Abstract
Background
Official guidelines from the Centers for Disease Control and Prevention and the American College of Sports Medicine state that every adult should accumulate 30 minutes or more of moderate-intensity physical activity on most, preferably all, days of the week.
Objective
To examine the dose-response relationship between coronary heart disease (CHD) risk factors and vigorous exercise above the recommended minimum levels to assess whether further benefits accrue.
Methods
Physician-supplied medical data were compared with reported distance run in a national cross-sectional survey of 8283 male recreational runners.
Results
Compared with runners who ran less than 16 km (10 miles) per week, long-distance runners (≥80 km/wk) showed an 85% reduced prevalence of high-density lipoprotein cholesterol levels that were clinically low {<0.9 mmol/L (<35 mg/dL)}, a 2.5-fold increased prevalence of clinically defined high levels of high-density lipoprotein cholesterol (ie, ≥1.55 mmol/L or ≥60 mg/dL), the level thought to be protective against CHD), a nearly 50% reduction in hypertension, and more than a 50% reduction in the use of medications to lower blood pressure and plasma cholesterol levels. Estimated age-adjusted 10-year CHD risk was 30% lower in runners who averaged more than 64 km/wk than in those who averaged less than 16 km/wk (42 vs 61 events per 1000 men). Each 16-km incremental increase in weekly distance run up to 64 to 79 km/wk was associated with significant increases in high-density lipoprotein cholesterol levels and significant decreases in adiposity, triglyceride levels, the ratio of total cholesterol to high-density lipoprotein cholesterol level, and estimated CHD risk.
Conclusions
Our data (1) suggest that substantial health benefits occur at exercise levels that exceed current minimum guidelines and (2) do not exhibit a point of diminishing return to the health benefits of running at any distance less than 80 km/wk.
The Centers for Disease Control and Prevention and the American College of Sports Medicine, in cooperation with the President’s Council on Physical Fitness and Sports, issued the following guidelines: “Every American adult should accumulate 30 minutes or more of moderate intensity physical activity over the course of most days of the week…. Activities that can contribute to the 30 minute total include walking upstairs (instead of taking the elevator), gardening, raking leaves, dancing, and walking part or all of the way to or from work…. One specific way to meet the standard is to walk two miles briskly”[1]. These recommendations targeted the 24% of adult Americans who are totally sedentary and the 54% who are inadequately active, and are therefore pragmatically helpful [2,3].
It is unfortunate that, in contrast to the intent of their authors, the recommendations have been construed to mean that there is little or no additional benefit to more vigorous exercise. Some of the July 30, 1993, newspaper headlines that followed the consensus conference press release read as follows: “Still don’t exercise? No sweat. A little at a time now called enough” (Chicago Tribune); “Gym workout? US says walking, gardening will do, too” (Boston Globe); “Study says you don’t have to sweat fitness routine” (Los Angeles Times); “If you can’t run for health, a walk will do, experts say” (New York Times); “A walk as good as a workout” (Atlanta Constitution). Similar sentiments have been expressed by scientists and opinion leaders [4–10]. Some question whether further health improvement occurs beyond 32 km (20 miles) of walking or jogging per week [4] or when total physical activity exceeds 1674 kJ/d [10].
Few of the prospective studies on which these recommendations are based involved useful numbers of very active men and women. Only 14% of the railroad workers studied by Slattery et al [6] expended more than 1046 KJ of intense physical activity per week, the equivalent of jogging 5 or more minutes per day; 4.7% of the men enrolled in the Multiple Risk Factor Intervention Trial reported an hour or more per week of heavy leisure-time physical activity [7]. An average of 4.4 and 12 minutes per day was spent in heavy physical activity in the Belgian Physical Fitness Study [11] and the Honolulu Heart Study [12], respectively, and less than 8% of the Norwegians studied by Sandvik et al [13] exercised strenuously at least twice per week or competed in sports. High physical activity of the Finnish men studied by Pekkanen et al [14] was primarily occupational and did not include “upper class joggers or recreational sports athletes.”
If the consensus statement [8] is correct in hypothesizing that the majority of health benefits can be achieved by walking 16 to 22 km weekly (the energy equivalent of running 8–12 km [4]) and in hypothesizing that the benefit of increasing activity status is 12-fold greater in sedentary than in active individuals [15], then there should be little additional improvement in risk factors for runners who exceed 16 km per week. We therefore examined the dose-response relationships between the reported distance run per week and coronary heart disease (CHD) risk factors in 8283 male runners who participated in the National Runners’ Health Study. The relationships between running distance and CHD risk factors in 1837 women runners from this study have been published previously [16].
SUBJECTS AND METHODS
A 2-page questionnaire, distributed nationally at races and to subscribers to the nation’s largest running magazine (Runners’ World, Emmaus, Pa), solicited information on demographics (age, race, and education), running history (age when the runner began running at least 19 km per week, average weekly running distance, number of marathons during the preceding 5 years, and best marathon and 10-km times), weight history (greatest and current weight, weight when running was started, least weight as a runner, and circumference of the chest, waist, and hips), diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit, ascorbic acid, vitamin E, and aspirin), current and past cigarette use, history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, or diabetes. The questionnaire also requested permission to obtain participants’ height, weight, resting heart rate, blood pressure, and fasting plasma concentrations of lipoprotein cholesterol, triglyceride level, uric acid, and glucose from the runners’ physicians. Approximately 42,000 of the 220,000 questionnaires sent to male subscribers were returned completed. Physician-supplied high-density lipoprotein (HDL) concentrations were obtained on 26% of the respondents. The remainder did not give permission to release medical data (27%), had no HDL cholesterol measurement in their medical records (22%), or had nonresponding physicians (25%). On the rare occasion that data from more than 1 clinic visit were provided by the physician, we used the values from the most recent clinic visit in which HDL cholesterol had been measured. The majority of the clinic visits were obtained within 2 years of the date of the questionnaire.
Average number of kilometers run per week was computed by averaging the reported yearly distances during the preceding 5 years. The test-retest correlations for self-reported distance run per week (r=0.89; P.T.W., unpublished data from 110 San Francisco Bay Area runners who completed a second questionnaire several months after the first questionnaire as part of a validation study, 1995) compares favorably with those reported for the Minnesota leisure-time physical activity questionnaire [17] and other questionnaires [18]. There is also a significant inverse relationship between reported distance run and resting heart rate (Table 1), a measure commonly used to validate physical activity assessment, since bradycardia is a cardiac manifestation of aerobic conditioning.
