Abstract
Background
Emerging evidence suggests that “fibroblast like cells” (FLC) may play a role in the regulation of gastrointestinal (GI) motor function. FLC are ultrastructurally distinct from other interstitial cells, including interstitial cells of Cajal (ICC), and express small-conductance Ca2+-activated K+ channels (SK3). In mice, platelet-derived growth factor receptor α (PDGFRα) antibody has also been shown to label FLC. The aims of this study were to determine the morphology and distribution of PDGFRα-immunoreactive (ir) FLC in human gastric muscle and to determine if FLC are altered in gastroparesis, where ICC are reduced.
Methods
Full thickness gastric body biopsies from 5 healthy subjects, 10 diabetic and 10 idiopathic gastroparesis patients were immunolabeled using SK3 and PDGFRα staining for FLC and Kit staining for ICC. Intramuscular FLC and ICC were quantified.
Key Results
Intramuscular PDGFRα-ir cells had slender cell bodies and long, thin processes and were more abundant in the longitudinal compared to the circular muscle. In the region of myenteric plexus, FLC had smaller, rounder cell bodies with 3-4 processes and formed networks, often around ganglia. All SK3-ir cell structures showed complete overlap with PDGFRα-ir. FLC were in close proximity to ICC, but their cell bodies did not overlap. No differences were seen in the distribution, morphology, or overall numbers of FLC in gastroparesis patients.
Conclusions & Inferences
In conclusion, PDGFRα identifies FLC in human gastric smooth muscle. FLC were not altered in distribution or overall numbers in gastroparesis. Further studies are required to determine their role in human GI function.
Keywords: enteric nervous system, interstitial cells of Cajal, smooth muscle, fibroblast-like cells, gastroparesis, platelet-derived growth factor receptor
Coordinated gastrointestinal (GI) motor activity requires a complex interplay of various cell types including inhibitory and excitatory nerves, glia, smooth muscle cells (SMC), immune cells and interstitial cells of Cajal (ICC).1 ICC generate slow waves, set the smooth muscle membrane potential gradient, and are mechanotransducers.2 ICC express Kit receptor tyrosine kinase.3 Another type III receptor tyrosine kinase expressed in the GI tract is platelet-derived growth factor receptor (PDGFR). This receptor has two subtypes (α and β) and four known ligands (A, B, C, and D as well as AB).4 Both receptor subtypes are expressed in mesenchymal cells which are common precursors of ICC and longitudinal SMC.5 PDGFRα is expressed in subepithelial myofibroblasts and PDGFRβ in vascular pericytes in the mature GI tract.4 PDGF signaling plays critical roles in mammalian organogenesis4, 6 and murine GI villous morphogenesis.7 Selective inhibition of PDGFR has been shown to suppress longitudinal SMC differentiation.5
Interstitial cells with fibroblast like ultrastructure have been described as “fibroblast-like cells (FLC)”. Their initial description conflicted on their contacts with SMC and nerve varicosities8, 9. Subsequently, Horiguchi et al. described gap junctions between FLC and SMC in Kit mutant W/Wv mice.10 At the same time, using sialylated glycoprotein CD34 immunoreactivity (ir), FLC were localized to be adjacent to but distinct from ICC in human small intestine.11 CD34-ir and Kit-ir cells were exclusive, but their processes closely intermingled.12 Later, SK3 (small conductance calcium-activated potassium channels type 3)-ir was described to closely overlap with CD34-ir in human GI musculature and these SK3-ir CD34-ir cells remained preserved in human diseases where ICC were lacking.13 SK channels are involved in control of neuronal excitability14, 15 and have potential functional significance in GI musculature,16-18 although conditional genomic deletion of SK3 did not result in overt phenotypic changes in mice.19 Recently, Iino et al. described PDGFRα-ir in FLC in murine GI tract which overlapped strongly with SK3-ir.20 Consistent with prior literature, these FLC were distinct but closely associated with ICC, enteric nerve fibers and myenteric ganglia, and these cells persisted in ICC deficient Wv/Wv mutant mice.20, 21 Recent work has suggested a potential role of PDGFRα- and SK3-ir FLC in inhibitory neurotransmission in mice.22, 23
We have recently shown that together with other cellular abnormalities, all patients studied with diabetic (DG) and idiopathic gastroparesis (IG) have ICC abnormalities24 with half of these patients showing significant decrease in ICC numbers.25 The distribution of the PDGFRα-ir FLC in human GI tract in health or disease is unknown. Nor is it known if there is co-localization of PDGFRα staining and SK3 staining cells in humans as seen in animal models. Furthermore, it is unknown if gastroparesis patients with and without preserved ICC numbers have a drop-out in the number of PDGFRα-ir FLC. The aims of this study were therefore to describe the distribution and morphology of the FLC in human gastric muscle (circular and longitudinal) and myenteric plexus region using antibodies against PDGFRα and SK3 and to determine if FLC are altered in number or distribution in DG and IG.
