Abstract
Monocytes are produced in the bone marrow and enter the blood. They generally leave the blood and enter a tissue, and then become macrophages. In healing wounds, circulating monocytes also enter the tissue and instead of becoming macrophages, can differentiate into fibroblast-like cells called fibrocytes. Fibrocytes are also present in the lesions associated with fibrosing diseases such as congestive heart failure, end stage kidney disease, and pulmonary fibrosis. We have found that culturing blood monocytes, or white blood cell preparations containing monocytes, in serum-free media permits some of the monocytes to differentiate into fibrocytes within 5 days, and that this differentiation is inhibited by the blood plasma protein serum amyloid P.
Keywords: Fibrocytes, Fibrosis, Inflammation, Monocytes, Pentraxin, Serum-free culture, Serum amyloid P
1. Introduction
Fibrocytes are spindle-shaped fibroblast-like cells that are involved in both tissue repair and fibrosis (1–6). Fibrocytes differentiate from peripheral blood monocytes, and express markers of both hematopoietic cells (CD34, CD45, LSP-1, and MHC class II) and stromal cells (collagen and prolyl-4-hydroxylase) (1, 2, 7–10). Fibrocytes have both beneficial and detrimental effects on health. Fibrocytes can regulate innate and adaptive immune responses, by the secretion of a variety of cytokines and growth factors (2, 11–14). Fibrocytes also promote wound healing both directly and also by secreting factors that activate fibroblasts (6, 15). Fibrocytes are also implicated in chronic inflammation and fibrosis, and have been detected in tumors, hypertrophic scars, bronchial asthma, pulmonary fibrosis, renal fibrosis, some diseased heart valves, and nephrogenic systemic fibrosis (3, 15–21).
The original in vitro culture conditions developed by Bucala and colleagues using DMEM-based culture media supplemented with 10% fetal bovine serum, has been used to generate human, murine, and porcine fibrocytes from peripheral blood (1, 2, 11, 13, 22). This system involves incubating peripheral blood mononuclear cells (PBMC), a mixture of monocytes, B, NK, and T cells, for 2 days before the removal of the non-adherent B, NK, and T cells, and then culturing the remaining adherent cells for 10–14 days (22). After 10–14 days, fibrocytes are then recovered from culture by trypsin-EDTA and used for subsequent experiments. In addition, fibronectin is often used to coat the tissue culture vessels prior to the addition of the PBMC. The fibronectin may promote fibrocyte differentiation and also permits the removal of the fibrocytes from the tissue culture plastic after the 14-day culture period (22).
We observed that human, murine, and rat PBMC cultured in serum-free medium gave rise to fibrocytes and some macrophages within 5 days. However, at 5 days in medium containing serum no fibrocytes appear, and more macrophages were observed (8, 9, 23, 24). This time-period appears closer to the 3–7 days that fibrocytes take to appear in wounds, inflammatory sites, and fibrotic lesions (1, 2, 12, 15–17, 25–27). The other major advantage of a serum-free culture system is that all the components are defined and there are no unknown compounds from complex biological fluids such as serum. The fibrocytes from serum-free cultures appear to be comparable to those generated over 2 weeks in the presence of serum as they express the same markers, and secrete and respond to the same range of cytokines and growth factors (10, 23, 24).
We have also been able to use the serum-free culture of fibrocytes to identify factors that affect fibrocytes differentiation. We have found that glucose, immunoglobulins, insulin, some cytokines, and some extracellular matrix proteins, can regulate fibrocyte differentiation (9, 23, 28). We have also found that the plasma protein serum amyloid P (SAP) inhibits fibrocyte differentiation in vitro, and that in vivo SAP can reduce experimentally induced fibrosis in both lungs and heart, and also inhibit dermal wound healing (8, 26, 27, 29, 30).
2. Materials
All materials and solutions used should be sterilized either by irradiation, 0.2 μm filtering, or exposure to ethylene oxide gas. Also, all materials used should be (if possible) “virgin plastic” disposable cell culture grade; standard laboratory glassware should be avoided to reduce exposure to endotoxins, detergents, or other contaminants. All reagents are stored at room temperature unless indicated.
2.1. Isolation of Peripheral Blood Mononuclear Cells
Heparin vacutainer tubes (#367874, 150 U per tube; BD Bioscience; San Diego, CA).
Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ).
Phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2 HPO4, 1.8 mM KH2 PO4 pH 7.4).
15 ml and 50 ml polypropylene tubes.
Sterile plastic transfer pipettes.
2.2. Cell Culture Reagents
FibroLife basal medium (Lifeline Cell Technology, Walkersville, MD). Store at 4°C.
