Abstract
Purpose.
To determine the order and components of the signaling pathway utilized by epidermal growth factor (EGF) to stimulate conjunctival goblet cell proliferation.
Methods.
Goblet cells from rat bulbar and forniceal conjunctiva and human bulbar conjunctiva were grown in organ culture. Goblet cells (GCs) were serum starved for 24 hours and preincubated with inhibitors for 30 minutes or small interfering RNA (siRNA) for 48 hours prior to addition of EGF. Proliferation was then measured or Western blot analysis was performed using antibodies against phosphorylated protein kinase B (AKT), extracellular signal-regulated kinase 1/2 (ERK1/2), or the non-receptor tyrosine kinase Src. Rat GCs were also incubated with adenoviruses expressing dominant negative protein kinase Cα (DNPKCα) or constitutively activated protein kinase Cα (myrPKCα), and activation of AKT and ERK1/2 was determined by Western blot analysis.
Results.
Inhibitors of phosphoinositol-3 kinase (PI-3K)/AKT pathway blocked EGF-stimulated ERK1/2 activation and GC proliferation. Inhibitors of EGF-stimulated ERK1/2 activity did not inhibit AKT activation but blocked proliferation. DNPKCα blocked EGF-stimulated activation of AKT and ERK1/2 while myrPKCα increased activation of these kinases. Inhibitors of PI-3K, ERK1/2, and protein kinase C (PKC) blocked myrPKCα-stimulated GC proliferation. EGF and myrPKCα increased phosphorylation of Src, and inhibition of Src with the chemical inhibitor PP1 or siRNA inhibited EGF-stimulated GC proliferation.
Conclusions.
We found that EGF activates a major pathway to stimulate goblet cell proliferation. This pathway consists of induction of phospholipase C (PLC)γ to activate PKCα. Active PKCα phosphorylates Src to induce PI-3K to phosphorylate AKT that subsequently activates the ERK1/2 cascade to stimulate goblet cell proliferation.
Keywords: cell proliferation, EGF, goblet cells, conjunctiva
EGF activates PLCγ to activate PKCα, which in turn phosphorylates Src. Src induces PI-3K to phosphorylate AKT that activates the ERK1/2 cascade to stimulate goblet cell proliferation.
Introduction
Epidermal growth factor (EGF) functions as the major stimulus of epithelial proliferation in health, and its aberrant regulation can drive tumor growth. The signaling pathways activated by EGF are complex and tissue specific. EGF binds to its receptor, the EGF receptor, and can form homodimers or heterodimers with other members of the EGF receptor family. Formation of dimers leads to autophosphorylation of specific tyrosine residues on the EGF receptor that serve as docking sites to recruit specific adapter proteins. Five major autophosphorylation sites are present: Y992, which binds phospholipase C (PLC)γ1; Y1045, which binds E3 ubiquitin-protein kinase (Cbl); Y1068 and Y1086, which interact with the adaptor proteins growth factor receptor-bound protein 2 (Grb2) and Grb2-associated binding protein 1 (Gab1); Y1148, which attaches to the adaptor protein Shc; and Y1173, which works with the tyrosine protein phosphatase Shp1, the Src homology 2 domain transforming protein Shc, and PLCγ1.1–3 Binding of individual adapter proteins leads to activation of specific signaling pathways. When activated, PLCγ1 produces inositol trisphosphate (InsP3), which releases intracellular Ca2+ and diacylglycerol that activates protein kinase C (PKC) isoforms. Cbl is a ubiquitin ligase and is believed to be involved in EGF receptor internalization.4 Phosphorylation of Grb2 and Shc activates the extracellular regulated kinase (ERK)1/2 cascade through the guanine nucleotide exchange factor Son of Sevenless (SOS) and the kinases Ras, Raf, mitogen-activated protein kinase kinase (MEK), and ERK1/2. Phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and c-Jun N-terminal kinase (JNK) can also be induced. Activation of the Grb2/Gab1 adaptor proteins induces the phosphoinositide-3 kinase (PI-3K) pathway that works through phosphorylation of AKT, also known as protein kinase B. When each of these signaling pathways is activated, the distal component of the pathway translocates to the nucleus where it activates immediate early genes to start the cellular proliferation program.2 In addition to causing cellular proliferation, EGF can also induce cellular migration, differentiation, or tumor formation.
Similarly to its effects in other epithelial cells, EGF stimulates conjunctival goblet cell proliferation.5–7 In addition, EGF induces secretion of high molecular weight glycoconjugates that include the large gel-forming mucin, MUC5AC.8 The amount of goblet cell secretory product, especially MUC5AC, is controlled by the rate of cell proliferation (the number of goblet cells in the conjunctiva), goblet cell secretion, and goblet cell secretory protein synthesis. As EGF stimulates both conjunctival goblet cell proliferation and secretion, it plays a critical role in the regulation of goblet cell mucin production from the conjunctiva.