Methods
Subject enrollment and histology studies
Gastroparesis patients enrolled in this study were patients >18 years of age with symptoms of at least 12 week duration, had delayed gastric emptying on scintigraphy (>60% retention at 2 h or >10% retention at 4 h), in the absence of evidence of gastric outlet obstruction. Exclusion criteria included presence of active inflammatory bowel disease, eosinophilic gastroenteritis, neurological conditions, acute liver or renal failure, and history of total or subtotal gastric resection. Full thickness gastric biopsy tissue was prospectively obtained from 10 DG and 10 IG patients undergoing surgery for placement of a gastric stimulator and from 5 age- and sex-matched patients undergoing sleeve gastrectomy/duodenal switch surgery after institutional review board-approved protocols. The biopsies were collected in all subjects from the anterior aspect of the stomach, midway between the greater and lesser curvatures where the gastroepiploic vessels meet using a standardized protocol. These patients are enrolled in the Gastroparesis Clinical Research Consortium (GpCRC) and extensive details on tissue acquisition and processing are provided elsewhere.25 The antibodies used for studying FLC are shown in Supplementary Table 1. Co-localization between FLC markers (both PDGFRα and SK3) and c-Kit was performed in all 5 normal controls. Co-localization between FLC marker (PDGFRα) and PGP9.5 was performed in all 5 normal controls. PDGFRα and SK3 co-localization was performed in 10 gastroparesis patients (5 diabetic and 5 idiopathic) and the controls.
Light microscopy
On arrival at the laboratory, samples were washed 5 times over 1 h in 1X phosphate-buffered saline (PBS) and immersed overnight at 4°C in 1X PBS solution containing 30% sucrose (Sigma-Aldrich, St Louis, MO). The specimens were cut in cross section and frozen in Tissue-Tek OCT Compound (Electron Microscopy Sciences, Hatfield, PA). Cryostat sections, 12 μm in thickness, were cut and slides were stored at −80°C until use. Further detailed protocols used are shown in Supplementary Table 2. Negative and positive controls for PDGFRα and SK3 immunoreactivity are shown in supplementary figures 1 and 2 respectively.
Quantification
Three nonadjacent slides were analyzed per patient in a blinded fashion by MG and CB. For quantitative assessment of FLC bodies, 38-40 images per subject at 40X magnification (each 348 μm by 262 μm) were analyzed. Cell bodies were then manually identified and counted. For FLC, a cell body was defined as a PDGFRα positive structure with a 4', 6-diamidino-2-phenylindole (DAPI)-positive nucleus and bipolar or multipolar processes. Due to the distribution and density of FLC in the myenteric plexus region, manual quantification in myenteric plexus was not possible and only the circular muscle layer was used for quantification.