Table 1.
Relationship between reported distance run per week and age, education, dietary intake, and running history in 7059 White male runners*
| Reported Distance Run/wk, km | Change per Kilometer Run/wk (Slope±SE) |
Significance of Linear Trend |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–15.9 (n=702) |
16–31.9 (n=2209) |
32–47.9 (n=2253) |
48–63.9 (n=1145) |
64–79.9 (n=495) |
≥80 (n=255) |
Probabily (P) |
t- statistic |
||
| Age, y | 44.8±10.5 | 46.4±9.6 | 46.1±9.3 | 45.9±10.0 | 44.8±9.2 | 45.1±10.0 | −0.006±0.006 | 0.28 | 1.1 |
| Education, y | 16.7±2.5 | 16.8±2.3 | 16.7±2.4 | 16.8±2.5 | 16.6±2.5 | 16.9±2.4 | 0.000±0.001 | 0.96 | 0.0 |
| Years run | 8.6±10.0 | 11.3±7.7 | 13.0±7.5 | 13.3±7.1 | 14.2±8.0 | 14.9±6.6 | 0.074±0.005 | <0.001 | 16 |
| Marathon participation>1 %±SE | 14.1 ±1.3 | 24.6 ±0.9 | 48.2 ±1.1 | 66.3 ±1.4 | 81.0 ±1.8 | 81.6 ±2.4 | <0.001 | 35 | |
| Average best time, min | 244.8 ±49.2 | 245.5 ±36.1 | 227.3 ±31.7 | 211.5 ±30.6 | 197.7 ±26.8 | 189.7 ±28.6 | −0.844±0.028 | <0.001 | 30 |
| 10-km race ≥1 %±SE | 61.8 ±1.8 | 79.0 ±0.9 | 88.7 ±0.7 | 91.6 ±0.8 | 94.1 ±1.1 | 91.8 ±1.7 | <0.001 | 15 | |
| Average best time, min | 48.6 ±7.4 | 46.2 ±6.0 | 43.3 ±5.0 | 40.8 ±4.4 | 39.1 ±4.9 | 37.7 ±4.4 | −0.146±0.004 | <0.001 | 42 |
| Resting pulse rate, beats/min | 65.3 ±9.7 | 63.8 ±10.3 | 61.7 ±10.4 | 60.7 ±10.6 | 60.1 ±10.6 | 58.1 ±10.9 | −0.089±0.008 | <0.001 | 12 |
| Alcohol intake, mL[oz]/wk | 72±102 [2.4 ±3.4] | 87±117 [2.9±3.9] | 90±120 [3.0±4.0] | 87±117 [2.9±3.9] | 84±123 [2.8±4.1] | 90±129 [3.0±4.3] | 0.003±0.002 | 0.14 | 1.5 |
| Dietary intake, servings/wk | |||||||||
| Beef, lamb or pork | 3.1 ±2.6 | 3.0±2.6 | 2.8±2.3 | 2.5±2.3 | 2.4±2.2 | 2.3±2.2 | −0.012±0.001 | <0.001 | 8.3 |
| Fish | 1.6±1.4 | 1.7±1.6 | 1.6±1.4 | 1.7±1.5 | 1.7±1.6 | 1.6±1.4 | 0.001 ±0.001 | 0.51 | 0.7 |
| Fruit | 9.9±7.5 | 10.6±7.7 | 11.3±8.4 | 11.8±8.7 | 13.2±11.7 | 12.9±11.0 | 0.044±0.005 | <0.001 | 8.8 |
| Aspirin intake, tablets/wk | 2.3±4.8 | 2.7±5.3 | 2.6±4.8 | 2.8±4.7 | 3.0±6.2 | 3.2±5.4 | 0.008±0.003 | ≤0.01 | 2.5 |
| Ascorbic acid, g/wk | 1.7±4.0 | 1.9±4.6 | 1.9±4.6 | 2.0±3.9 | 2.0±5.6 | 2.5±5.0 | 0.006±0.003 | ≤0.02 | 2.2 |
| Vitamin E, U/wk | 561.8 ±1278.1 | 588.8 ±1393.6 | 582.1 ±1527.2 | 665.2 ±1657.7 | 787.5 ±2142.0 | 918.5 ±2057.9 | 3.917±0.922 | <0.001 | 4.3 |
AII were nonsmoking, nonvegetarlan, and without history of heart disease or cancer. None used drugs for the control of blood pressure, cholesterol, insulin, or thyroid levels. Data are mean±SD unless otherwise indicated.
Amount of alcohol consumed per week was calculated on the basis of 14.4 mL per 360 ml (0.48 oz per 12-oz) bottle of beer, 14.4 mL per 120-mL (0.48 oz per 4-oz) glass of wine, and 18 mL (0.60 oz) per drink of hard liquor [19]. The reliability of the self-reported usual weekly intakes of alcohol, red meat, fruit, and fish were validated against 4-day food records (r=0.65 for alcohol intake, r=0.46 for servings of red meat, r=0.38 for servings of fruit, and r=0.19 for servings of fish in 110 runners; P.T.W., unpublished data, 1995). Body mass index was calculated as the weight in kilograms divided by the height in meters squared. Self-reported height and weight from the questionnaire were strongly correlated with their clinic measurements (unpublished correlations in 110 men were r=0.96 for both). Self-reported greatest lifetime weight was also strongly correlated in duplicate questionnaires (r=0.98).
The HDL cholesterol values were obtained from the medical records of 9920 male runners. We excluded 572 runners because of histories of cancer or heart attacks, 137 because of smoking, and 871 because of vegetarian diets. An additional 1,052 men (1,002 white and 50 nonwhite) were excluded for most analyses because they used medications for diabetes or to control cholesterol, blood pressure, or thyroid levels, which are known to affect plasma lipoprotein concentrations. This left 7059 white, 100 Hispanic, 35 African-American, 19 Native American, 47 Asian, and 21 other nonwhite, nonvegetarian, nonsmoking runners who were without a history of heart disease or cancer and who were not using medications that might affect lipoprotein levels (7 runners did not report their race). Thus, the analyses of the 8283 nonsmoking, nonvegetarian runners presented herein include 7,059 white and 222 nonwhite runners not taking medications and 1,002 white runners who used medication. Similar results were obtained for the complete sample of 9,920 runners.
