Abstract
The current study examined the relations between individual differences in sustained attention in infancy, the temperamental trait behavioral inhibition in childhood, and social behavior in adolescence. The authors assessed 9-month-old infants using an interrupted-stimulus attention paradigm. Behavioral inhibition was subsequently assessed in the laboratory at 14 months, 24 months, 4 years, and 7 years. At age 14 years, adolescents acted out social scenarios in the presence of an unfamiliar peer as observers rated levels of social discomfort. Relative to infants with high levels of sustained attention, infants with low levels of sustained attention showed increasing behavioral inhibition throughout early childhood. Sustained attention also moderated the relation between childhood behavioral inhibition and adolescent social discomfort, such that initial levels of inhibition at 14 months predicted later adolescent social difficulties only for participants with low levels of sustained attention in infancy. These findings suggest that early individual differences in attention shape how children respond to their social environments, potentially via attention’s gate-keeping role in framing a child’s environment for processing.
Keywords: sustained attention, temperament, social behavior, infancy, childhood and adolescence
Individual differences in attention may shape socioemotional development (McConnell & Bryson, 2005; Rothbart & Posner, 2006). Consistent with this possibility, infants selected for extremes in behavioral reactivity preferentially attend as infants to novelty and uncertainty (Marshall, Reeb, & Fox, 2009), show greater difficulty as young children deploying attention when under stress (Pérez-Edgar & Fox, 2005), and, by adolescence, preferentially attend to threat cues (Pérez-Edgar et al., 2010) and closely monitor their performance for errors (Bar-Haim et al., 2009; McDermott et al., 2009). Whereas these children often preferentially attend to threat or novelty, paradoxically their initial behavioral response is to withdraw. This is in contrast to more approach-oriented children who, upon noting a novel stimulus, will tend to approach and explore further. At the broader, pheno-typic level, behavioral reactivity is often a precursor to behavioral inhibition. Behavioral inhibition, in turn, is marked by withdrawal from novelty and salient environmental stimuli in infancy, social reticence with peers in childhood, and anxiety in adolescence and early adulthood (Fox, Henderson, Pérez-Edgar, & White, 2008).
Beyond this large body of developmental work, an equally compelling parallel set of adult studies finds that anxious individuals preferentially attend to threat cues in their environment relative to positive or neutral stimuli (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). That is, they show an attention bias to threat. In addition, anxious individuals have difficulty engaging regulatory attention mechanisms when processing threat-related (Bishop, Duncan, Brett, & Lawrence, 2004) and neutral (Bishop, 2009) stimuli. Taken together, these cognitive and phenotypic data in infants, children, and adults suggest that attentional and socioemotional processes may interact throughout development.
The current study examined the degree to which individual differences in sustained attention among 9-month-olds predicted patterns of behavior in the laboratory over the next 14 years. In particular, we focused on the prospect that early life individual differences in attention are associated with diverging trajectories of behavioral inhibition during early childhood. We then examined the possibility that these relations extend to adolescent social functioning. The broader literature supports this supposition as infants who have difficulty controlling attention also show greater difficulty in regulating emotional states (Posner & Rothbart, 1998). This link is also evident within atypical populations. For example, spontaneous patterns of attention are linked to social and communicative deficits among autistic adolescents (Klin, Jones, Schultz, Volkmar, & Cohen, 2002).
The current study relied on a task previously used to assess early infancy components of sustained attention (Richards, 1985, 1987) in which infants are shown an engaging video intermittently interrupted by a static, suddenly appearing stimulus in the visual periphery. Prior studies indexed individual differences in attention on the basis of the degree to which the infant monitors the distracter stimulus (Casey & Richards, 1988; Richards & Cronise, 2000; Richards & Turner, 2001). In these studies, sustained attention was marked by fewer shifts to the distracter stimulus. Here, we charted the infants’ spontaneous tendencies in deploying visual attention. Continued orienting to the distracter stimulus could reflect an underlying bias to attend to novelty (Marshall, Reeb, & Fox, 2009) or a lack of attention control because the orienting persists long after one could classify the distracter as novel or unexpected. In this regard, continued orienting to an irrelevant stimulus could also be considered an early life analogue of hyper-vigilance (Hill & Braungart-Rieker, 2002).