Statistical analysis
Quantification data are represented as mean ± SEM. Statistical significance was determined using Kruskal–Wallis one-way analysis of variance with Newman–Keuls multiple comparison test. A P value of < 0.05 was considered statistically significant.
Results
Distribution of PDGFRα-ir FLC in the gastric body of normal human subjects
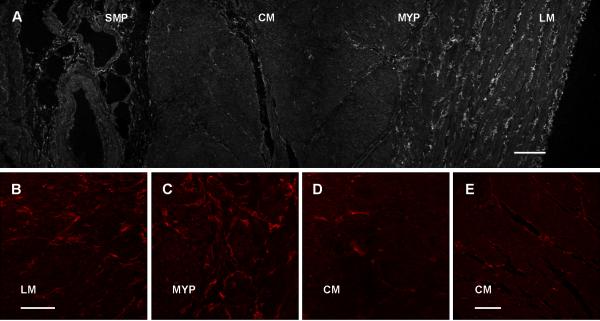
PDGFRα-ir cells were found to be distributed in the circular and longitudinal muscle layers and in the myenteric plexus region. PDGFRα-ir cells were clearly more abundant in longitudinal muscle compared to the circular muscle (Fig. 1A, 1B, 1D and 1E). In the muscle layers, PDGFRα-ir cells were found in the interstitium between the muscle bundles with processes that ran parallel to muscle fibers (Fig. 1A for longitudinal muscle, 1E for circular muscle). The cells had slender cell bodies with 2-4 long, thin processes (Fig. 1B, D, E). Together with the long processes emerging from the cell body, several of the cells also had smaller processes that projected for a shorter distance from the cell bodies. PDGFRα-ir cells in the myenteric plexus region had smaller, rounder, multipolar cell bodies with 3-4 long, thin processes that appeared to form networks (Fig. 1C). PDGFRα-ir cells were also present in the submucosa (Fig. 1A) where they had a flat stellate shaped appearance with several processes running parallel to the epithelial membrane. The submucosal PDGFRα-ir cells had weaker labeling compared to the cells in the muscle layers.
Figure 1.
PDGFRα immunoreactivity shown as a low power tiled image across the thickness (A) of the human gastric body (Scale = 200 μm). Higher power images are shown for the longitudinal muscle (B), myenteric plexus region (C), circular muscle (D) {Scale = 100 μm}. Panel E shows circular muscle at the same orientation and magnification as longitudinal muscle in panel A (E) {Scale=200 μm}. SMP=submucosal plexus, CM=circular muscle, MYP=myenteric plexus, LM=longitudinal muscle.
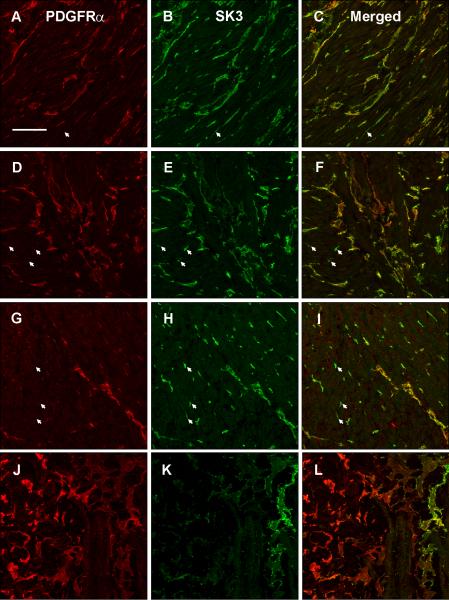
Overlap between PDGFRα- and SK3-ir FLC in the gastric body of normal human subjects
SK3-ir was stronger in general than PDGFRα-ir but SK3-ir and PDGFRα-ir tended to occur in the same structures (Fig. 2 C, F, I). However there were cells that were SK3-ir (arrowhead, Fig. 2B, 2E, 2H) in both the circular and longitudinal muscle layers as well as in the myenteric plexus region and not PDGFRα-ir. In the muscle layers and myenteric plexus region, all PDGFRα-ir cells expressed SK3-ir both in the cell bodies and processes (Fig. 2C, F, I). In contrast, in the submucosa, most PDGFRα-ir cells (Fig. 2J) were negative for SK3-ir (Fig. 2L); indeed SK3-ir was markedly diminished in the submucosa (Fig. 2K).