Multiple regression analyses were used to test for linear relationships between the distance run and risk factor levels and to assess the significance of these relationships when adjusted for other variables. Linear trends for proportions were tested by means of the estimated trends and SEs for a linear contrast among the 6 distance categories [20]. Ten-year CHD risk was estimated from the equation derived from the Framingham Study [21]. This estimate should be interpreted cautiously given its derivation from a principally sedentary population. The age-adjusted mean 10-year risk for each distance group was obtained by directly standardizing each distance category to the age distribution of the total sample.
RESULTS
Table 1 shows that men who ran greater weekly distances tended to adopt behaviors that may indicate a greater health consciousness: reduced weekly servings of beef, lamb, or pork and greater weekly intakes of fruit, aspirin, ascorbic acid, and vitamin E. Age, education, and intakes of fish and alcohol were unrelated to running distance. As expected, the higher-distance runners were more likely to have participated in at least 1 marathon or 10-km race during the preceding 5 years and to complete those races more quickly. Longer weekly distance was also associated with more years spent running at a level of 19 km or more per week.
Plasma Lipoproteins
Higher HDL cholesterol levels were among the most pronounced effects of higher weekly running distance. Table 2 shows that mean plasma HDL concentrations increased significantly with each 16-km increment up to 64 to 79 km run per week. The higher HDL level could not be attributed to age, education, or the intakes of alcohol, aspirin, red meat, fish, fruit, ascorbic acid, or vitamin E (ie, the regression slope remained essentially unchanged when adjusted). Plasma HDL cholesterol level of 1.55 mmol/L (60 mg/dL) or more has been recognized by the National Cholesterol Education Program as protective against CHD [22]. An HDL cholesterol value at this level is purported to be sufficiently protective to compensate for the detrimental effects of 1 risk factor (eg, diabetes, hypertension, cigarette smoking, family history of premature CHD, or being a man aged >45 years [22]). Figure 1 shows that running more kilometers is associated with as much as a 2.5-fold increase in the proportion of men having a high HDL cholesterol level (≥1.55 mmol/L [≥60 mg/dL]; t= 10.2 for the linear trend), and that the proportion increases significantly for each 16-km increment up to 64 to 79 km per week. Figure 1 also shows that the percentage of men with clinically significant low HDL cholesterol levels {<0.91 mmol/L (<35 mg/dL) [22]} decreased significantly with the distance run (t=6.7 for the linear trend) and decreased incrementally for each 16-km increment up to 48 to 63 km/wk. The percentage of men with clinically defined low HDL cholesterol level was 6-fold greater in the lowest distance runners as compared with the highest-distance runners. Table 2 shows that the ratio of total cholesterol level to HDL cholesterol level also decreases with the distance run per week, with significant incremental reductions for every 16-km increase up to 64 to 79 km/wk.
Table 2.
Relationship between reported distance run per week and adiposity, blood pressure, and plasma concentrations of lipoproteins, fasting glucose, and uric acid in 7059 White Male Runners*
| Reported Distance Run/wk, km | Increment per km/wk (Slope±SE)† | Significance of Linear Trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–15.9 | 16–31.9 | 32–47.9 | 48–63.9 | 64–79.9 | ≥80 | P | t-stat | ||
| HDL cholesterol, mmol/L | 1.24±0.01 | 1.28±0.01‡ | 1.34±0.01§ | 1.40±0.01§ | 1.49±0.02§ | 1.49±0.02 | 0.004±0.000 | <0.001 | 17 |
| (mg/dL) | 47.9±0.5 | 49.6±0.3‡ | 51.9±0.3§ | 54.3±0.4§ | 57.4±0.7§ | 57.7±0.9 | 0.136±0.008 | ||
| LDL cholesterol, mmol/L | 3.27±0.03 | 3.25±0.02 | 3.23±0.02 | 3.19±0.03 | 3.11 ±0.04 | 3.10±0.05 | −0.002±0.001 | <0.001 | 4.5 |
| (mg/dL) | 126.5±1.2 | 125.7±0.7 | 124.9±0.7 | 123.2±1.0 | 120.1±1.4 | 119.8±1.8 | −0.085±0.019 | ||
| Triglycerides, mmol/L | 1.33±0.03 | 1.25±0.02‡ | 1.15±0.01§ | 1.04±0.02§ | 0.95±0.03|| | 0.98±0.03 | −0.005±0.001 | <0.001 | 13 |
| (mg/dL) | 118.0±3.0 | 110.6±1.5‡ | 101.8±1.3§ | 92.3±1.5§ | 84.3±2.3|| | 86.4±3.0 | −0.476±0.038 | ||
| Total/HDL cholesterol | 4.40±0.05 | 4.22±0.03§ | 4.01±0.02§ | 3.79±0.03§ | 3.56±0.04§ | 3.54±0.06 | −0.012±0.001 | <0.001 | 17 |
| Fasting glucose, mmol/L | 5.12±0.03 | 5.09±0.02 | 5.11±0.02 | 5.06±0.02 | 5.05±0.04 | 4.96±0.04 | −0.001±0.000 | 0.001 | 3.2 |
| (mg/dL) | 92.2±0.6 | 91.7±0.3 | 92.0±0.3 | 91.2±0.4 | 90.9±0.8 | 89.3±0.8 | −0.025±0.008 | ||
| Uric acid, μmol/L | 333±4 | 333±2 | 327±2 | 323±3 | 314±5 | 306±5 | −0.36±0.06 | <0.001 | 5.9 |
| (mg/dL) | 5.60±0.07 | 5.60±0.03 | 5.50±0.03 | 5.43±0.05 | 5.28±0.08 | 5.14±0.09 | −0.006±0.001 | ||
| Systolic BP, mm Hg | 122.8±0.6 | 122.0±0.3 | 122.1±0.3 | 121.1±0.4 | 119.1±0.6‡ | 119.8±0.9 | −0.040±0.009 | <0.001 | 4.7 |
| Diastolic BP, mm Hg | 77.2±0.4 | 77.6±0.2 | 77.4±0.2 | 76.2±0.3§ | 75.8±0.4 | 75.9±0.6 | −0.027±0.006 | <0.001 | 4.9 |
| BMI, kg/m2 | 24.78 ±0.12 | 24.25 ±0.05§ | 23.75 ±0.05§ | 23.09 ±0.07§ | 22.77±0.09§ | 22.12 ±0.12§ | −0.033±0.001 | <0.001 | 23 |
| ΔBMI since greatest weight, kg/m2 | −2.38 ±0.08 | −2.46 ±0.04 | −2.78 ±0.05§ | −3.17 ±0.07§ | −3.39 ±0.11 | −3.88 ±0.16‡ | −0.020 ±0.001 | <0.001 | 15 |
All were nonsmoking, nonvegetarian, and without history of heart disease or cancer. None used drugs to control blood pressure (BP), cholesterol, insulin, or thyroid levels. All values are mean±SE. HDL indicates high density lipoprotein; LDL, low-density lipoprotein; and BMI body mass index.