Whereas relatively few studies have examined the relations between measures of attention in infancy and behavioral trajectories in children, considerable work has examined these associations at later ages. Specifically, research among adolescents and adults finds concurrent relations between attention biases to threat and levels of anxiety, manifested either as an overt anxiety disorder or as increased anxiety symptoms (Mogg, Philippot, & Bradley, 2004; Monk et al., 2006; Roy et al., 2008). Anxious adults also have difficulty ignoring a distracter and inhibiting processing even when the stimuli are affect neutral and the task demands are low (Bishop, 2009).
Our work builds on prior literature examining mechanisms that moderate developmental trajectories. Whereas studies of behavioral inhibition find signs of both discontinuity and continuity (Degnan & Fox, 2007; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001), prior work suggests that neurocognitive factors predict continuity by moderating underlying behavioral tendencies (Fox, Nichols, et al., 2005; Henderson, Fox, & Rubin, 2001; Pérez-Edgar, Schmidt, Henderson, Schulkin, & Fox, 2008). Children who persist in displaying stable extreme behavioral inhibition are at increased risk for psychopathology, particularly anxiety in adolescence and young adulthood (Biederman et al., 1993; Chronis-Tuscano et al., 2009). In addition, early inhibition appears to broadly impact social functioning, negatively impacting social relationships and social transitions into adulthood (Asendorpf, Denissen, & van Aken, 2008). As such, finding early appearing markers of risk, before difficulties become entrenched, will aid in identification and intervention in childhood. The current study capitalized on the availability of comprehensive longitudinal data on socioemotional behavior (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001) to examine the degree to which early individual differences in attention are associated with the developmental course of social behavior into early childhood and then adolescence.
To summarize, this study grouped infants on the basis of individual differences in sustained attention; these individual differences were first linked to measures of childhood behavioral inhibition. We then examined the relations among infant attention, childhood behavioral inhibition, and adolescent social behavior. We expected infants with low levels of sustained attention to also show increased levels of behavioral inhibition in childhood. We further expected these attention differences to moderate the relations between childhood behavioral inhibition and adolescent social behavior, paralleling recent work showing that early behavioral inhibition is linked to increased social withdrawal in adolescence only among individuals who display attention biases to threat (Pérez-Edgar et al., 2010).
Method
Participants
Subjects were recruited from a large metropolitan area for a longitudinal study of the behavioral and physiological correlates of temperament. The sample predominately involved Caucasian (95%) middle- to upper middle-class families. All infants were born within 2 weeks of due date and reported no significant medical difficulties. At 4 months of age, 207 children (95 boys) were screened for levels of behavioral reactivity with a standard battery of novel visual and auditory stimuli originally designed by Kagan and colleagues (e.g., Kagan & Snidman, 1991). Of these children, 81 (47 boys) were selected for the longitudinal study on the basis of extreme levels of (a) high negative reactivity, (b) high positive reactivity, and (c) low reactivity (Calkins, Fox, & Marshall, 1996). The selected sample was subsequently seen at 9 months, 14 months, 24 months, 4 years, and 7 years of age. At each of these visits, numerous physiological and behavioral measures were collected (Fox et al., 2001).
Beginning in the toddler years, the focus of the longitudinal study turned to patterns of behavioral inhibition over time. Due to attrition, additional children (n = 13, 6 boys) were recruited at ages 4 or 7 years to complete the quartet play sessions used to characterize behavioral inhibition (see below). The newly recruited children continued to participate in future waves of the longitudinal study. All participants were then invited to return in adolescence (13–15 years of age) for an assessment of the social, physiological, and cognitive consequences of behavioral inhibition (Fox & Reeb-Sutherland, 2010).
The current report focuses on the predictive relations between data collected at 9 months of age, when 70 children (40 boys) completed the sustained attention task. Data from two girls were lost due to technical errors. Eleven of the infants selected at 4 months did not attempt the sustained attention task, either because they were too fatigued at time of testing or they could not be scheduled for a laboratory visit within the narrow age window (plus or minus 1 week from the 9-month birthday).
Although we had success in retaining the selected sample, the number of available participants varied across measures and ages, as children missed individual waves within the larger study. All children were invited to participate in each wave of the study even if data were missing from prior visits. We report the total number of participants for each individual measure in the sections below.
Sustained Attention Task
We adapted the procedure from Casey and Richards (1988) for use in the current study.
Apparatus
At 9 months, an infant was seated on his or her mother’s lap with a 19-in. television monitor placed approximately 51 cm in front of the infant. The plane of the television was parallel to the infant’s eyes. The fixation stimuli were recorded, muted scenes from Sesame Street (see Richards, 1997). A projection screen was placed approximately 51 cm to the right of the infant. A slide projector was then used to present either a blank slide or a slide with a black-and-white bull’s-eye, which served as the dis-tracter stimulus. The bull’s-eye filled a 30-cm-square area on the projection screen. A video camera located above the television was used to record infant gaze direction and duration for later behavioral coding.