Figure 2.
PDGFRα immunoreactivity in longitudinal muscle (A), myenteric plexus region (D), circular muscle (G) and submucosal plexus (J). SK3 immunoreactivity in longitudinal muscle (B), myenteric plexus region (E), circular muscle (H) and submucosal plexus (K). Overlap between PDGFRα and SK3 immunoreactivity is shown in panel C for longitudinal muscle, panel F for the myenteric plexus region, panel I for circular muscle and panel L for the submucosal plexus. Arrows represent SK3-ir cells that are not PDGFRα-ir. Tissue obtained from the normal gastric body of a human subject. Scale = 100 μm
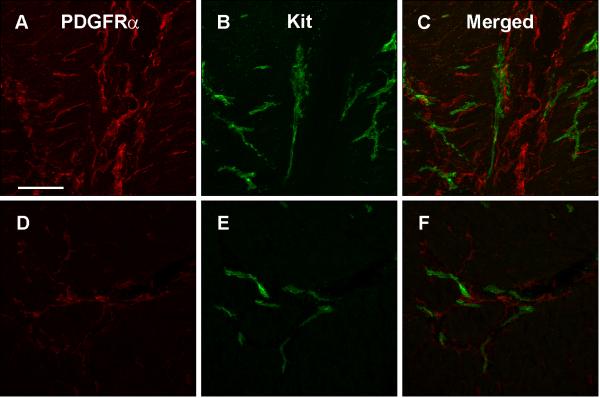
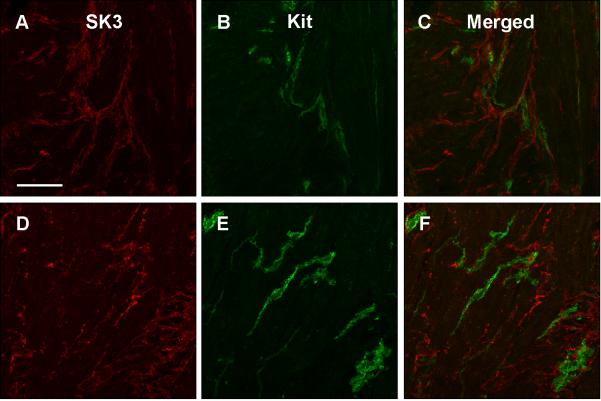
Relationship between Kit-ir ICC and PDGFRα-ir FLC in the gastric body of normal human subjects
In the muscle layers and the myenteric plexus region, ICC and FLC were often in close proximity to each other but their cell bodies did not overlap as determined with both PDGFRα-(Fig. 3C, F) and SK3-ir (Fig. 4C, F). The processes of ICC and FLC often came in close proximity and seemed to overlap at places but no definite direct connection between ICC and FLC cell processes was seen within the magnification allowed by light microscopy (Fig. 3C, F and Fig. 4C, F).
Figure 3.
PDGFRα immunoreactivity in myenteric plexus region (A) and circular muscle (D). Kit immunoreactivity in myenteric plexus region (B) and circular muscle (E). Overlap between PDGFRα and Kit immunoreactivity is shown for the myenteric plexus region (C) and the circular muscle (F). Tissue obtained from the normal gastric body of a human subject. Scale = 100 μm
Figure 4.