1 M HEPES (Sigma-Aldrich, St. Louis, MO). Store at 4°C.
100× Nonessential amino acids (NEAA, Sigma-Aldrich). Store at 4°C.
100 mM sodium pyruvate (Sigma-Aldrich). Store at 4°C.
200 mM glutamine (Hyclone, Thermo Scientific, Logan, UT). Store at −20°C.
Mixture of penicillin (10,000 U/ml) and streptomycin (10,000 μg/ml) (Hyclone). Store at −20°C.
100× ITS-3 (Sigma-Aldrich). Store at 4°C.
Flat-bottomed 24, 48, or 96-well tissue culture plates (BD Biosciences).
Eosin and methylene blue solutions diluted 1:5 (v:v) in water (Hema 3 Stain, Fisher Scientific, Middletown, VA).
2.3. Preparation of Serum Amyloid P
Human, mouse, rat, or pig serum (Gemini Bio-Products, West Sacramento, CA). Store at −80°C.
50 ml and 1000 ml, 0.2 μm tissue culture filter units (Nalgene, Thermo Scientific).
ECH Sepharose 4B beads (GE healthcare Life Sciences, Piscataway, NJ). Store at 4°C.
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, also called N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC or EDAC) (Sigma-Aldrich). Store desiccated at −20°C.
Phosphoethanolamine (Sigma-Aldrich). Store at −20°C.
Phosphocholine beads (Pierce, Thermo Scientific, Rockford, IL). Store at 4°C.
Agarose beads (SP Sepharose FF, GE-Healthcare Biosciences). Store at 4°C.
Amicon Ultra-15 ml centrifugal filters with 10 kDa cutoff (Millipore, Billerica, MA).
Amicon Ultra-0.5 ml centrifugal filters with 10 kDa cutoff (Millipore).
Solution A. 500 ml H2 O, pH 4.5 (∼1 μl of 12 N HCl in 500 ml H2 O).
Solution B. 500 ml of 500 mM NaCl (14.6 g in 500 ml H2 O).
Acetate buffer. 61.5 ml of 0.2 M acetic acid (11.55 ml of glacial acetic acid per liter), 13.5 ml of 0.2 M Na acetate (27.2 g Na acetate trihydrate per liter), and 4.38 g of NaCl (500 mM). Adjust to 150 ml total with H2 O.
100 mM Tris buffer. 15 ml of stock 1 M Tris–HCl pH 8 and 4.38 g NaCl, adjust to 150 ml with H2 O.
Tris/NaCl/CaCl2 binding buffer. 20 mM Tris pH 8, 140 mM NaCl, 2 mM CaCl2.
Tris/NaCl/EDTA elution buffer. 20 mM Tris pH 8, 140 mM NaCl, 10 mM EDTA.
20 mM sodium phosphate buffer pH 7.4. For 200 mM sodium phosphate buffer, mix 19 ml of 200 mM sodium phosphate monobasic (27.6 g NaH2 PO4·H2 O per liter) with 81 ml of 200 mM sodium phosphate dibasic (53.65 g Na2 HPO4·7H2 O per liter). Dilute to 20 mM with water.
Filter sterilize all solutions with 0.2 μm Nalgene vacuum filter sterilization units.
3. Methods
As Bucala and colleagues have recently published methods on the culture of fibrocytes in serum-containing conditions (22), we have focused this chapter on the culture of fibrocytes in serum-free conditions. This chapter also focuses on the culture of human fibrocytes, but we have also recently published protocols for the serum-free culture of murine fibrocytes from blood (24) and we and others have cultured fibrocytes from murine spleen preparations (20, 50).
3.1. Preparation of Human Peripheral Blood Mononuclear Cells
All procedures must comply with and have the specific approval of your institution's Institutional Review Board. All procedures must use appropriate BSL-2 laboratory facilities, in accordance with your institution's Of fice of Biosafety.
20 ml of human peripheral blood is collected by venipuncture from healthy adult volunteers using heparin as an anticoagulant (see Note 1). For 20 ml of blood, two 10 ml vacutainer tubes will be needed (see Note 2).
After the blood is collected, invert tubes 3–4 times to mix the blood and heparin.
If the PBMC are not to be isolated from the blood immediately, the tubes can be stored for 1–3 h in the dark at room temperature (20–23°C).
Transfer the blood from the vacutainer tube to a 50 ml polypropylene tube, either using a sterile plastic transfer pipette, or by simply pouring the blood into the 50 ml polypropylene tube. Then add an equal volume of PBS to a final volume of approximately 40 ml (see Note 3).