P<0.01 relative to preceding exercise category;
P<0.001 relative to preceding exercise category;
P<0.05 relative to preceding exercise category.
regression slopes remained significant when adjusted for age, education, years run, red meat, fish, fruit, and vitamins C and E.
Figure 1.
Percentage of men with clinically defined low high-density lipoprotein (HDL) cholesterol levels {<0.91 mmol/L (<35 mg/dL), considered at risk of coronary heart disease} and high HDL cholesterol levels {≥1.55 mmol/L (≥60 mg/dL), considered protective for coronary heart disease [22]} in 7059 white, nonvegetarian, nonsmoking runners who were without a history of heart disease or cancer and who were not using medications that might affect lipoprotein levels.
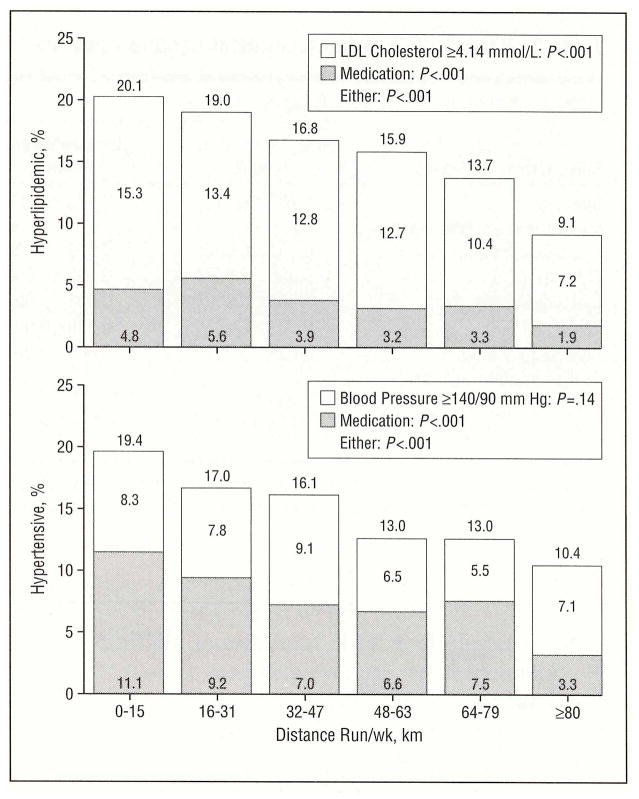
Plasma triglyceride concentrations are well known to be inversely related to plasma HDL levels [23]. Table 2 shows that triglyceride levels decreased significantly with each 16-km increment up to 64 to 79 km per week. The association between kilometers run and plasma low density lipoprotein (LDL) cholesterol concentrations was significant albeit weaker than that observed for either HDL cholesterol or triglyceride concentrations. Figure 2 displays the percentage of men who were hyperlipidemic, defined as either having a high-risk LDL cholesterol level (≥4.14 mmol/L [≥160 mg/dL]) or using cholesterol lowering medication. Running 80 km or more per week was associated with a 50% lower frequency of hyperlipidemia (t=5.74 for linear trend). Use of medication for control of plasma cholesterol level was also significantly less frequent with increased-distance run (t=3.49 for trend). This shows that LDL cholesterol reduction in higher-distance runners was not an artifact of improved compliance with cholesterol-lowering drugs.
Figure 2.
Percentage of hyperlipidemic men (top; low-density lipoprotein [LDL] cholesterol level ≥4.14 mmol/L [≥160 mg/dL] or taking cholesterol-lowering medication) and hypertensive men (bottom; systolic blood pressure > 140 or diastolic blood pressure >90 mm Hg or taking medication for blood pressure control) in 8054 white, non vegetarian, nonsmoking runners who were without a history of heart disease or cancer. (Untreated hypertension may be overestimated because it is based on readings from a single clinic visit.) These analyses cannot distinguish between an effect of medication use on running level and an effect of running level on medication use.
Blood Pressure
Higher weekly running distance was associated with significant apparent reductions in both systolic and diastolic blood pressures (Table 2). Figure 2 shows that running more than 80 km/wk was associated with nearly a 50% apparent reduction in hypertension (t=4.5 for linear trend) and a 70% apparent reduction in the use of medication for blood pressure control (t=4.8 for linear trend). Untreated hypertension (blood pressure > 140/90 mm Hg but not treated with medication) was relatively constant across distance categories, suggesting that reduced use of hypertensive medication did not increase the number of men with untreated hypertension among high-distance runners.
Body Mass Index
Men who ran further each week were significantly leaner. The leanness is presumably the consequence of running, because higher-distance runners exhibited the greatest difference between their current and their greatest lifetime weight (change in body mass index). Table 3 examines the relative contributions of their current body mass index, change in body mass index (ie, deviation from greatest weight), weekly running distance, number of years of running, and alcohol intake to the runners’ blood pressure and plasma concentrations of HDL cholesterol, LDL cholesterol, and triglycerides. Body mass index was strongly related to all five risk factors; the leaner runners had higher HDL cholesterol levels and lower levels of LDL cholesterol, triglycerides, and systolic and diastolic blood pressures. Levels of HDL cholesterol, triglycerides, and systolic blood pressure were all strongly related to change in body mass index, suggesting that the effects of adiposity depend not only on the leanness achieved but also on the amount of weight lost in achieving the acquired level of leanness (eg, at the same weekly running distance, alcohol intake, and leanness, HDL cholesterol level will be higher for previously overweight runners than for runners who were always lean [24]).