SK3 immunoreactivity in myenteric plexus region (A) and circular muscle (D). Kit immunoreactivity in myenteric plexus region (B) and circular muscle (E). Overlap between SK3 and Kit immunoreactivity is shown for the myenteric plexus region (C) and circular muscle (F). Tissue obtained from the normal gastric body of a human subject. Scale = 100 μm
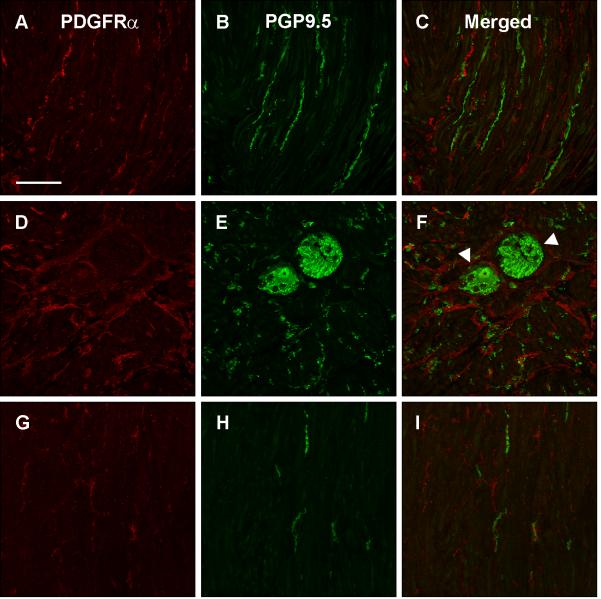
Relationship between nerves fibers (PGP9.5) and PDGFRα-ir FLC in the gastric muscle layers of normal human subjects
The relationship between PDGFRα-ir cells and PGP9.5-ir nerve fibers and ganglion cells is shown in Fig. 5. The PDGFRα-ir cells formed networks around ganglia in several areas of the myenteric plexus (arrowhead, Fig. 5F). In the intramuscular layers, PDGFRα-ir processes appeared to come in close proximity to some PGP9.5-ir nerve fibers (Fig. 5C, I).
Figure 5.
PDGFRα immunoreactivity in longitudinal muscle (A), myenteric plexus region (D), and circular muscle (G). PGP9.5 immunoreactivity in longitudinal muscle (B), myenteric plexus region (E), and circular muscle (H). Overlap between PDGFRα and PGP9.5 immunoreactivity is shown for the longitudinal muscle (C), myenteric plexus region (F), and circular muscle (I). Arrowhead represents PDGFRα-ir cells forming networks around ganglia in several areas of the myenteric plexus. Tissue obtained from the normal gastric body of a human subject. Scale = 100 μm
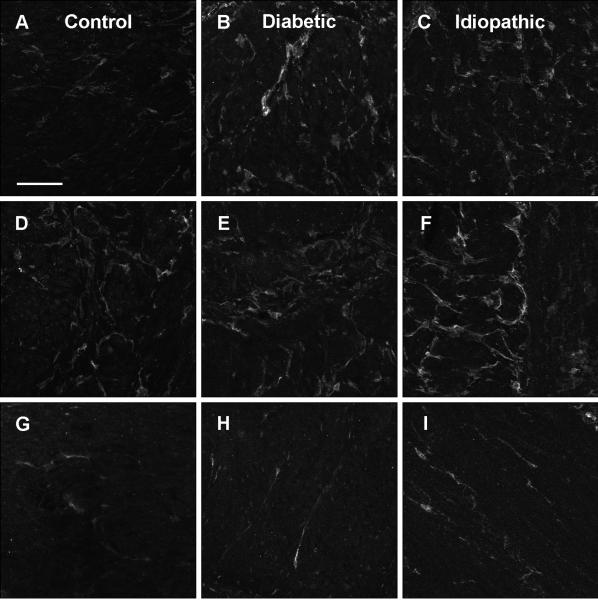
Distribution of PDGFRα-ir FLC in gastroparesis
PDGFRα-ir FLC had a similar distribution and morphology in diabetic and idiopathic gastroparesis subjects as in normal human subjects in both the muscle layers (Fig. 6A-C and G-I) and the myenteric plexus region (Fig. 6D, E, F). On quantification, there were no differences in circular muscle PDGFRα-ir FLC between gastroparetic subjects and controls (Supplementary Table 3). When patients with and without decrease in ICC counts were compared, no differences in circular muscle PDGFRα-ir FLC were seen for both diabetic (Supplementary Fig. 3) and idiopathic gastroparesis (Supplementary Fig. 4).