The signaling pathways used by EGF to cause conjunctival goblet cell proliferation have begun to be investigated. Using both human and rat conjunctival goblet cells in culture, our laboratory has shown that the EGF receptor is present in these goblet cells and that exogenous EGF transactivates the EGF receptor and activates PLC to increase intracellular [Ca2+] and activate PKCα and -ε.5,7 Active PKCα then interacts with the ERK1/2 pathway, translocating ERK/2 to the nucleus where it induces cellular proliferation.9
Autophosphorylation of the EGF receptor can also activate PI-3K, whose major substrate is the protein kinase AKT. PI-3K is predominantly a lipid kinase that upon activation translocates to the plasma membrane.10 At this location it is brought into proximity with phosphoinositides from which PI-3K generates D-3 phosphorylated lipid products. These products bind to the pleckstrin homology (PH) domain of AKT and the serine-threonine kinase PDK1, bringing them in proximity so that PDK1 can phosphorylate AKT. Mammalian target of rapamycin complex (mTORc), another serine-threonine kinase, also phosphorylates AKT, locking AKT into its active conformation. Active AKT can then induce multiple processes including proliferation, angiogenesis, cell survival, metabolism, and translation. AKT works when its PH domain binds to protein substrates. The Ras guanosine triphosphatase (GTPase)-activating protein RasGAP can bind to AKT.11
Src is a nonreceptor tyrosine kinase that plays a key role in cell differentiation, motility, proliferation, and survival.12 Src has multiple phosphorylation sites. Dephosphorylation of phosphotyrosine 527 increases Src activity; phosphorylation of phosphotyrosine 416 increases Src activity; phosphorylation of tyrosine 138 does not change its activity; and phosphorylation of tyrosine 213 increases Src activity.12 Src kinase can also be phosphorylated by protein serine-threonine kinases such as PKC (serine 12), protein kinase A (serine 17), and CDK1/cdc2 (threonine 34 and 46, serine 72).12
In the present study we determined whether EGF activates PI-3K/AKT pathway to cause proliferation, where in the EGF-stimulated signaling pathway this complex fits, and if PKCα activates the nonreceptor tyrosine kinase Src to stimulate PI-3K/AKT activity. We found that there is a major pathway that causes goblet cell proliferation consisting of EGF inducing PLCγ to activate PKCα. Active PKCα phosphorylates Src to induce PI-3K to phosphorylate AKT that subsequently activates the ERK1/2 cascade to stimulate goblet cell proliferation.
Materials and Methods
Materials
EGF was purchased from PeproTech, Inc. (Rocky Hill, NJ). Ser473-phospho AKT monoclonal antibody, AKT polyclonal antibody, phospho c-Src monoclonal antibody (Tyrosine [Y] 416), and Src antibody were purchased from Cell Signaling Laboratories (Beverly, MA). Antibodies for phospho-ERK1/2, ERK 1, PKCα, and horseradish peroxidase–conjugated goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PP1, an inhibitor of Src family tyrosine kinases, was purchased from Enzo Life Sciences (Plymouth Meeting, PA). The inhibitors U0126 (blocks MEK and prevents activation of ERK1/2), LY294002 (blocks PI-3K and activation of AKT), and GÖ6983 (blocks activation of PKC) were purchased from EMD Chemicals (Madison, WI). Wortmannin was purchased from Cell Signaling Technology (Danvers, MA). The secondary antibody used for Western blot analysis was horseradish peroxidase–conjugated goat anti-rabbit IgG from Jackson Immunoresearch Laboratories, Inc. (West Grove, PA). β-actin monoclonal antibody was purchased from Sigma-Aldrich (St. Louis, MO). ERK2 siRNA and c-Src small interfering RNA (siRNA) were purchased from Dharmacon RNA Technologies (Lafayette, CO). Cell Counting Kit-8 for measurement of cell proliferation was purchased from Dojindo Molecular Technologies (Gaithersburg, MD). RPMI 1640 cell culture medium, penicillin/streptomycin, and L-glutamine were purchased from Lonza (Walkersville, MD). Fetal bovine serum (FBS) was obtained from HyClone Laboratories (Logan, UT). PKCα dominant negative adenovirus (Ad-DN PKCα) was the gift of George King of Joslin Diabetes Institute, Harvard Medical School (Boston, MA). The myristoylated (myr) PKCα construct was a gift from Alex Toker of Beth Israel Hospital, Harvard Medical School, Boston.
Animals
Male Sprague-Dawley rats weighing between 150 and 250 g (4–6 weeks old) were obtained from Taconic Farms (Germantown, NY) and used in the experiments. The rats were killed by CO2 inhalation. The bulbar and forniceal conjunctiva was removed from both eyes. The procedure was in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Human Tissue
Human conjunctival tissue was obtained from patients during ocular surgery using a protocol that adhered to the tenets of the Declaration of Helsinki and was approved by the Schepens Eye Research Institute Human Studies Internal Review Board. The tissue, which was normally discarded during surgery, was donated by three patients and was placed in PBS solution containing penicillin-streptomycin (300 μg/mL; Lonza).