Procedure
In four control trials, the fixation stimulus was presented to the infant until he or she looked away. These trials were used to prevent an association between presentation of the fixation stimulus and the appearance of the distracter stimulus and were therefore not coded. For the eight experimental trials that followed, the fixation stimulus was presented for 3 s. The slide projector was then advanced to present the distracter stimulus, with the fixation stimulus still presented. The trial continued until the infant met the disengagement criteria: looking at neither the fixation point nor the distracter stimulus for 5 consecutive s. Compared with using a set time limit for each trial, this procedure allowed us to maximize variability in gaze pattern among the infants.
Behavioral coding
Coding began once the distracter stimulus was presented. We noted the total time attending to the fixation stimulus and the total time attending to the distracter stimulus across the experimental trials. The total time spent in the task was noted, as was the number of visual shifts from fixation stimulus to distracter stimulus.
The agreement for overall look duration and visual shifts was in line with previous studies (Casey & Richards, 1988; Richards & Cronise, 2000; Richards & Turner, 2001). The correlation in look time between two independent coders was .96, the agreement on a look toward the peripheral stimulus was 97%, and a comparison of trial-by-trial durations (defined by time until meeting disengagement criteria) was not significant (p = .80).
Calculation of sustained attention score
Across trials, infants spent a mean of 144.31 s (range = 21.98 to 347.79, SD = 57.35) in the task before reaching the disengagement criteria. As expected (Richards & Cronise, 2000), the infants exhibited a lognormal distribution of looking time and spent significantly more time visually attending to the fixation stimulus than to the distracter stimulus (60.49 vs. 22.30), t(67) = 12.85, p < .001, d = 3.14. However, a review of the data indicated that the infants differed in the degree to which they did so. In order to quantify this variability with a single variable, we calculated a difference score by subtracting the time spent attending to the distracter stimulus from the time spent attending to the fixation stimulus (range = −30.3 to 74.21, M = 38.1, SD = 24.45). Given the distribution of the raw looking times, our measure of sustained attention was also skewed. To reflect this distribution and note the extreme groups evident in the data, we mean split participants into low (n = 28, M = 14.03, SD = 18.80) and high (n = 40, M = 54.96, SD = 8.66) sustained attention groups.
Behavioral Inhibition in Early Childhood
At 14 (n = 80) and 24 (n = 78) months, the participants’ reactions to unfamiliar stimuli in the laboratory were coded to provide an index of behavioral inhibition (Calkins, Fox, & Marshall, 1996; Kagan, Reznick, & Snidman, 1988). At 14 months, the stimuli consisted of the following: (a) an unfamiliar room/ environment, (b) an adult stranger, and (c) a novel toy/object. At 24 months, the children were presented with identical stimuli, with the addition of an adult stranger dressed in a clown costume and an inflatable tunnel. These changes were designed to incorporate more developmentally appropriate stressors for the older children.
At 14 months, the behavioral inhibition scores were standardized (range = −1.85 to 3.00). Intercoder reliability was computed for 15% of the sample; Pearson correlations between coders for the subcomponents ranged from .85 to 1.00. At 24 months, a single behavioral inhibition score was similarly computed (range = −2.30 to 2.56). Intercoder reliability was computed for 24% of the sample; Pearson correlations ranged from 0.77 to 0.97.
At 4 and 7 years, children participated in a group play session with three unfamiliar, same-sex, same-age peers. At the 4-year visit, children (n = 58 from the original selected sample) were assigned to quartets with other children from the longitudinal study. At age 7, children (n = 49 from the original selected sample) were reassigned to new quartet combinations (for details, see Fox et al., 2001). At each age, additional children were recruited as needed in order to fully form the quartets (see above).
Scores at each age were derived for each child in the quartet from two 15-min free play sessions coded with Rubin’s (1989) Play Observation Scale (POS) focusing on onlooking and unoccupied behavior (Coplan, Rubin, Fox, Calkins, & Stewart, 1994). Three independent observers coded the tapes and reached acceptable reliability with Cohen’s kappa greater than 0.80.