Figure 6.
PDGFRα immunoreactivity in longitudinal muscle (A), myenteric plexus region (D), and circular muscle (G) from a control subject. PDGFRα immunoreactivity in longitudinal muscle (B), myenteric plexus region (E), and circular muscle (H) from a diabetic gastroparesis subject. PDGFRα immunoreactivity in longitudinal muscle (C), myenteric plexus region (F), and circular muscle (I) from an idiopathic gastroparesis subject. No differences were seen between the 3 groups. Scale = 100 μm
Discussion
This current study provides the first description of the morphology and distribution of FLC in human GI smooth musculature using PDGFRα-ir. All PDGFRα-ir cells in the muscle layer and in the myenteric plexus region expressed SK3-ir, previously used to localize FLC in both humans and mice.13, 20, 21 Thus, PDGFRα-ir can also be utilized to localize FLC in human GI smooth muscle. FLC in the human stomach were distinct from but were in close apposition to ICC processes. This observation is similar to the observations made by others in recent descriptions of FLC in mice.11, 20, 21, 23 In our study, we were unable to show co-labeling of PDGFRα-ir and Kit-ir. This observation is different from what has been reported by Klemm et al in guinea pig stomach smooth muscle where 2.5% of SK3-ir cells displayed Kit-ir and 4.2% of Kit-ir cells displayed SK3-ir.26 Whether a small subset of such cells exists in the human GI tract will require further study. However given that the majority of the PDGFRα-ir FLC were exclusive from Kit-ir ICC, these cells represent a new cell compartment in human GI smooth muscle.
A new finding emerging from this study was that the density of FLC was greater in longitudinal smooth muscle when compared to the circular smooth muscle. This finding may have important functional implications. The bi- or multipolar processes of these cells appear to form networks with other FLC and come in close proximity with ICC processes suggesting that these cells may be in communication. In addition, their close proximity to the PGP9.5-ir nerve fibers and networking around the ganglia suggests a potential role in neuromuscular transmission.23 There is strong evidence that ICC play a role in nitrergic neurotransmission,27-29 however contrary evidence also exists.30, 31 Both ICC and FLC express nitric oxide-sensitive soluble guanylate cyclase and both have proximity to the nNOS-ir nerve fibers, suggesting that FLC might also be responsive to nitrergic signals.32 Indeed, expression of nitric oxide-sensitive guanylate cyclase in ICC and FLC is sufficient to sustain normal nitrergic responses and whole-gut transit in mice with conditional deletion of this gene in GI smooth muscles.33 Recently, the PDGFRα-ir cells from mice colon were shown to generate large amplitude transient outward K+ current spontaneously and in response to purines that was blocked by apamin.22 The apamin-sensitive inhibitory junction potential was maintained in W/Wv mice where intramuscular ICC are greatly diminished. Also, gap junctions between FLC and FLC-smooth muscle were preserved in the small intestine of ICC deficient Kit mutant mice.10 The relative paucity of FLC in human circular smooth muscle compared to the longitudinal muscle will need to be considered when elucidating the electrophysiologic function of FLC in human gastric inhibitory neurotransmission. Furthermore, these differences may explain in part the variable effects of caveolin-1 deletion on the response to NO and apamin-sensitive mediators in circular or longitudinal smooth muscle34 and different relative roles of NO between circular and longitudinal muscle compartment.35, 36