Add 3 ml of Ficoll-Paque to a 15 ml polypropylene tube, repeat for three additional tubes (see Note 4).
Gently layer 10 ml of diluted blood over the 3 ml Ficoll-Paque (see Note 5), repeat for the remaining three tubes. These volumes leave 2 ml of extra space which will help in the following steps when a transfer pipette is inserted through the plasma to remove the PBMC at the Ficoll interface, see step 11.
Isolate the PBMC by centrifugation at room temperature for 40 min at 400 × g, using swing-out buckets with screw top lids attached.
Carefully remove the centrifuge buckets from the centrifuge, so as not to disturb the PBMC at the Ficoll interface. For this step, and all subsequent steps, place the centrifuge buckets with the lids still attached inside a class II cabinet, and only then unscrew the lids and remove the tubes from the centrifuge bucket (see Note 6).
Carefully remove the PBMC from the diluted serum-Ficoll interface with a sterile plastic transfer pipette (see Note 7). This procedure should remove approximately 2–3 ml of liquid containing the PBMC. Add the PBMC to a new 15 ml polypropylene tube. Repeat the procedure for the three remaining tubes. We generally combine two tubes of recovered material into one 15 ml tube. Therefore, for four tubes of blood-Ficoll mix, two new 15 ml polypropylene tubes will be required.
Add 10 ml of PBS to each tube, mix by inversion, and collect the PBMC by centrifugation for 10 min at 300 × g at room temperature (see Note 8).
Pour off the excess PBS and resuspend the cell pellet with 1–2 ml of PBS, and then add an additional 10 ml of PBS to each tube. Recentrifuge cells for 10 min at 300 × g.
Again resuspend the cell pellet, and then combine the four cell pellets into one tube, and add PBS to 13 ml total (see Note 9).
Repeat the centrifugation step.
Repeat the process of resuspending the cells in PBS and collecting the cells by centrifugation for a total of six times (see Note 10).
After the sixth centrifugation step, resuspend the PBMC in 10 ml of PBS and count the cells with a hemocytometer. Collect the cells by centrifugation as above. At this point the cells can be resuspended in 1 ml Fibrolife serum-free medium (see recipe below) and stored on ice. If necessary, the PBMC can be kept in a covered ice bucket in a cold-room for up to 24 h, although we rarely store cells for more than 3 h. We recover approximately 20 × 106 cells from 20 ml of blood.
3.2. Differentiation of Fibrocytes in Serum-Free Culture
Prepare FibroLife serum-free medium (FibroLife-SFM) by mixing 47.0 ml FibroLife basal medium in a 50 ml polypropylene tube with 0.5 ml of each of the following supplements; HEPES, NEAA, sodium pyruvate, glutamine, penicillin/streptomycin, and ITS-3 (see Note 11).
Resuspend the PBMC to 5 × 105 cells/ml in FibroLife-SFM (see Note 12). We have also shown that density has an effect on fibrocyte differentiation, and that PBMC cultured between 2.5 and 5 × 105 cells/ml is optimal for fibrocyte differentiation (24).
Add 0.1 ml cell suspension per well to 96-well plates, along with 0.1 ml of Fibrolife-SFM or Fibrolife-SFM containing a test compound.
Incubate cells in a humidified incubator containing 5% CO2 at 37°C. Fibrocytes will begin to be visible by microscopy in approximately 2 days.
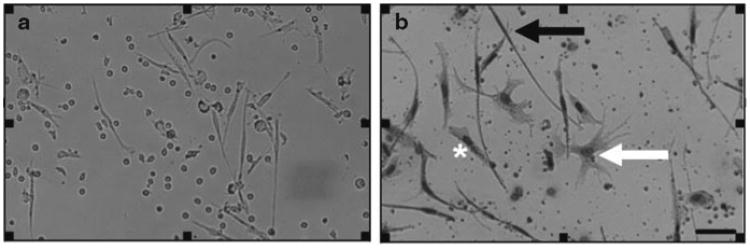
After 5 days, fibrocytes will be very obvious on a low power inverted microscope, due to their elongate morphology (50–200 μm long spindle-shaped cells), compared to small round lymphocytes (8–10 μm cells), dendritic cells (10–15 μm cells, with many dendritic-like processes protruding from the cell), and larger irregular-shaped macrophages (15–20 μm cells, with a large nucleus and pronounced cytoplasm) (Fig. 1a). We observe approximately 1,000–2,000 fibrocytes per 1 × 105 PBMC (see Note 13).
At this point the cells can be air-dried, fixed, stained, and then stored at room temperature in the dark. We have plates fixed and stained in 2001 that are still easily viewable in 2010.