Table 3.
Multiple regression analysis of cardiovascular risk factor levels in White male runners*
| Dependent Variables | |||||
|---|---|---|---|---|---|
| Independent Variables | HDL Cholesterol, mmol/L | LDL Cholesterol, mmol/L | Triglycerides, mmol/L | Systolic Blood Pressure, mm Hg | Diastolic Blood Pressure, mm Hg |
| Intercept | 1.733±0.043† | 2.123±0.109† | −0.160±0.093 | 106.76±1.89† | 64.04±1.23† |
| Weight loss (ΔBMI since greatest weight), kg/m2 | −0.017±0.002† | 0.004±0.005 | 0.023±0.004† | −0.46±0.08† | 0.04±0.05 |
| BMI, kg/m2 | −0.026±0.002† | 0.042±0.004† | 0.063±0.004† | 0.59±0.07† | 0.55±0.05† |
| Distance run/wk, km | 0.002±0.000† | −0.001±0.001‡ | −0.003±0.001† | −0.03±0.01† | −0.01±0.01 |
| Years run | 0.002±0.001§ | 0.010±0.001† | 0.000±0.001 | 0.01±0.02 | −0.01±0.01 |
| Alcohol intake, oz/wk | 0.024±0.001† | 0.008±0.003§ | −0.004±0.002‡ | 0.24±0.04† | 0.16±0.03† |
| Test of model, F-statistic | 235.5 | 37.1 | 97.2 | 31.4 | 37.4 |
| R | 0.39 | 0.17 | 0.26 | 0.16 | 0.18 |
AII were nonsmoking, nonvegetarian, and without history of heart disease or cancer. None used drugs for the control of blood pressure, cholesterol, insulin, or thyroid levels. All values are slopes±SE. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; and BMI, body mass index.
P<0.001.
<0.05.
<0.01.
Estimated 10-year CHD risk
Figure 3 assesses the impact of the improved lipoprotein and blood pressure levels by calculating the estimated CHD risk for each runner from the equations derived from the Framingham Study [21]. The mean 10-year risk for each distance group was obtained by directly standardizing each distance category to the age distribution of the total sample. There was a significant reduction in the predicted number of events with increasing weekly distance (t=12.3 for trend). Estimated CHD risk decreased significantly with each 16-km increment in weekly distance up to 64 to 79 km per week. During the next 10 years, men who ran 64 km or more per week are estimated to have 30% less risk of CHD (42 events per 1000 men) than those who ran less than 16 km per week (61 events per 1000 men).
Figure 3.
Ten-year risk of coronary heart disease (CHD) (estimated by the equation derived from the Framingham Study [21] and age adjusted) in 7059 white, nonvegetarian, nonsmoking runners who were without a history of heart disease or cancer and who were not using medications that might affect lipoprotein levels.
Nonwhite male runners
Table 4 shows that reported distance run per week in nonwhite men was associated with increasing levels of plasma HDL cholesterol and decreasing total cholesterol-HDL cholesterol ratio, body mass index, change in body mass index, and blood pressure (P<0.01). The change in risk factor levels per kilometer run was the same in nonwhites and whites (Table 2) for all variables except systolic blood pressure, where the decrease per kilometer run was significantly greater in the nonwhites (P=0.04). Although there is limited statistical power to detect relationships within specific ethnic groups, the distance run was significantly related (P≤0.05) in African-American runners to HDL cholesterol, total cholesterol-HDL cholesterol ratio, and change in body mass index, in Hispanic runners to systolic blood pressure, body mass index, and change in body mass index, and in Asian runners to systolic and diastolic blood pressures, total cholesterol-HDL cholesterol ratio, and body mass index.
Table 4.
Regression slope for Change in Cardiovascular Risk Factor Levels per Kilometer Run per Week in Nonwhite Men*
| All Nonwhite (N=222) | Hispanics (N=100) | Asian (N=47) | African American (N=35) | Native American (N=19) | |
|---|---|---|---|---|---|
| HDL cholesterol (mmol/L) | 0.003 ± 0.001† | 0.003 ± 0.002 | 0.003 ± 0.002 | 0.010 ± 0.005‡ | 0.005 ± 0.003 |
| LDL cholesterol (mmol/L) | −0.005 ± 0.003 | −0.005 ± 0.004 | −0.011 ± 0.006 | −0.011 ± 0.007 | −0.004 ± 0.011 |
| Triglycerides (mmol/L) | −0.003 ± 0.002 | −0.003 ± 0.004 | −0.009 ± 0.005 | −0.004 ± 0.006 | 0.011 ± 0.006 |
| Total/HDL-cholesterol | −0.013 ± 0.005† | −0.011 ± 0.006 | −0.019 ± 0.010‡ | −0.056 ± 0.027‡ | −0.012 ± 0.013 |
| Systolic BP mm Hg | −0.140 ± 0.045† | −0.121 ± 0.060‡ | −0.202 ± 0.091‡ | −0.104 ± 0.144 | 0.077 ± 0.233 |
| Diastolic BP mm Hg | −0.086 ± 0.034† | −0.055 ± 0.046 | −0.189 ± 0.069† | 0.057 ± 0.106 | 0.097 ± 0.145 |
| BMI kg/m2 | −0.025 ± 0.008† | −0.030 ± 0.013‡ | −0.039 ± 0.013† | −0.027 ± 0.029 | −0.032 ± 0.036 |
| Weight loss (ΔBMI since greatest weight, kg/m2 | −0.031 ± 0.008§ | −0.028 ± 0.013‡ | −0.007 ± 0.013 | −0.063 ± 0.021† | −0.014 ± 0.031 |
All were nonsmoking, nonvegetarian, and without history of heart disease or cancer. None used drugs for the control of blood pressure, cholesterol, insulin, or thyroid levels. All nonwhite includes 21 runners who identified their race as other. All values are slopes±SE. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; and BMI, body mass index.