Although there were no repeated meetings between children, quartet formation from within the participants in the longitudinal sample did not fully meet the independence assumption. As such, we ran separate unconditional models at each age predicting behavior at 4 years and then at 7 years. Unconditional models account for the nesting (only) in predicting the outcome. Results indicate that nesting was not a significant source of variance in the outcomes (Age 4: intraclass correlation < .01, p = ns; Age 7: intraclass correlation = 0.05, p = ns). Therefore, nesting was not accounted for in subsequent analyses.
Although the laboratory measures of behavioral inhibition were quantified through different procedures, each was conceptualized as a developmentally appropriate marker for the same underlying temperamental trait. Previous studies have used these measures in analyses of stability and change in behavioral inhibition across childhood (Fox et al., 2001; Fox et al., 2005; McDermott et al., 2009).
Social Behavior in Adolescence
Participants returned to the laboratory in adolescence (n = 62, M = 14.02 years, SD = 0.27) and completed a social scenarios task with a same-age, same-sex, unfamiliar peer recruited from outside the longitudinal cohort solely for the dyad visit.
The participants were seated at a table with the unfamiliar peer and asked to act out three social scenarios: (a) inviting a (hypothetical) unfamiliar teen to join in a group activity, (b) receiving a compliment from a hypothetical teen, and (c) offering to help a hypothetical teen. The tasks and coding procedures were modeled on previous work employing role play to examine social skill deficits (Beidel, Turner, & Morris, 2000; Bellack, Hersen, & Turner, 1979). Participants were videotaped during the role play. Coding focused on the affective quality and level of social discomfort separately for each social scenario. This included ratings of the adolescent’s level of smiling (5-point Likert scale from not at all smiling to smiling throughout), the clarity and volume of voice (4-point Likert scale from not at all clear to very loud and clear), speaking time (duration in seconds), and the overall level of anxiety for each scenario (4-point Likert scale from not at all anxious/nervous to extremely anxious/nervous). Intercoder reliability was computed for 20% of the sample; the intercorrelations between coders for the individual codes ranged from 0.79 to 0.93. Scores were standardized and averaged (after reverse scoring smiling, voice clarity, and speaking time) to create a single measure of social discomfort (range: −1.74 to 1.13, M = 0.030, SD = 0.65). Higher scores represent greater difficulty and unease during the task.
Statistical Analyses
The initial analysis examined the impact of individual differences in attention on the developmental trajectory of behavioral inhibition in early childhood. To do so we relied on latent basis growth models using Mplus (Muthén & Muthén, 2007). Relative to alternate data analytic approaches, this method held two main advantages. First, in line with our theoretical interests, structural equation modeling (SEM) captures differences in intraindividual change over time as a function of interindividual characteristics (MacCallum & Austin, 2000; McArdle, 2009). Second, at the analytical level, SEM uses full information maximum likelihood estimation rather than list-wise deletion. Therefore, we were able to retain participants who were missing a data point at one or more of the collection waves.
With the baseline model, we examined changes in the measured variables of behavioral inhibition at 14 months, 24 months, 4 years, and 7 years using standardized scores. We expected that this model would show little or no growth, given that we were using standardized scores from the whole sample. True evaluation of the research questions involved a second model involving a multi-group analysis examining the impact of sustained attention groups at 9 months on behavioral inhibition trajectories. In this model, we expected there to be group-related differences in growth.
For each model, we estimated the initial status of behavioral inhibition at 14 months (i.e., intercept) and the change over time through age 7 (i.e., slope). The parameterization for the slope factors was set to zero at Time 1 (14 months), allowed to vary at Times 2 and 3 (24 months and 4 years), and set to 1 at Time 4 (7 years). We were therefore able to note the proportional growth in behavioral inhibition across each time point. Model quality was assessed with three measures of fit: (a) the Bentler-Bonett normed fit index (NFI), (b) comparative fit index (CFI), and (c) root-mean-square error of approximation (RMSEA). Values of 0.90 to 1.00 are considered indicators of good fit for the NFI and CFI, whereas for RMSEA scores less than 0.10 are desirable.
After running the initial model and the multigroup model through age 7 years, we then tested models to predict social discomfort in adolescence. The first of these models focused on the predictive value of initial behavioral inhibition, sustained attention, and the interaction between the two measures. In parallel, the second model focused on change (i.e., slope) in behavioral inhibition (after controlling for the initial level), sustained attention, and the interaction of the two terms in predicting adolescent social discomfort.
Results
Descriptive scores for the two attention groups and correlations between measures of interest are presented in Table 1 and Table 2.
Table 1.