Remove the liquid either by aspirating off the liquid with an eight (or 12)-well multichannel pipette, or simply by flicking out the liquid into a biosafety bag containing paper tissues to soak up the excess liquid. Then invert the plate over the vents inside a class II safety cabinet and air dry the plates for at least 2 h. Cells can then be fixed by adding 0.1 ml methanol to each well using a multichannel pipette and leaving the plate for 10 min at room temperature. Methanol is then flicked out as above, and the plates air dried again (see Note 14).
Stain the cells by adding 0.1 ml of the diluted solution of eosin to each well with a multichannel pipette. Incubate the plate for 1–2 min. Then flick out the eosin and add 0.1 ml of the diluted solution of methylene blue to each well. Again leave for 1–2 min and then flick out the liquid, and gently rinse the whole plate with standard distilled water, and then air dry the plate. Store plates in the dark at room temperature.
Fibrocytes can then be counted using a standard inverted microscope (Fig. 1b). We routinely count duplicate wells for fibrocytes from five different 900 μm diameter fields per well. All cultures are counted by at least two independent observers blinded to the experimental design. If necessary, macrophages, lymphocytes, and dendritic cells can also be enumerated in the same wells. Fibrocytes are defined as adherent 50–200 μm long spindle-shaped cells with an oval nucleus, as described previously (8– 10, 23).
Fig. 1.
Appearance of human fibrocytes in serum-free conditions. (a) Viable human PBMC cultured in SFM for 5 days. (b) PBMC air-dried and stained with eosin and methylene blue. Solid arrow points to a fibrocyte, white arrow points to a dendritic cell, and asterisk is to the left of a macrophages. Bar is 50 μm.
3.3. Preparation of Serum Amyloid P from Serum or Plasma
We have previously shown that fibrocyte differentiation is inhibited by a variety of molecules but that SAP appears to be the dominant inhibitor of fibrocyte differentiation (8, 23, 26, 29). The original methods used to isolate SAP from plasma, serum, or amyloid deposits used a variety of techniques. These methods included using ultracentrifugation through sucrose gradients (31); chromatography using cross-linked dextran (Sephadex) (32) or polyacrylamide (Bio-Gel P) (33) beads; and calcium-dependent binding to agarose (34, 35) or phosphoethanolamine (36, 37). However, agarose varies in the ability to bind SAP (37, 38), due to the differential amounts of pyruvate present in the agarose preparations (39). Therefore, as the calcium-dependent binding of human and murine SAP to phosphoethanolamine beads is specific, this method has become the standard matrix to purify SAP from human and murine serum (37, 40).
3.4. Preparing Phosphoethanolamine Beads
Day 1
Take 25–30 ml of ECH-Sepharose 4B gel slurry and place it in a 50 ml tube top filter device (Nalgene). Apply vacuum, and rinse first with 40 ml of the pH 4.5 H2 O wash (solution A), followed by 40 ml of the 500 mM NaCl wash (solution B). Repeat this alternating wash process four times.
Dissolve 100–150 mg of phosphoethanolamine in 25 ml pH 4.5 H2 O (solution A) and add to the ECH-Sepharose slurry contained in the top of the filtration device. Transfer slurry to a 50 ml polypropylene tube.
Add 400–500 mg EDC (Sigma) powder to the Sepharose/phosphoethanolamine slurry and mix using a rotary mixer.
Leave overnight at 4°C on a rotary mixer.
Day 2
5. Recover the beads in the 50 ml polypropylene tube by centrifugation at 300 × g for 60 s. Pipette off the excess liquid, do not pour off liquid as beads will be lost. Then wash the beads with 25 ml acetate buffer. Mix for 2 min, spin 300 × g for 60 s, and pipette off excess liquid. Next, add 25 ml Tris buffer, and repeat mixing, centrifugation, and removal of liquid steps.
6. Repeat washes three additional times (4 × 25 ml washes for each wash buffer), alternating between the acetate and Tris wash buffers.
7. Wash beads as above twice with 25 ml H2 O by centrifugation at 300 × g for 60 s, and remove excess liquid. Follow with two washes in 25 ml of Tris/NaCl/CaCl2 binding buffer.
8. Store beads at 4°C in an equal volume Tris/NaCl/CaCl2 binding buffer.
All batches of phosphoethanolamine beads should be tested for their ability to bind to commercial SAP preparations. Take 50 μl of the phosphoethanolamine bead slurry and mix with 200 μl of 50 μg/ml SAP. Incubate for 60 min and then separate the beads and non-bound material by centrifugation. Analyze the presence of SAP in the unbound fraction, following the protocols in Subheading 3.5, step 9.