P<0.01.
<0.05.
<0.001.
Discussion
The number of male runners we studied was large and adds substantially to the total number in all previously published cross-sectional studies of running and lipoproteins in men. Previous studies have not had the statistical power required to test for incremental changes in risk factors between running levels as small as 16 km over a range of 80 km. This is required for a precise assessment of the dose-response relationship between the distance run and CHD risk. Table 2 and Figures 1 through 3 provide strong evidence of apparent improvements in cardiovascular risk factors beyond the recommended level (walking 16–22 km per week, the approximate energy equivalent of running 8–12 km). Each 16-km incremental increase in weekly distance run up to 64 to 79 km/wk was associated with a significant increase in HDL cholesterol levels and significant decreases in adiposity, triglyceride levels, total cholesterol-HDL cholesterol ratio, and estimated CHD risk. Thus, our data do not exhibit a point of diminishing return to the health benefits of running at any distance less than 80 km/wk. These results agree with results of meta-analysis that showed no attenuation in the rate of reduction in cardiovascular mortality risk at higher levels of leisure-time physical activity [13]. It may be premature, however, to conclude from our data that the benefits of running taper off above 80 km/wk, since the 80 km or more category included only 3.6% of the sample and encompassed distances between 80 and 171 km/wk.
The National Runners’ Health Study also included 1,837 female runners whose CHD risk factors have been reported separately [16]. The increase in HDL cholesterol level per 1 km run in men (slope±SE, β=0.004±0.0002 mmol/L per kilometer per week) is nearly identical to that previously reported for women in this study (β = 0.003±0.0005 mmol/L per kilometer per week). Per kilometer run, there were also similar decreases in systolic blood pressure (men vs women, β =−0.04±0.009 vs −0.06±0.02 mm Hg), diastolic blood pressure (β = −0.027±0.006 vs −0.028±0.013 mm Hg), and body mass index (β = −0.033±0.001 vs −0.036±0.003 kg/m2). As compared with women, male runners exhibited significantly greater decreases in plasma LDL cholesterol levels (β = −0.002±0.001 vs −0.0008±0.001 mmol/L per kilometer per week), triglyceride concentrations (β = −0.005±0.001 vs −0.0006±0.0008 mmol/L per kilometer per week), and the ratio of total cholesterol-HDL cholesterol (β = −0.012±0.001 vs −0.005±0.001 per kilometer per week). The decreases per kilometer run per week were also greater in men than in nonmenstruating women for plasma LDL cholesterol level (women’s slope±SE, β = −0.002±0.002 mmol/L per kilometer per week) and triglyceride concentrations (β = −0.001 ±0.002 mg/dL per kilometer per week) and in the ratio of total cholesterol-HDL cholesterol (β = −0.001±0.003 per kilometer per week) than men.
The lower participation of minorities in leisure-time physical activity has been noted elsewhere [8] and could result in part from a lack of perceived benefit. There were no significant differences in the apparent effects of running distance on risk factor levels between white and nonwhite runners for all variables except systolic blood pressure. More specifically, our analyses suggest that Asian, African-American, and Hispanic men who ran greater distances tended to show more desirable risk factor levels.
The revised recommendations from the Centers for Disease Control and Prevention and the American College of Sports Medicine deemphasize the importance of sustained vigorous exercise. The new recommendations are prescribed in terms of moderate physical activity performed at an intensity of 3 to 6 METS (work metabolic rate divided by resting metabolic rate, equivalent to 17–29 kJ/min) [8]. This is in contrast to their previous recommendations of 30 to 60 minutes of moderate- to high-intensity exercise (60% to 90% of maximum heart rate) performed 3 or more times per week [8]. More intense activity is classified as hard or vigorous (>6 METS or >17–29 kJ/min) and includes running at a speed of 9.6 km/h (10.2 METS [4]). The activity goal of the new recommendations can be achieved through the accumulation of intermittent short bouts of moderate activity (eg, cleaning house, mowing the lawn with a power mower, and gardening [8]).
Blair et al [23] and others [7,26] have demonstrated the benefits of increased physical activity among the least physically fit. Their results dismiss the perception once held that physical activity is unprofitable unless a minimum threshold is exceeded [5]. Whereas the basis for emphasizing moderate activity is apparent, the scientific rationale for deemphasizing vigorous activity is less established. Not all studies find that the greatest improvements in risk from physical activity occurs at the lowest activity strata [12,27]. The observation by Morris et al [28] that men who participated in vigorous exercise during leisure time had significantly lower rates of CHD than those not participating, agrees with the observation by Paffenbarger et al [29] that “at any given level of energy expenditure, the risk of heart attack tends to be lower with strenuous sports than with casual activities.” Paffenbarger et al [29] found that the break point for optimum benefit was 14,651 kJ/wk, a level much higher than current recommendations, but consistent with the data presented herein. More recently, Paffenbarger et al [30] showed that Harvard alumni who took up moderately vigorous sports activity (≥4.5 METS) significantly reduced their mortality risk from all causes (23% reduction) and from CHD (41% reduction) compared with men who continued not to engage in such activity. In contrast, increasing the overall daily activity (both vigorous and nonvigorous) had no significant impact on overall mortality [30]. Paffenbarger and coworkers’ observations [29,30] and, more recently, those by Lee et al [31] appear to contradict the opinion that the health benefits of physical activity are linked principally to the total amount of physical activity performed [8] and that the amount of activity is more important than mode, intensity, or duration of the activity bouts [8].
Our surveys show that 45% of runners take aspirin regularly and that 28% take 3.5 or more aspirin tablets per week. These rates are higher than general usage (10% for 3.5 aspirin tablets per week [32]). None of the previous epidemiologic studies controlled for aspirin use. Their analyses could overestimate the reduction in CHD risk by physical activity, since aspirin also lowers risks. The Physicians’ Health Study showed that men randomly assigned to receive 3.5 aspirin tablets per week reduced their risk of myocardial infarction by 44% when compared with placebo [33]. Aspirin use decreased nonfatal myocardial infarction by 33% for the combined analyses of the British Doctors’ Trial and the Physicians’ Health Study [33,34]. Curiously, the two chronic diseases most strongly affected by physical activity (CHD and colon cancer [36,37]) are also affected by aspirin [32–35].