Individual Sustained Attention, Early Inhibition, and Social Behavior Scores for the Low and High Sustained Attention Groups
| Measure | Overall | Low sustained attention |
High sustained attention |
t-test | p-values |
|---|---|---|---|---|---|
| Sustained attention | 38.11 (24.45) | 14.03 (18.80) | 54.96 (8.66) | −10.75 | <0.001 |
| 14 months | −0.03 (0.95) | −0.10 (0.61) | 0.02 (1.13) | −0.524 | 0.600 |
| 24 months | −0.01 (0.98) | 0.06 (0.98) | −0.05 (0.98) | 0.458 | 0.649 |
| 4 years | −0.04 (1.03) | 0.35 (0.89) | −0.22 (1.05) | 1.974 | 0.043 |
| 7 years | 0.20 (1.08) | 0.80 (1.24) | −0.05 (0.91) | 2.450 | 0.019 |
| Adolescent social discomfort | 0.03 (0.65) | 0.06 (0.56) | 0.16 (0.61) | −0.510 | 0.612 |
Note. Standard deviations are noted in parentheses. The last column notes statistical differences between the two attention groups in independent sample t tests. Significant findings are in bold.
Table 2.
Intercorrelations Among the Central Measures in the Presented Analyses
| Measure | Sustained attention | 14 months | 24 months | 4 years | 7 years | Adolescent discomfort |
|---|---|---|---|---|---|---|
| Sustained attention | —— (68) | |||||
| 14 months | 0.015 (64) | —— (80) | ||||
| 24 months | −0.039 (60) | 0.373** (73) | —— (78) | |||
| 4 years | −0.251† (55) | 0.060 (68) | 0.265 (68) | —— (75) | ||
| 7 years | −0.338* (41) | 0.093 (51) | 0.189 (51) | 0.371** (55) | —— (59) | |
| Adolescent discomfort | 0.097 (45) | 0.191 (52) | 0.181 (51) | 0.076 (50) | 0.059 (42) | —— (62) |
Note. Degrees of freedom for the r statistic are noted in parentheses.
p < .10.
p < .05.
p < .01.
For the baseline model, which was an unconditional growth model, the fit statistics indicated the model accounted for the data well, χ2 = 0.098, p = .99, NFI = 0.99, CFI = 1.00, RMSEA = 0.00. As expected, the model showed no significant change in behavioral inhibition scores over time when using the whole sample, B < 0.001, SE = 0.14, p = .99.
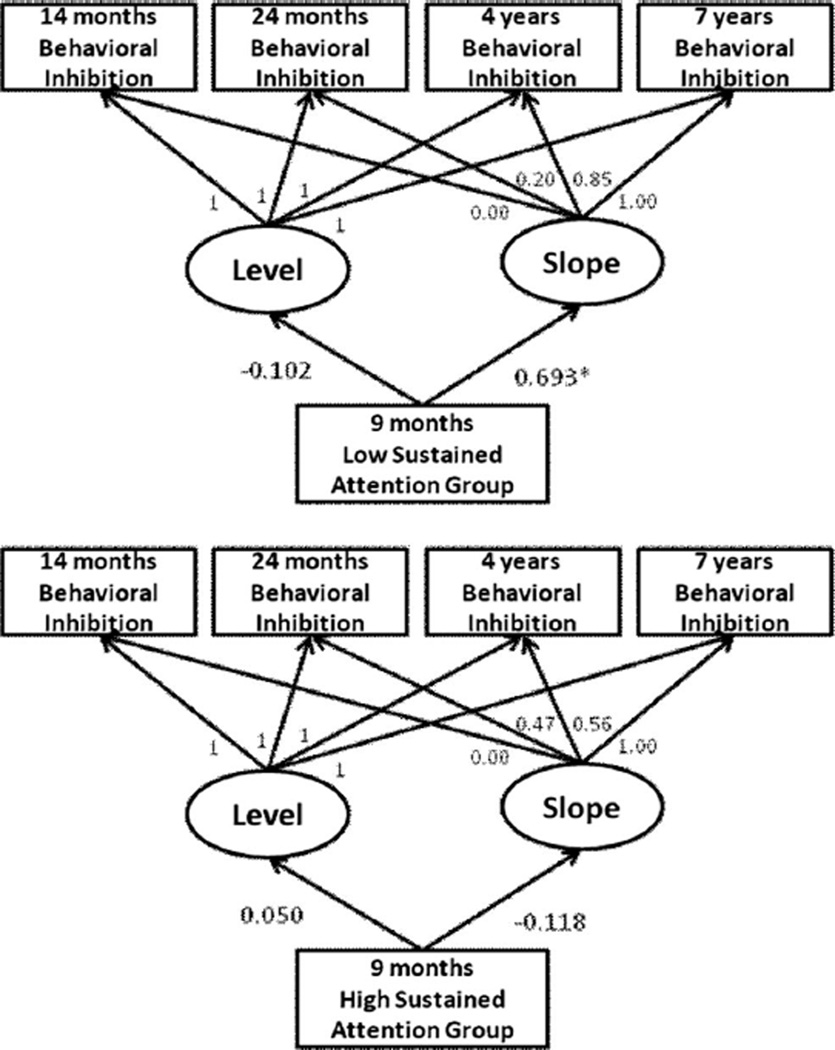
A multigroup analysis was then performed that compared the parameter estimates in the model for the low and high sustained attention groups. The unconditional multigroup model (see Figure 1) was a good fit to the data, χ2 = 4.42, p = .62, with NFI = 0.84, CFI = 1.00, and RMSEA = 0.00. For the children in the low sustained attention group, the initial level of inhibition was not significant, B = −0.102, SE = 0.12, p = .40, reflecting that the means were not different from zero at 14 months (see Table 1). Notably, the rate of growth in behavioral inhibition was now significant, B = 0.693, SE = 0.32, p = .03. In particular, the basis estimate, which was set to zero at 14 months, grew to 0.20 at 24 months, 0.85 at 4 years, and 1.00 at 7 years (where it was set). For the high sustained attention group, neither the initial level, B = 0.050, SE = 0.16, p = .75, nor slope, B = −0.118, SE = 0.19, p = .54, was significant. Thus, infants with low sustained attention at 9 months were more likely to show increases in behavioral inhibition over the course of early childhood.1
Figure 1.