In this study we also explored a potential role for PDGFRα-ir FLC in gastroparesis. However, PDGFRα-ir FLC were not altered in morphology or distribution in gastroparesis subjects with or without ICC loss. On quantification, the average number of PDGFRα-ir FLC was similar in gastroparetics with or without ICC loss and to those in controls. These data suggest that PDGFRα-ir FLC may not be involved in the pathophysiology of gastroparesis. Previously Vanderwinden et al. had shown that interstitial SK3-ir cells were abundantly present in the interstitium between the hypertrophied muscle fibers in patients with infantile hypertrophic pyloric stenosis where ICC were lacking.13 Additionally, they also looked at the aganglionic colonic segment of patients with Hirschsprung's disease where the distribution of SK3-ir cells was similar to the ganglionic segment whereas ICC were depleted in the aganglionic segments. Similarly work from ICC deficient Wv/Wv mutant mice showed preserved presence of FLC.21 In contrast, a study of small and large bowel tissue from patients with chronic intestinal pseudo-obstruction, showed CD34-ir was depleted in a proportion of patients.37
In conclusion, PDGFRα-ir FLC are present in the normal human stomach in both muscle layers and in the myenteric plexus region. However, unlike other cell types such as ICC, smooth muscle and enteric nerves that have been shown to be affected in patients with gastroparesis, the PDGFRα-ir FLC population appears to be intact in gastroparesis. PDGFRα-ir FLC were relatively more abundant in the longitudinal muscle layer compared to the circular muscle layer. Their morphologic features such as long processes and formation of networks with other FLC and presence of gap junctions with smooth muscle cells, their topographic localization in close proximity to ICC, nerve fibers and ganglia raise the possibility that this cell type may also participate in the regulation of normal human gastrointestinal motility, as proposed in mice, however further studies will be required to determine if this is indeed the case and their relative roles in circular and longitudinal muscle.
Supplementary Material
Acknowledgment
Authors thank Kristy Zodrow and Peter Strege for an excellent technical support. This work was supported by P01DK68055, R01DK57061, P30DK84567, and the Gastroparesis Clinical Research Consortium (GpCRC) (grants U01DK073983, U01DK073975, U01DK073985, U01DK074007, U01DK073974, U01DK074008).
Footnotes
Disclosures: none to declare for all authors
Authors Contributions:
Madhusudan Grover, Cheryl E. Bernard, Gianrico Farrugia: performed the research, designed the research study, contributed essential reagents or tools, analyzed the data, wrote the paper Pankaj J. Pasricha, Henry P. Parkman, Thomas L. Abell, Linda A. Nguyen, William Snape: performed the research, designed the research study, analyzed the data, wrote the paper K. Robert Shen, Michael Sarr, James Swain, Michael Kendrick, Simon Gibbons, Tamas Ordog: designed the research study, analyzed the data, wrote the paper
Contributor Information
Madhusudan Grover, Mayo Clinic, Rochester, MN.
Cheryl E. Bernard, Mayo Clinic, Rochester, MN
Pankaj J. Pasricha, Stanford University, Palo Alto, CA
Henry P. Parkman, Temple University, Philadelphia, PA
Thomas L. Abell, University of Mississippi, Jackson, MS
Linda A. Nguyen, California Pacific Medical Center, San Francisco, CA
William Snape, California Pacific Medical Center, San Francisco, CA.
K. Robert Shen, Mayo Clinic, Rochester, MN.
Michael Sarr, Mayo Clinic, Rochester, MN.
James Swain, Mayo Clinic, Rochester, MN.
Michael Kendrick, Mayo Clinic, Rochester, MN.
Simon Gibbons, Mayo Clinic, Rochester, MN.
Tamas Ordog, Mayo Clinic, Rochester, MN.
Gianrico Farrugia, Mayo Clinic, Rochester, MN.
