or smooth muscle cells) undergo apoptosis. Our results demonstrated that ECs from old mice, tested in vitro, are more sensitive to apoptosis induction (Fig. 3a). We postulated that the decrease in eNOS we observed in the hindlimb of older mice by western blot may partially explain the increased sensitivity to apoptosis [32]. Interestingly, when ECs were preincu-bated with SNP, an NO donor, the apoptotic responses were attenuated such that there was no difference between the percent of old vs. young cells that became apoptotic (Fig. 3b). These findings are supported by the data of Hoffmann et al. who demonstrated that late passaged ECs (not, however, derived from old mice) exhibited increased sensitivity to TNF-induced apoptosis compared with early passaged cells, an effect attenuated by exogenous NO [10].
We found that SMCs derived from old mice also exhibit increased sensitivity to apoptosis induction (Fig. 3c), an effect attenuated by pre-incubating the cells with tadalafil, a PDE-5 inhibitor. Tadalafil inhibits PDE-5-induced breakdown of cyclic GMP, which is a critically important downstream product of NO signaling (Fig. 3d). Thus, these two findings—rescue of ECs derived from old mice from apoptosis by an NO donor, and rescue of SMCs derived from old mice by a PDE-5 inhibitor—suggest aging leads to an increased sensitivity of key vascular wall cells to apoptosis, and that such an effect is mediated, at least in part, by a dysfunctional eNOS pathway.
An unexpected, novel, and potentially important finding of our study was that PDE-5 mRNA was increased in the SMCs derived from old mice (Fig. 3e). If protein and activity levels follow mRNA levels, we will have demonstrated a new mechanism contributing to aging-related dysfunctional eNOS signaling. Such findings would also suggest that PDE-5 inhibitors may improve collateral remodeling in the elderly.
Because of findings we recently reported in a parallel study, we attribute particular importance to our current observations that aging increases the propensity of cells of the vascular wall (ECs and SMCs) to apoptosis, and that this effect is rescued by NO-enhancing drugs. In that independent study, we found in male mice that aging is associated with both collateral loss and impaired collateral remodeling following acute ligation of the parent artery. Collateral dropout was determined by two independent experiments. First, we found that the number of α-SMA-positive vessels crossing the midpoint of the semimembranosis muscle, the main adductor muscle and a collateral-rich zone, declined with aging. Consistent with this finding was the observation that hindlimb flow immediately after femoral artery ligation (which largely reflects flow through pre-existing collaterals) is lower in old mice. Second, we demonstrated by direct measurement that aging significantly reduces cerebral collateral number, and third, we found by direct measurement that aging impairs cerebral collateral remodeling.
These findings, taken together, strongly suggest that an aging-induced increased propensity to apoptosis of ECs and SMCs lining collateral vessels contributes to collateral rarefaction and to impaired collateral remodeling, effects mediated, at least in part, by an aging-induced dysfunction of eNOS/NO signaling. Additional studies will have to be performed, however, to prove a direct causal link between aging-induced apoptosis and these two critical aging-related abnormalities of the collateral circulation.
The above findings have important clinical therapeutic implications, as any dropout of collaterals occurring during aging could impair responsiveness of the target tissue (i.e., the collaterals) to any collaterogenic therapeutic intervention. Impaired target tissue responsiveness is further suggested by two additional experiments performed in this investigation.
Aging-Related Impairment of Endothelial Cell Responsiveness
Endothelial cells are critically important in both angiogenesis and collateral remodeling, as they must migrate and proliferate in order to contribute to either new vessel development or existing vessel enlargement. We sought to determine if aging might impair endothelial cell function. In a Matrigel plug assay, we demonstrated that aging impairs the capacity of ECs to respond to the potent angiogenic agent bFGF. Thus, cellular infiltration in response to bFGF-impregnated Matrigel plugs was significantly less in older mice (Fig. 4b) as was the number of red blood cell-containing vessels (p=0.03) (Fig. 4a, c) and vessels lined by CD31-positive cells (Fig. 4a).