In this article, we have described the dose-response relationship between exercise level and CHD risk factors in terms of the distance run per week. This was done to evaluate official government guidelines [8], which are prescribed in terms of quantity of physical activity. Elsewhere, however, we have argued that exercise may principally affect HDL cholesterol level through reduced adiposity, expressed in terms of either the leanness achieved or deviations from sedentary weight [24,38,39]. In this study, we find that the HDL cholesterol levels were significantly related not only to weight and distance run, but also to the difference between the runners’ current and greatest weight. Other studies also show that the difference between the runners’ self-reported greatest weight and their current weight is an important determinant of HDL cholesterol concentrations [24]. A more complete exercise recommendation may need to include in its prescription exercise-induced weight loss and current adiposity in addition to the distance run.
Although these cross-sectional associations do not prove that running greater distances causes these improvements in risk factors, other well-designed randomized, controlled intervention studies prove causality [40–43]. The important contribution of the cross-sectional relationships presented herein is the dose-response relationship between exercise level and risk factors at substantially higher exercise levels than previously achieved in intervention studies. Higher-distance runners may follow a somewhat healthier lifestyle as reflected by their lower red meat and higher fruit consumption. However, adjustment for fruit, red meat, vitamin, and alcohol intake had little effect on the relationship between CHD risk factors and the distance run. Self-selection may also contribute partially to the association between running level and HDL cholesterol level. That men with higher levels of HDL cholesterol will tend to run more when undertaking an exercise program has been demonstrated in two studies [44,45]. Higher HDL cholesterol levels at baseline may be a marker for men genetically endowed with muscle fiber types that make running easier and thereby facilitates weight loss [46]. Compliance with medication for the control of blood pressure, cholesterol level, thyroid hormone levels, or diabetes did not contribute to the favorable risk factor profiles associated with running distance, since cholesterol level and blood pressure medication were more commonly used in the lowest distance group (Figure 2).
Our analyses, based on physician-supplied CHD risk measurements, show detectable improvements in risk factors as commonly measured in medical practice. Thus, the association reported herein between the distance run and CHD risk factors are in terms of conventional clinical measurements, which are expected to be less precise than measurements made under usual research conditions. A larger measurement error of the dependent variable does not bias the estimated mean levels within each distance category, nor does it bias the regression slope between distance run and risk factor levels. The added error will increase the probability of a type II statistical error (ie, false-negative result) rather than a type I error (ie, a false-positive result) and is therefore conservative.
An unbiased estimate of the dose-response relationship between CHD risk factors and the distance run does not require that the runners be selected at random with respect to running mileage. However, volunteers are expected to differ from nonvolunteers, and therefore caution is warranted in extrapolating our estimates to all runners. Only 42,000 of the approximately 220.000 men mailed questionnaires responded, and, of these, 8283 were used in the analysis.
It is important to emphasize that there are some risks associated with vigorous exercise that may increase with intensity and duration [47]. Men who intend to begin a running program should have a recent physical examination [4]. Current guidelines recommend a symptom-limited, maximum exercise test for men who are 40 years of age or older, men with abnormal physical examinations, and men with two or more coronary risk factors [4].
Acknowledgments
This study was supported in part by grant HL- 45652 from the National Heart Lung and Blood Institute and was conducted at the Lawrence Berkeley National Laboratory, Berkeley, Calif (Department of Energy grant DE-AC03-76SF00098 to the University of California).
My appreciation to Amby Burfoot and Vern Walther of Runners’ World for their assistance in contacting many of the runners who participated in this study, and to Davina Moussa, Elizabeth Noceti, Alison Collins, Melissa King, Lisa Geüig, Kimberly Starr Reid, and Stephanie Proctor for their assistance in data collection.
References
- 1.Centers for Disease Control and Prevention and American College of Sports Medicine. cooperation with the President’s Council on Physical Fitness and Sports. Summary Statement: Workshop on Physical Activity and Public Health; July 29, 1993.Indianapolis, Ind: American College of Sports Medicine; [Google Scholar]
- 2.Hahn RA, Teutsch SM, Rothenberg RB, Marks JS. Excess deaths from nine chronic diseases in the United States, 1986. JAMA. 1990;264:2654–2659. [PubMed] [Google Scholar]
- 3.McGinnis JM, Foego WLL. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- 4.Fletcher GF, Froelicher VF, Hartley H, Haskell WL, Pollock ML. Exercise standards: a statement for health professionals from the American Heart Association. Circulation. 1990;82:2286–2322. doi: 10.1161/01.cir.82.6.2286. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, Gordon NF, Paffenbarger RS. How much physical activity is good for health? Ann Rev Public Health. 1992;13:99–126. doi: 10.1146/annurev.pu.13.050192.000531. [DOI] [PubMed] [Google Scholar]
- 6.Slattery ML, Jacobs DR, Nichaman MZ. Leisure time physical activity and coronary heart disease death: the US Railroad Study. Circulation. 1989;79:304–311. doi: 10.1161/01.cir.79.2.304. [DOI] [PubMed] [Google Scholar]
- 7.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure time physical activity levels and risk of coronary heart disease and death: the Multiple Risk Factor Intervention Trial. JAMA. 1987;258:2388–2395. [PubMed] [Google Scholar]
- 8.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 9.Cooper KH. Antioxidant Revolution. Nashville, Tenn: Thomas Nelson Publishers; 1994. pp. 45–91. [Google Scholar]
- 10.Haskell WL. Physical activity and health: need to define the required stimulus. Am J Cardiol. 1985;55:4D–9D. doi: 10.1016/0002-9149(85)91048-3. [DOI] [PubMed] [Google Scholar]