3.5. Purification of SAP from Human, Murine, Rat, or Pig Serum
This procedure describes a simple batch process. The following purification can also be performed using column chromatography following the recommended protocols of the manufacturers of the specific columns.
Remove 100 ml of human, mouse, rat, or pig serum from −80°C and defrost overnight at 4°C.
Mix serum by inversion and then pipette 12 ml of serum into eight 15 ml polypropylene tubes, and add 3 ml of phosphoethanolamine bead slurry.
Mix at room temperature for at least 1 h, using a rotary mixer.
Spin down at 300 × g for 60 s and remove supernatant (see Note 15).
Wash beads by adding 10 ml Tris/NaCl/CaCl2 binding buffer. Mix for 10 min at room temperature, using a rotary mixer. Centrifuge at 300 × g for 60 s and remove supernatant. To increase bead recovery, one can perform an extra centrifugation step on the supernatant before discarding the supernatant.
Add 2 ml of Tris/NaCl/CaCl2 binding buffer to resuspend the beads in each tube and then combine the beads from two tubes. This reduces the amount of buffer used for subsequent steps. Repeat wash procedure with 10 ml Tris/NaCl/CaCl2 binding buffer at least four times.
Add an equal volume of Tris/NaCl/EDTA elution buffer to the beads and combine beads into one tube. Mix overnight at 4°C using a rotary mixer. Next day, centrifuge at 300 × g to pellet the beads and remove elution buffer. The elution buffer now contains the purified SAP. Repeat elution by adding fresh elution buffer to the beads and mix for 30 min at room temperature. Centrifuge beads and recover the elution buffer and combine with the first elution. Then centrifuge the elution buffer at 1,000 × g to remove any remaining beads.
Desalt and concentrate the elution buffer containing the SAP using a 15 ml centrifugal filter device with 10 kDa cutoff. Prewash the device with 10 ml H2 O by centrifugation for 5 min at 3,000 × g to remove residual glycerol present on the filter. Repeat the wash step twice with 10 ml of 20 mM sodium phosphate buffer pH 7.4. Apply the combined elution buffer containing the SAP to a centrifugal filter and add 20 mM Na phosphate buffer to 15 ml final volume. Centrifuge for 10 min at 3,000 × g, so that only approximately 1 ml of material is in the upper filter chamber. Add 14 ml of 20 mM Na phosphate buffer and repeat the buffer exchange at least three times.
At this point the material can be tested for purity and concentration using PAGE and/or western blotting using anti-SAP antibodies, as described previously (8, 27, 29, 38). Additionally, to quickly assess the protein concentration take 4 μl of sample and dilute with 396 μl of H2 O into an Eppendorf tube, and take 4 μl of sodium phosphate buffer and dilute with 396 μl of H2 O into an Eppendorf tube. Take the two tubes and perform an UV scan at 280 and 340 nm, using the phosphate buffer as the blank control. Additionally, the protein content can be assessed using a standard Bradford protein assay.
We find that a repeat purification step using phosphoethanolamine beads is usually necessary. Dilute the concentrate from step 8 with 20 mM Na phosphate buffer, pH 7.4, to a final volume of 4 ml. Add 6 ml of PE bead slurry with additional CaCl2 (8 μl of 1 M solution) to obtain a final concentration of 2 mM CaCl2 for 10 ml, and mix for at least 1 h at RT. Repeat steps 4–8.
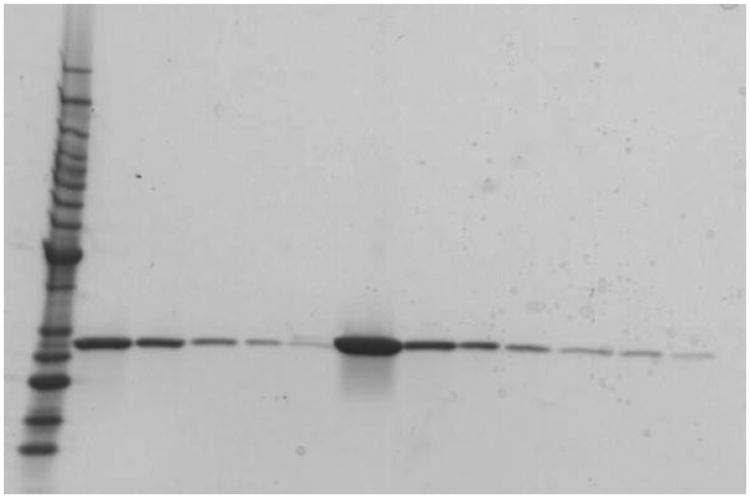
Dilute the final concentrated sample to 500 μg/ml SAP using 20 mM sodium phosphate buffer and store at 4°C. The final SAP preparation should be a single 27 kDa band when analyzed by PAGE on a 4–15% reducing gel and stained with Coomassie or silver stain (Fig. 2).