Aging-Related Impairment of Bone Marrow-Derived Cells
There is extensive literature [33–35] demonstrating that mobilization of progenitor cells residing in the BM and their homing to regions of developing collaterals importantly contribute to collaterogenesis and that aging compromises mobilization/homing [36]. First, we confirmed that aging impaired mobilization and homing of BM cells existed in our particular mouse model of aging. We examined gene expression in the BM of CD26 (DPPIV), a key modulator of the CXCR4/SDF-1 binding interaction.
CD26 is expressed on many hematopoietic cells, including stem and progenitor cells [18]. A membrane-bound extracellular peptidase, CD26 cleaves certain dipeptides from the N terminus of polypeptide chains. One of its substrates is SDF-1, and when this is cleaved by CD26 the chemoattractant properties of SDF-1 for CXCR4 are abolished, thereby abolishing the anchoring properties exerted by the bond created between SDF-1 and CXCR4-expressing cells. Consequently, such cells are released from their BM niche and into the circulation.
In our model, we found that old mice have decreased levels of CD26 mRNA in their BM under baseline conditions (data not shown) and had significantly fewer CD26-positive cells present 2 h post-hindlimb ischemia induction (Fig. 5), findings compatible with impaired CD26-mediated mobilization. We also found significantly fewer white blood cells in peripheral blood 2 h post-ischemia onset, indicating generalized aging-induced impaired cell mobilization (data not shown) and that when young mice were transplanted with old BM (see below), homing of BM cells to ischemic tissue was compromised (Fig. 6b).
We next determined whether BMC mobilization and homing, impaired with aging, improve in old mice receiving BMC transplantation from young donors and, if they are, whether this improves blood flow recovery of old mice. This would be expected unless collateral responsiveness was impaired by aging.
We did find increased levels of CD26 mRNA in BM (Fig. 6b), and significantly more BM-derived cells in the ischemic tissue of mice, whether old or young, when BM was reconstituted with young BM (Fig. 6b). However, despite the greater homing of cells to ischemic tissue when transplanting young BM into old mice, blood flow recovery failed to improve (Fig. 6c, d). These results are compatible with our hypothesis that aging impairs the responsiveness of collaterals to stimuli that would be expected to enhance collateral function. This conclusion is supported by the results in a very different model. Thus, Chang et al., using an ischemic flap model, demonstrated that when young BM is transplanted into aged hosts perfusion of the flap is identical to that of aged controls [37].
Conclusions
Our results suggest that aging-induced impaired collateral remodeling and collateral dropout may result in part from an increased sensitivity of ECs and SMCs to apoptosis ensuing from a dysfunctional NOS/NO signaling pathway. Furthermore, aging impairs mobilization of bone marrow progenitor cells and their homing to the region of developing collaterals. While mobilization and homing improve when old mice are transplanted with young BMCs, the presence of these young cells at the site of collateral development do not lead to improved flow recovery following femoral artery ligation, suggesting that aging also impairs the responsiveness of collateral vessels to angiogenic intervention.
Acknowledgments
This work was partially funded by the National Institutes of Health, NIH RO1 HL085003.
Footnotes
Competing Interests: The authors have no competing interests to disclose.
Contributor Information
Jinsong Wang, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China 510080.
XinZhi Peng, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China 510080, The Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China 510120.
Roberta M. Lassance-Soares, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA
Amir H. Najafi, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA
Lee O. Alderman, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA
Subeena Sood, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA.
Zhenyi Xue, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA.
Rosanna Chan, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA.
James E. Faber, Department of Cell and Molecular Physiology and McAllister, Heart Institute, University of North Carolina, Chapel Hill, NC 27599, USA
Stephen E. Epstein, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA
Mary Susan Burnett, Email: mary.s.burnett-miller@medstar.net, Cardiovascular Research Institute, MedStar Health Research Institute, Washington, DC 20010, USA.