Proportional growth in the parameter estimates of initial inhibition and growth over the course of early childhood associated with individual differences in sustained attention in infancy. Normed fit index = 0.84; comparative fit index = 1.00; root-mean-square error of approximation = 0.00, * p < .05.
Building on the baseline growth model, we then examined the relations between sustained attention and behavioral inhibition in predicting levels of social discomfort in adolescence. The first model estimated the effects of initial behavioral inhibition (i.e., intercept), sustained attention group, and the interaction of intercept and attention group on social discomfort in adolescence. Overall, the addition of these paths had a significant impact on the model in terms of the change in log likelihoods (McArdle, 2007), Δχ2(3) = 28.67, p < .001, as well as the Bayesian information criterion (BIC), 830.01 versus 849.58 (baseline). Within the model, the intercept and slope estimates showed significant individual variance (intercept: M = −0.03, SD = 0.73, pvariance < 0.001; slope: M = 0.08, SD = 0.93, pvariance < 0.001). In addition, adolescent social discomfort was significantly predicted by the interaction of initial behavioral inhibition and attention group, B = −0.46, z = −2.33, p = .02. To interpret this interaction, we reran the analysis with attention group centered to the low group and then again with attention group centered to the high group, in accordance with Aiken and West (1991). For the children with low sustained attention, children’s initial behavioral inhibition (intercept) was significantly and positively associated with their social discomfort in adolescence, B = 0.53, z = 3.41, p = .001. However, when sustained attention was high, initial behavioral inhibition was not significantly associated with social discomfort, B = 0.08, z = 0.68, p = .50.
The second model estimated the effects of initial inhibition (i.e., intercept), change in inhibition (i.e., slope), attention group, and the interaction of slope and attention group on social discomfort in adolescence. Overall, the addition of the paths involving slope had a significant impact on the baseline model in terms of both the change in log likelihoods (McArdle, 2007), Δχ2(4) = 29.41, p < .001, as well as the Bayesian information criterion (BIC), 833.49 versus 849.58 (baseline).2 Within this model, the intercept and slope estimates showed significant individual variance (intercept: M = −0.03, SD = 0.72, pvariance < .001; slope: M = 0.08, SD = 0.89, pvariance < .001). However, the interaction between change in behavioral inhibition and attention group was not significantly associated with adolescent social discomfort, B = 0.37, z = 1.81, p = .07.
Discussion
The current study evaluated the degree to which sustained attention in infancy is associated with the trajectory of socioemo-tional development over the course of early childhood. Prior evidence suggested that individual differences in attention are linked to patterns of socioemotional behaviors over time, perhaps by setting in motion a unique pattern of cognitive or affective processing (Fox, Henderson, Marshall, Nichols, & Ghera, 2005).