As an additional purification step use phosphocholine beads from Pierce/Thermo Scientific (Cat #20307; immobilized p-aminophenyl phosphoryl choline) to remove any residual levels of CRP from the purification. In humans and mice, the concentration of CRP in normal serum is only 1–2 μg/ml, therefore only small amounts of resin are needed. For rat serum, where the CRP levels can be as high as 300–500 μg/ml (41) large volumes of p-aminophenyl phosphoryl choline agarose beads may be required (see Note 16). Alternatively, pneumococcal C-polysaccharide linked beads can be used to remove any contaminating CRP from preparations of SAP (37, 42, 43).
Fig. 2.
Example of purified SAP from murine serum. Purified mouse SAP was analyzed by PAGE, on a 4–15% reducing gel, and stained with Coomassie. Lane 1 Protein molecular weight markers (BenchMark protein standards, Invitrogen, Carlsbad, CA). Lanes 2–6 300, 100, 30, 10 and 3 μg/ml human SAP loading controls. Lane 7 Undiluted murine SAP preparation. Lanes 8–13 Murine SAP preparation diluted 1/10, 1/20, 1/40, 1/80, 1/100, and 1/160 with sodium phosphate buffer, respectively.
3.6. Alternative Protocol Using Sepharose Beads
As discussed above, SAP binds to agarose in a calcium-dependent manner, but different preparations, brands, and batches of agarose have differing capacities to bind SAP. However, we have found that SP Sepharose FF appears to bind SAP efficiently irrespective of the batches tested between 2003 and 2010 (38).
Wash 30 ml pre-hydrated agarose beads (SP Sepharose FF) four times in 10 volumes of Tris/NaCl/CaCl2 binding buffer, by collecting the beads by centrifugation at 300 × g for 60 s. Store beads in Tris/NaCl/CaCl2 buffer at 4°C.
Resuspend the bead slurry in 10 ml Tris/NaCl/CaCl2 buffer and add 3 ml to a 15 ml conical polypropylene tube containing 12 ml serum.
Mix at room temperature for at least 1 h, using a rotary mixer.
Collect the beads by centrifugation and remove supernatant as in step 4 of Subheading 3.5. Add 10 ml Tris/NaCl/CaCl2 buffer and mix for 10 min at RT. Spin at 300 × g for 60 s and discard supernatant, as in step 5 of Subheading 3.5. To increase bead recovery, one can perform an extra centrifugation step on the supernatant before discarding the supernatant.
Repeat wash procedure four times.
Add an equal volume of Tris/NaCl/EDTA elution buffer to beads and combine into 1–2 tubes. Mix overnight at 4°C. Next day, collect the beads by centrifugation and remove supernatant. Repeat elution by adding fresh elution buffer to the beads and mix for 15 min at room temperature. Centrifuge the beads, collect the supernatant, and combine with the first elution.
Desalt and concentrate the eluted material as in step 8 of Subheading 3.5. Apply sample to centrifugal filter and fill to 15 ml using 20 mM Na phosphate buffer, pH 7.4. Concentrate by centrifugation to 1 ml and repeat buffer exchange at least three times.
Dilute the final concentrated samples to 500 μg/ml SAP using 20 mM sodium phosphate buffer and store at 4°C.
3.7. Desalting Commercial Human SAP
Many commercial preparations of human SAP (#565190, EMD-Calbiochem) or rat SAP (#1895-SA-050, R&D Systems) contain azide, detergent, or EDTA, which may interfere with many biological processes. Either a buffer control has to be used in all experiments, or a better solution is to desalt the SAP preparation by buffer exchange.
Wash two Amicon Ultra-0.5 ml centrifugal filters (this is a 10 kDa cut-off, so for a 130 kDa protein the loss is negligible) with 0.5 ml H2 O for 5 min at 10,000 × g. Then wash twice with 20 mM sodium phosphate pH 7.4. The liquid should pass through the filter into the bottom Eppendorf tube. Running at slower speeds and shorter time periods will prevent the filter from drying out, which is to be avoided (see Note 17).
Add 0.4 ml of the commercial SAP solution to the centrifugal filter. Centrifuge for 5–10 min, or until approx. 0.3 ml of the buffer has passed through the filter into the bottom Eppendorf.