References
- 1.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716–26. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–46. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 3.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 4.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurahashi M, Niwa Y, Cheng J, et al. Platelet-derived growth factor signals play critical roles in differentiation of longitudinal smooth muscle cells in mouse embryonic gut. Neurogastroenterol Motil. 2008;20:521–31. doi: 10.1111/j.1365-2982.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–66. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- 8.Rumessen JJ, Thuneberg L. Interstitial cells of Cajal in human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology. 1991;100:1417–31. [PubMed] [Google Scholar]
- 9.Rumessen JJ, Thuneberg L, Mikkelsen HB. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Rec. 1982;203:129–46. doi: 10.1002/ar.1092030112. [DOI] [PubMed] [Google Scholar]
- 10.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–7. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 11.Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest. 1999;79:59–65. [PubMed] [Google Scholar]
- 12.Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–53. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- 13.Vanderwinden JM, Rumessen JJ, de Kerchove d'Exaerde A, Jr., et al. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–58. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini R, Benton DC, Dunn PM, Jenkinson DH, Moss GW. SK3 is an important component of K(+) channels mediating the afterhyperpolarization in cultured rat SCG neurones. J Physiol. 2001;535:323–34. doi: 10.1111/j.1469-7793.2001.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunnet M, Jespersen T, Angelo K, et al. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–87. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- 16.Ro S, Hatton WJ, Koh SD, Horowitz B. Molecular properties of small-conductance Ca2+-activated K+ channels expressed in murine colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2001;281:G964–73. doi: 10.1152/ajpgi.2001.281.4.G964. [DOI] [PubMed] [Google Scholar]
- 17.Fujita A, Takeuchi T, Saitoh N, Hanai J, Hata F. Expression of Ca(2+)-activated K(+) channels, SK3, in the interstitial cells of Cajal in the gastrointestinal tract. Am J Physiol Cell Physiol. 2001;281:C1727–33. doi: 10.1152/ajpcell.2001.281.5.C1727. [DOI] [PubMed] [Google Scholar]
- 18.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- 19.Bond CT, Sprengel R, Bissonnette JM, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–6. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- 20.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 21.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–15. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- 22.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faussone-Pellegrini MS, Grover M, Pasricha P, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis: The NIDDK gastroparesis clinical research consortium (GpCRC). J Cell Mol Med. 2011 Sep 14; doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–85. e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemm MF, Lang RJ. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exp Pharmacol Physiol. 2002;29:18–25. doi: 10.1046/j.1440-1681.2002.03601.x. [DOI] [PubMed] [Google Scholar]
- 27.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 28.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi KM, Gibbons SJ, Roeder JL, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–95. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 30.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G14–24. doi: 10.1152/ajpgi.00266.2009. [DOI] [PubMed] [Google Scholar]
- 32.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008;152:437–48. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 33.Groneberg D, Konig P, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology. 2011;140:1608–17. doi: 10.1053/j.gastro.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 34.El-Yazbi AF, Cho WJ, Boddy G, Schulz R, Daniel EE. Impact of caveolin-1 knockout on NANC relaxation in circular muscles of the mouse small intestine compared with longitudinal muscles. Am J Physiol Gastrointest Liver Physiol. 2006;290:G394–403. doi: 10.1152/ajpgi.00321.2005. [DOI] [PubMed] [Google Scholar]
- 35.Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G231–41. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ueno T, Duenes JA, Zarroug AE, Sarr MG. Nitrergic mechanisms mediating inhibitory control of longitudinal smooth muscle contraction in mouse small intestine. J Gastrointest Surg. 2004;8:831–41. doi: 10.1016/j.gassur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Streutker CJ, Huizinga JD, Campbell F, Ho J, Riddell RH. Loss of CD117 (c-kit)- and CD34-positive ICC and associated CD34-positive fibroblasts defines a subpopulation of chronic intestinal pseudo-obstruction. Am J Surg Pathol. 2003;27:228–35. doi: 10.1097/00000478-200302000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.