Temperament models clearly separate the reactive and regulatory mechanisms shaping behavior (Rothbart & Posner, 2006). In these models, individual differences in reactivity to stimuli are evident early in the first months of life and form the core of individual differences in early socioemotional behavior. With time, children can begin to regulate these initial biases in reactivity, helping to soften the markers of extreme temperament (Rothbart, Ellis, Rueda, & Posner, 2003). Attention, in this light, is considered a central mechanism of regulation. Since the focus is on later appearing regulatory mechanisms, much of the literature has examined higher order processes, such as effortful and inhibitory control.
However, the research suggests that attention mechanisms not only moderate initial reactive tendencies from the top down but also may elicit from the bottom up the same behaviors normally associated with negative reactivity. For example, recent work has demonstrated attentional biases to threat in adolescents with high levels of behavioral inhibition (Pérez-Edgar et al., 2010) or anxiety (Roy et al., 2008). These biases, in turn, may play a causal role in the emergence of anxiety (Eldar, Ricon, & Bar-Haim, 2008; Wilson, MacLeod, Mathews, & Rutherford, 2006).
The current study found an analogous pattern of results even though the core behavior assessed during infancy was not linked to a specific class of stimuli (i.e., threat cues). The observed developmental trajectories may be a reflection of the important gatekeeper role that attention plays in day-to-day psychological processes, influencing the initiation, deployment, and termination of a wide range of functions at the behavioral and neural level. The relations demonstrated here allow for the possibility that early appearing differences in attention orienting and control may work to bias development by shaping interactions with, and interpretations of, emotionally salient components of the environment. Future studies directly examining the proposed causal mechanisms will be needed to fully address this working hypothesis.
The study’s limitations should be noted when one is reviewing the findings. First, the overall number of participants was somewhat small. While this was partially offset by the richness of the developmental data available, future work would benefit by systematically collecting measures of both attention and social behavior across multiple time points with a larger sample. Second, the design of the attention task did not allow us to determine if the observed performance differences were due to the child’s relative inability to control attention (i.e., attention was involuntarily captured by the distracter stimulus) or biases in how the child actively deployed his or her somewhat limited attentional resources. In-triguingly, data suggest that nascent top-down (i.e., cortical) executive attention mechanisms begin to emerge in the second half of the first year of life (Johnson, 1990), perhaps reflecting the development of effortful control processes that shape socioemotional functioning (Rothbart et al., 2003). Examining this distinction will require a more complex task or set of tasks. Third, the current study did not incorporate psychophysiological measures, such as heart-rate deceleration (Casey & Richards, 1988), that are often used to mark periods of sustained attention. As such, the current study could address behavior only over the course of the entire task.
The current study spanned a time period marked by important changes in socioemotional functioning. Capturing this change in a developmentally appropriate manner can be quite challenging. Finding an early mechanism whose imprint is detectible throughout this period may be even more difficult. However, our data suggest that early attention may act as a marker of hypervigilance or increased sensitivity to novelty, shaping socioemotional trajectories through age 7 and into adolescence. Attention may impact functioning by determining which aspects of the environment the child will focus on and subsequently process. Future work will further refine the links between attention and other known mechanisms of risk, potentially signaling its role as an endophenotypic marker amenable to both early detection and intervention.
Acknowledgments
The authors would like to thank Kenneth H. Rubin and Amy Kennedy Root for the coding and analysis of the peer interaction data at ages 4 and 7 years. We would also like to thank Stacey Barton, Melissa Ghera, Dalit H. Marshall, Kirsten VanMeenen, Ariana Shahinfar, Genevieve Erb, Patricia Peters, Shari K. Young, and Lisa Perry for their assistance in the longitudinal data collection. We would especially like to thank the parents of the children who participated and continue to participate in our studies. Funding for the study was provided by National Institute of Mental Health Grant MH073569 to Koraly Pérez-Edgar, by National Institutes of Health Grants MH074454 and HD17899 to Nathan A. Fox, and by the National Alliance for Research on Schizophrenia and Depression’s Distinguished Investigator Award to Nathan A. Fox.
Footnotes
We also completed a separate 4 × 2 repeated measures analysis of variance with the standardized behavioral inhibition scores and sustained attention groups for children with full data across childhood (n = 38). Here we found a significant Age × Group linear contrast, F(1, 36) = 7.22, p = .01, d = 0.90, confirming the results of the structural equation modeling analyses despite the limited sample size.