Replace the lost liquid with 20 mM sodium phosphate pH 7.4 in the top filter and repeat four times.
When approximately 0.3 ml has passed through a fifth time, carefully remove the SAP solution from the top unit and replace the lost volume with 20 mM phosphate buffer.
Check the concentration of the solution by protein gel/Coomassie, using albumin and the original commercial SAP preparations as protein standards. This leaves the SAP in 20 mM sodium phosphate and in this buffer SAP is stable for many weeks/months at 4°C (see Note 18).
SAP is very stable in its 130 kDa pentameric form and only dissociates into its individual 27 kDa protomers when exposed to temperatures above 100°C in the presence of SDS (44, 45). In the presence of 10 mM calcium, SAP is also resistant to a variety of proteases (44). However, SAP will form decamers or aggregates above 1 mM calcium, or in the presence of 140 mM NaCl (46– 48). Nevertheless, in plasma and in solutions containing at least 10 mg/ml albumin SAP remains a pentamer, even in the presence of 2 mM calcium (48, 49). Therefore, SAP should be stored either in the presence of albumin or EDTA (to chelate calcium), or in 20 mM sodium phosphate (not PBS) to reduce the formation of decamers or aggregates.
Acknowledgments
We would like to thank Nehemiah Cox and Jeff Crawford for critical reading of the manuscript. This work was supported by NIH grant HL083029.
Footnotes
This procedure is usually performed by a licensed and/or trained phlebotomist.
We have also used EDTA tubes to collect blood for fibrocyte studies.
All procedures involving blood are performed inside class II biological safety cabinets, using sterile technique and materials. Diluting the blood reduces the viscosity of the blood which helps in the following separation steps.
Store Ficoll-Paque at room temperature, as Ficoll-Paque stored at 4°C has a density greater than the defined density of 1.077.
Care is to be taken to prevent mixing of the two liquids, as the PBMC are collected at the Ficoll-blood interface and a disturbed interface will be less efficient—read manufacturer's leaflets for additional directions on layering blood on density gradient liquids. These volumes can be scaled up for larger blood volumes using 10 ml Ficoll in 50 ml polypropylene tubes, and adding 40 ml of blood diluted with PBS.
This reduces the risk of exposure to aerosols if the tubes break during centrifugation.
Read manufacturer's leaflets for additional directions on removing the cell interface from density gradient liquids.
This process removes excess Ficoll which is toxic to cells after long exposure, along with any dead cells and platelets. These molecules and cells may also interfere in biological assays.
This reduces the amount of PBS need for the subsequent washes.
We have found that this number of steps is necessary to remove platelets.
These supplements are designed to promote cell survival and differentiation in the absence of serum. We have previously shown that glucose, sodium pyruvate, and insulin all affect fibrocyte differentiation (28). HEPES is an additional buffering agent, as serum has the capacity to buffer biological fluids. NEAA prolongs the viability of cells in culture and may reduce the biosynthetic burden on cells. Sodium pyruvate is an additional energy source, which is necessary for fibrocyte differentiation. ITS-3 contains insulin to promote the uptake of glucose; transferrin, an iron-transport protein necessary for cell differentiation and proliferation; selenium, an essential trace element normally found in serum; and albumin with oleic and linoleic acids, which promote cell differentiation.
As fibrocytes differentiate from highly adherent peripheral blood monocytes, it is an advantage to use medium that is cold to reduce the possibility of the monocytes adhering to the polypropylene tubes before being added to the cell culture plates.
These protocols can also be used to culture fibrocytes on glass slides for staining with antibodies (8, 10, 24).
Due to the inhalation hazard of methanol, ethanol can be used instead of methanol to fix the cells. Methanol and ethanol are not only effective fixatives but potent anti-biological agents.
Retain supernatant to check that the majority of the SAP has been removed.
Read manufacturer's data sheets for the amount of CRP bound per mg of p-aminophenyl phosphoryl choline beads.
Read manufacturer's data sheets for additional information on centrifugation speeds and times.
This procedure should only take a few 5 min centrifuge steps at room temperature, for a buffer exchange.