- 11.Sobolski J, Kornitzer M, De Backer G, et al. Protection against ischemic heart disease in the Belgian Physical Fitness Study: physical fitness rather than physical activity? Am J Epidemiol. 1987;125:601–610. doi: 10.1093/oxfordjournals.aje.a114573. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez BL, Curb D, Burchfiel CM, et al. Physical activity and 23-year incidence of coronary heart disease morbidity and mortality among middle aged men: the Honolulu Heart Program. Circulation. 1994;89:2540–2544. doi: 10.1161/01.cir.89.6.2540. [DOI] [PubMed] [Google Scholar]
- 13.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 14.Pekkanen J, Nissinen A, Marti B, Tuomilehto J, Punsar S, Karvonen MJ. Reduction of premature mortality by high physical activity: a 20-year follow-up of middle-aged Finnish men. Lancet. 1987;1:1473–1477. doi: 10.1016/s0140-6736(87)92218-5. [DOI] [PubMed] [Google Scholar]
- 15.Williams PT. Physical activity and public health. JAMA. 1995;274:533–534. doi: 10.1001/jama.1995.03530070031016. [DOI] [PubMed] [Google Scholar]
- 16.Williams PT. High density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med. 1996;334:1298–1303. doi: 10.1056/NEJM199605163342004. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Casperson CJ, Taylor HJ, et al. Leisure time physical activity and its relationship to coronary risk factors in a population-based sample: the Minnesota Heart Survey. Am J Epidemiol. 1985;121:570–579. doi: 10.1093/oxfordjournals.aje.a114035. [DOI] [PubMed] [Google Scholar]
- 18.Yasim S. Measuring habitual leisure-time physical activity by recall record questionnaire. In: Karvonen MJ, Barry AJ, editors. PhysicalActivity and the Heart. Springfield, Ill: Charles C Thomas Publisher; 1967. pp. 372–373. [Google Scholar]
- 19.Haskell WL, Camargo C, Jr, Williams PT, et al. The effect of cessation and resumption of moderate alcohol intake on serum high-density lipoprotein subfractions: a controlled study. N Engl J Med. 1984;310:805–810. doi: 10.1056/NEJM198403293101301. [DOI] [PubMed] [Google Scholar]
- 20.Mood AM, Graybill FA, Boes DC. Introduction to the Theory of Statistics. 3. New York, NY: McGraw-Hill International Book Co; 1974. pp. 178–181. [Google Scholar]
- 21.Anderson KM, Wilson PWF, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 22.National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) Bethesda, Md: National Institutes of Health; 1993. p. 11. NIH publication 93–3096. [Google Scholar]
- 23.Tall AR. Plasma lipid transfer proteins. J Lipid Res. 1986;27:361–367. [PubMed] [Google Scholar]
- 24.Williams PT. Weight set-point theory predicts HDL-cholesterol levels in previously\x=req-\ obese long-distance runners. Int J Obes. 1990;14:421–427. [PubMed] [Google Scholar]
- 25.Blair SN, Kohl HW, Paffenbarger RS, et al. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB. Habitual level of physical activity and risk of coronary heart disease: the Framingham Study. Can Med Assoc J. 1967;96:811–812. [PMC free article] [PubMed] [Google Scholar]
- 27.Ekelund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: the Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 28.Morris JN, Pollard R, Everitt MG, Chave SPE. Vigorous exercise in leisure\x=req-\time: protection against coronary heart disease. Lancet. 1980;2:1207–1210. doi: 10.1016/s0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 29.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 30.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 31.Lee I-M, Hsieh C-C, Paffenbarger RS. Exercise intensity and longevity in men: the Harvard Alumni Health Study. JAMA. 1995;273:1179–1184. [PubMed] [Google Scholar]
- 32.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 33.Steering Committee of the Physicians’ Health Study Research Group. Final report of the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 34.Peto R, Gray R, Collins R, et al. Randomized trial of prophylactic daily aspirin in British male doctors. BMJ. 1988;296:313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennekens CH, Peto R, Hutchison GB, Doll R. An overview of the British and American aspirin studies. N Engl J Med. 1988;318:923–924. doi: 10.1056/NEJM198804073181412. [DOI] [PubMed] [Google Scholar]
- 36.Vena JE, Graham S, Zielezny M, Swanson MK, Barnes RE, Nolan J. Lifetime occupational exercise and colon cancer. Am J Epidemiol. 1985;122:357–365. doi: 10.1093/oxfordjournals.aje.a114116. [DOI] [PubMed] [Google Scholar]
- 37.Gerhardsson M, Floderus B, Norell SE. Physical activity and colon cancer risk. Int J Epidemiol. 1988;17:743–746. doi: 10.1093/ije/17.4.743. [DOI] [PubMed] [Google Scholar]
- 38.Williams PT. Weight-set point theory and the high-density lipoprotein concentrations of long-distance runners. Metabolism. 1990;39:460–467. doi: 10.1016/0026-0495(90)90003-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT, Krauss RM, Vranizan KM, Albers JJ, Wood PDS. Effects of weight loss by exercise and by diet on apolipoprotein A-1 and A-11 and the particle size distribution of high-density lipoproteins in men. Metabolism. 1992;41:441–449. doi: 10.1016/0026-0495(92)90082-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood PD, Williams PT, Haskell WL. Physical activity and high density lipoproteins. In: Miller NE, Miller GJ, editors. Clinical and Metabolic Aspects of High Density Lipoproteins. Amsterdam, the Netherlands: Elsevier Science Publishers; 1984. pp. 133–165. [Google Scholar]
- 41.Krummel D, Etherton TD, Peterson S, Kris-Etherton PM. Effects of exercise on plasma lipids and lipoproteins of women. Proc Soc Exp Biol Med. 1993;204:123–137. doi: 10.3181/00379727-204-43644. [DOI] [PubMed] [Google Scholar]
- 42.Wood PD, Stefanick ML, Dreon D, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 43.Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325:461–466. doi: 10.1056/NEJM199108153250703. [DOI] [PubMed] [Google Scholar]
- 44.Williams PT, Wood PD, Haskell WL, Vranizan KM. The effects of running mileage and duration on plasma lipoprotein levels. JAMA. 1982;247:2674–2679. [PubMed] [Google Scholar]
- 45.Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PDS. Effects of weight loss by exercise or by dieting on plasma high density lipoprotein levels in men with low, intermediate and normal-to-high HDL at baseline. Metabolism. 1994;43:917–924. doi: 10.1016/0026-0495(94)90277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams PT. High-density lipoproteins and lipase activity in runners. Atherosclerosis. 1993;98:251–252. doi: 10.1016/0021-9150(93)90134-g. [DOI] [PubMed] [Google Scholar]
- 47.Siscovick DS, Weiss NS, Fletcher RH. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]