However, this model was not significantly different from the initial intercept-only model.
Contributor Information
Koraly Pérez-Edgar, George Mason University.
Jennifer N. Martin McDermott, University of Maryland
Katherine Korelitz, University of Maryland.
Kathryn A. Degnan, University of Maryland
Timothy W. Curby, George Mason University
Daniel S. Pine, National Institute of Mental Health
Nathan A. Fox, University of Maryland
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Asendorpf JB, Denissen JJA, van Aken MAG. Inhibited and aggressive preschool children at 23 years of age: Personality and social transitions into adulthood. Developmental Psychology. 2008;44:997–1011. doi: 10.1037/0012-1649.44.4.997. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Ernst M. Neural correlates of reward processing in adolescents with a history inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, van IJzendoorn M. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM, Morris TL. Behavioral treatment of childhood social phobia. Journal of Consulting and Clinical Psychology. 2000;68:1072–1080. [PubMed] [Google Scholar]
- Bellack AS, Hersen M, Turner SM. Relationship of role playing and knowledge of appropriate behavior to assertion in the natural environment. Journal of Consulting and Clinical Psychology. 1979;47:670–678. doi: 10.1037//0022-006x.47.4.670. [DOI] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. A 3-year follow-up of children with and without behavioral inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Casey BJ, Richards JE. Sustained visual attention in young infants measured with an adapted version of the visual preference paradigm. Child Development. 1988;59:1514–1521. [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: Distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research & Therapy. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Pérez-Edgar K, White L. The biology of temperament: An integrative approach. In: Nelson C, Luciana M, editors. The handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2008. pp. 839–854. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin KH, Schmidt LA, Hamer DH, Pine DS. Evidence for a gene environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Reeb-Sutherland BC. Biological moderators of infant temperament and social withdrawal. In: Rubin KH, Coplan RJ, editors. The development of shyness and social withdrawal. New York, NY: Guilford Press; 2010. pp. 84–106. [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hill AL, Braungart-Rieker JM. Four-month attentional regulation and its prediction of three-year compliance. Infancy. 2002;3:261–273. doi: 10.1207/S15327078IN0302_9. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Annual Progress in Child Psychiatry & Child Development. 1988:102–127. [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Five steps in the structural factor analysis of longitudinal data. In: Cudeck R, MacCallum RC, editors. Factor analysis at 100: Historical developments and future directions. Mahwah, NJ: Erlbaum; 2007. pp. 99–130. [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McConnell BA, Bryson SE. Visual attention and temperament: Developmental data from the first 6 months of life. Infant Behavior & Development. 2005;28:537–544. [Google Scholar]
- McDermott JM, Pérez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox N. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus 5.0 user’s guide. Los Angeles, CA: Author; 2007. [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, Martin McDermott J, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. A behavioral and electrophysiolog-ical study of children’s selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–118. [Google Scholar]
- Pérez-Edgar K, Schmidt LA, Henderson HA, Schulkin J, Fox NA. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008;33:916–925. doi: 10.1016/j.psyneuen.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. The development of sustained visual attention in infants from 14 to 26 weeks of age. Psychophysiology. 1985;22:409–416. doi: 10.1111/j.1469-8986.1985.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Development. 1987;58:488–496. [PubMed] [Google Scholar]
- Richards JE. Peripheral stimulus localization by infants: Attention, age, and individual differences in heart rate variability. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:667–680. doi: 10.1037//0096-1523.23.3.667. [DOI] [PubMed] [Google Scholar]
- Richards JE, Cronise K. Extended visual fixation in the early preschool years: Look duration, heart rate changes, and attentional inertia. Child Development. 2000;71:602–620. doi: 10.1111/1467-8624.00170. [DOI] [PubMed] [Google Scholar]
- Richards JE, Turner ED. Extended visual fixation and distractibility in children from six to twenty-four months of age. Child Development. 2001;72:963–972. doi: 10.1111/1467-8624.00328. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1144. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Handbook of developmental psychopathology. 2nd ed. Vol. 2. Hoboken, NJ: Wiley; 2006. pp. 465–501. [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, Pine DS. Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) Waterloo, ON: University of Waterloo; 1989. [Google Scholar]
- Wilson EJ, MacLeod C, Mathews A, Rutherford EM. The causal role of interpretive bias in anxiety reactivity. Journal of Abnormal Psychology. 2006;115:103–111. doi: 10.1037/0021-843X.115.1.103. [DOI] [PubMed] [Google Scholar]