References
- 1.Yang EH, Barsness GW, Gersh BJ, Chandrasekaran K, Lerman A. Current and future treatment strategies for refractory angina. Mayo Clinic Proceedings. 2004;79(10):1284–1292. doi: 10.4065/79.10.1284. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis— how many patients might be eligible? The American Journal of Cardiology. 1999;84(5):598–600. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 3.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation. 2010;122(18):1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 4.Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy oftherapeutic interventions. Cardiovascular Research. 2008;78(2):257–264. doi: 10.1093/cvr/cvm116. [DOI] [PubMed] [Google Scholar]
- 5.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: patho-physiological mechanisms. Annual Review of Pathology. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 6.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circulation Research. 2002;90(11):1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 8.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation. 2008;15(3):225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 9.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. Journal de Physiologie. 2009;587(Pt 15):3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circulation Research. 2001;89(8):709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 11.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. The American Journal of Pathology. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 12.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108(2):205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Marx S, Chen H, Poon M, Marks A, Rabbani L. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation. 2001;103:2967–2972. doi: 10.1161/01.cir.103.24.2967. [DOI] [PubMed] [Google Scholar]
- 14.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiological Reviews. 2009;89(2):481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 15.Luque CD, Vargas RH, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacology & Therapeutics. 2006;112(2):553–563. doi: 10.1016/j.pharmthera.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Baker CS, Rimoldi O, Camici PG, Barnes E, Chacon MR, Huehns TY, et al. Repetitive myocardial stunning in pigs is associated with the increased expression of inducible and constitutive nitric oxide synthases. Cardiovascular Research. 1999;43(3):685–697. doi: 10.1016/s0008-6363(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. American Journal of Physiology Heart and Circulatory Physiology. 2009;297(5):H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. Journal of Immunology. 2002;169(12):7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 19.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101(12):4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 20.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. American Journal of Physiology Heart and Circulatory Physiology. 2000;278(1):H85–H93. doi: 10.1152/ajpheart.2000.278.1.H85. [DOI] [PubMed] [Google Scholar]
- 21.Westvik TS, Fitzgerald TN, Muto A, Maloney SP, Pimiento JM, Fancher TT, et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. Journal of Vascular Surgery. 2009;49(2):464–473. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-a specifies formation of native collaterals and regulates collateral growth in ischemia. Circulation Research. 2008;103(9):1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalothorn D, Faber JE. Formation and maturation of the native cerebralcollateral circulation. Journal of Molecular and Cellular Cardiology. 2010;49(2):251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey intosubclinical arterial disease. Current Opinion in Nephrology and Hypertension. 2010;19(2):201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms ofvascular aging: new perspectives. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen A, Thorin-Trescases N, Thorin E. Working under pressure: coronary arteries and the endothelin system. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2010;298(5):R1188–R1194. doi: 10.1152/ajpregu.00653.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. American Journal of Physiology Heart and Circulatory Physiology. 2009;297(1):H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimmeler S, Fisslthaler B, Fleming I, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells via Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 29.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. Journal of Molecular and Cellular Cardiology. 2007;42(2):271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10(6):1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 32.Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacology & Therapeutics. 2005;106(2):147–162. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 34.Napoli C, Williams-Ignarro S, de Nigris F, de Rosa G, Lerman LO, Farzati B, et al. Beneficial effects of J. of Cardiovasc. Trans. Res. concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sica V, Williams-Ignarro S, de Nigris F, D'Armiento FP, Lerman LO, Balestrieri ML, et al. Autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the diabetic mouse hindlimb. Cell Cycle. 2006;5(24):2903–2908. doi: 10.4161/cc.5.24.3568. [DOI] [PubMed] [Google Scholar]
- 36.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circulation Research. 2007;101(12):1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 37.Chang EI, Loh SA, Ceradini DJ, Lin SE, Bastidas N, Aarabi S, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116(24):2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]