References
- 1.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 3.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 5.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 6.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 8.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;17:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 13.Balmelli C, Ruggli N, McCullough K, Summer field A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005;77:923–933. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- 14.Balmelli C, Alves MP, Steiner E, Zingg D, Peduto N, Ruggli N, Gerber H, McCullough K, Summer field A. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology. 2007;212:693–699. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 17.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 19.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth PJ, Koster H, Moosdorf R. CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract. 2005;201:301–304. doi: 10.1016/j.prp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Quan TE, Bucala R. Culture and analysis of circulating fibrocytes. Methods Mol Med. 2007;135:423–434. doi: 10.1007/978-1-59745-401-8_28. [DOI] [PubMed] [Google Scholar]
- 23.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351:62–70. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik-Mathuria B, Pilling D, Crawford JR, Gay AN, Smith CW, Gomer RH, Olutoye OO. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16:266–273. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilling D, Gomer RH. Regulatory pathways for fibrocyte differentiation. In: Bucala R, editor. Fibrocytes-new insights into tissue repair and systemic fibroses. World Scientific; Singapore: 2007. pp. 37–60. [Google Scholar]
- 29.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA. 2008;105:10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cathcart ES, Wollheim FA, Cohen AS. Plasma protein constituents of amyloid fibrils. J Immunol. 1967;99:376–385. [PubMed] [Google Scholar]
- 32.Thompson AR, En field DL. Human plasma P component: isolation and characterization. Biochemistry. 1978;17:4304–4311. doi: 10.1021/bi00613a030. [DOI] [PubMed] [Google Scholar]
- 33.Binette P, Binette M, Calkins E. The isolation and identification of the P-component of normal human plasma proteins. Biochem J. 1974;143:253–254. doi: 10.1042/bj1430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepys MB, Dash AC. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977;1:1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- 35.Painter RH. Evidence that C1t (amyloid P-component) is not a subcomponent of the first component of complement (C1) J Immunol. 1977;119:2203–2205. [PubMed] [Google Scholar]
- 36.Pontet M, Engler R, Jayle MF. One step preparation of both human C-reactive protein and CIt. FEBS Lett. 1978;88:172–175. doi: 10.1016/0014-5793(78)80167-7. [DOI] [PubMed] [Google Scholar]
- 37.de Beer FC, Pepys MB. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50:17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- 38.Gomer RH, Pilling D, Kauvar L, Ellsworth S, Pissani S, Real L, Ronkainen SD, Roife D, Ma F, Davis SC. A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs. Wound Repair Regen. 2009;17:397–404. doi: 10.1111/j.1524-475X.2009.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hind CR, Collins PM, Renn D, Cook RB, Caspi D, Baltz ML, Pepys MB. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984;159:1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwalbe RA, Dahlback B, Coe JE, Nelsestuen GL. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry. 1992;31:4907–4915. doi: 10.1021/bi00135a023. [DOI] [PubMed] [Google Scholar]
- 41.de Beer FC, Baltz ML, Munn EA, Feinstein A, Taylor J, Bruton C, Clamp JR, Pepys MB. Isolation and characterization of C-reactive protein and serum amyloid P component in the rat. Immunology. 1982;45:55–70. [PMC free article] [PubMed] [Google Scholar]
- 42.Tillett WS, Francis T. Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bach BA, Gewurz H, Osmand AP. C-reative protein in the rabbit: isolation, characterization and binding affinity to phosphocholine. Immunochemistry. 1977;14:215–219. doi: 10.1016/0019-2791(77)90197-5. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita CM, Gewurz AT, Siegel JN, Ying SC, Hugli TE, Coe JE, Gupta RK, Huckman R, Gewurz H. A protease-sensitive site in the proposed Ca(2+)-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1992;1:700–709. doi: 10.1002/pro.5560010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E. Amyloid P component. A critical review. Amyloid. 1997;4:274–295. [Google Scholar]
- 46.Baltz ML, de Beer FC, Feinstein A, Pepys MB. Calcium-dependent aggregation of human serum amyloid P component. Biochim Biophys Acta. 1982;701:229–236. doi: 10.1016/0167-4838(82)90118-2. [DOI] [PubMed] [Google Scholar]
- 47.Coker AR, Purvis A, Baker D, Pepys MB, Wood SP. Molecular chaperone properties of serum amyloid P component. FEBS Lett. 2000;473:199–202. doi: 10.1016/s0014-5793(00)01530-1. [DOI] [PubMed] [Google Scholar]
- 48.Hutchinson WL, Hohenester E, Pepys MB. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol Med. 2000;6:482–493. [PMC free article] [PubMed] [Google Scholar]
- 49.Sorensen IJ, Andersen O, Nielsen EH, Svehag SE. Native human serum amyloid P component is a single pentamer. Scand J Immunol. 1995;41:263–267. doi: 10.1111/j.1365-3083.1995.tb03562.x. [DOI] [PubMed] [Google Scholar]
- 50.Crawford JR, Pilling D, Gomer RH. Improved serum-free culture conditions for spleen-derived murine fibrocytes. J Immunol Methods. 2010;363(1):9–20. doi: 10.1016/j.jim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]