Culture of Rat and Human Conjunctival Goblet Cells
Conjunctival goblet cell cultures were established from rat or human conjunctival tissue as described previously.13,14 Excised conjunctiva was cut into small pieces that were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin-streptomycin, and 2 mM glutamine. The cells were incubated at 37°C with 5% CO2 in 6-well plates. The tissue plug and nongoblet cells were removed after nodules of cells were formed. After 7 days, the goblet cells were trypsinized and plated in either 48- or 6-well culture plates for cell proliferation or Western blot analysis, respectively.
Constitutively Active and Dominant Negative PKCα Adenoviral Constructs
Adenoviral constructs expressing PKCα with an attached myristic acid (Ad-myrPKCα) that makes it constitutively active by attaching it to the membrane15 and dominant negative PKCa (Ad-DNPKCα) were used to transfect rat goblet cells as described previously.5 First-passage goblet cells were serum starved for 24 hours and then incubated with adenoviral constructs Ad-myrPKCα at 1 × 107 plaque-forming units (pfu) or Ad-DNPKCα at 1 × 106 pfu for 22 hours. The amount of PKCα present was determined by Western blot analysis using anti-PKCα antibody. β-actin protein was measured to determine the amount of cellular protein.
Transfection of Rat Goblet Cells With ERK2 siRNA and c-Src siRNA
Predesigned ERK2 and c-Src siRNA were obtained from Thermo Scientific Dharmacon RNAi Technologies (Lafayette, CO) as previously described.8 Irrelevant siRNA (sc siRNA) without sequence similarity to any known gene sequences was used as a negative control. First-passage conjunctival goblet cells were grown in 10% FBS containing RPMI 1640 medium for 24 hours. Rat Src siRNA, rat ERK2 siRNA, or irrelevant siRNA (100 nM each) was used with the DharmaFECT 1 siRNA Transfection Reagent. siRNA constructs were added in antibiotic-free media, and cells were incubated for 18 hours. Media were removed, and cells were transfected for 48 hours with antibiotic-free media. To determine the silencing effect of ERK2 siRNA, Src siRNA, and sc siRNA, cells were homogenized and analyzed by Western blotting. Measurement of endogenous β-actin protein expression was used to normalize ERK2 or Src expression.
Cellular Proliferation Assay
Primary rat conjunctival goblet cells were trypsinized and seeded on 48-well culture plates at a density of 400 to 500 cells/well. Cells were grown for approximately 48 to 72 hours until they reached subconfluency. Cells were serum starved using RPMI 1640 with 0.35% bovine serum albumin (BSA) for 24 hours prior to the start of each experiment. Cells were preincubated with buffer alone (RPMI 1640 with 0.35% BSA) or the inhibitors U0126, LY294002, and GÖ6983 for 30 minutes or siRNA for c-Src or ERK2 for 48 hours. Following preincubation, cells were stimulated with EGF (10−7 M) for 24 hours or Ad-myrPKCα (1 × 107 pfu) for 22 hours, as we had previously demonstrated that these concentrations in this time frame significantly increased goblet cell proliferation.7,9 Each condition was performed in triplicate. Incubation was terminated by the removal of supernatant, and cell proliferation was measured using the Cell Counting Kit-8 Assay (Dojindo Molecular Technologies). This assay measures the number of cells using the water-soluble salt WST-8. The absorbance was read at 465 nm using a microplate reader (Synergy MX Biotek, Winooski, VT) after the plate had been incubated at 37°C for 1 hour.
Western Blot Analysis
First-passage goblet cells grown on six-well plates were serum starved for 24 hours. Inhibitors were added for 30 minutes followed by incubation with EGF for 5 minutes. In select experiments, Ad-myrPKCα was added for 22 hours prior to stimulation with EGF. Ice-cold PBS was added to the wells to terminate the reaction. Western blot analysis was performed as previously described.5 In brief, radio-immunoprecipitation assay (RIPA) lysate buffer contained phosphate-buffered saline pH 7.4, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/mL phenylmethylsulfonylfluoride (PMSF), 42 μg/mL aprotinin, and 1 mM sodium orthovanadate. Cells were removed, sonicated, and homogenized. After determination of protein concentration, the proteins were separated by SDS-PAGE. The separated proteins were transferred from the gel onto a nitrocellulose membrane. The membrane was incubated overnight at 4°C with the appropriate primary antibody followed by secondary antibody conjugated to horseradish peroxidase (HRP). Detection was performed with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL), and the immunoreactive bands were analyzed by ImageJ (National Institutes of Health, Bethesda, MD).
Data Presentation and Statistical Analysis
Each experiment was repeated in goblet cells cultured from separate animals or humans; n is the number of individuals. Data are expressed as the fold increase over the basal value, which was set to 1.0. Results are expressed as the mean ± SEM. Data were analyzed by Student's t-test, and P ≤ 0.05 was considered statistically significant.
Results
EGF Activates PI-3K to Stimulate Proliferation of Rat and Human Goblet Cells
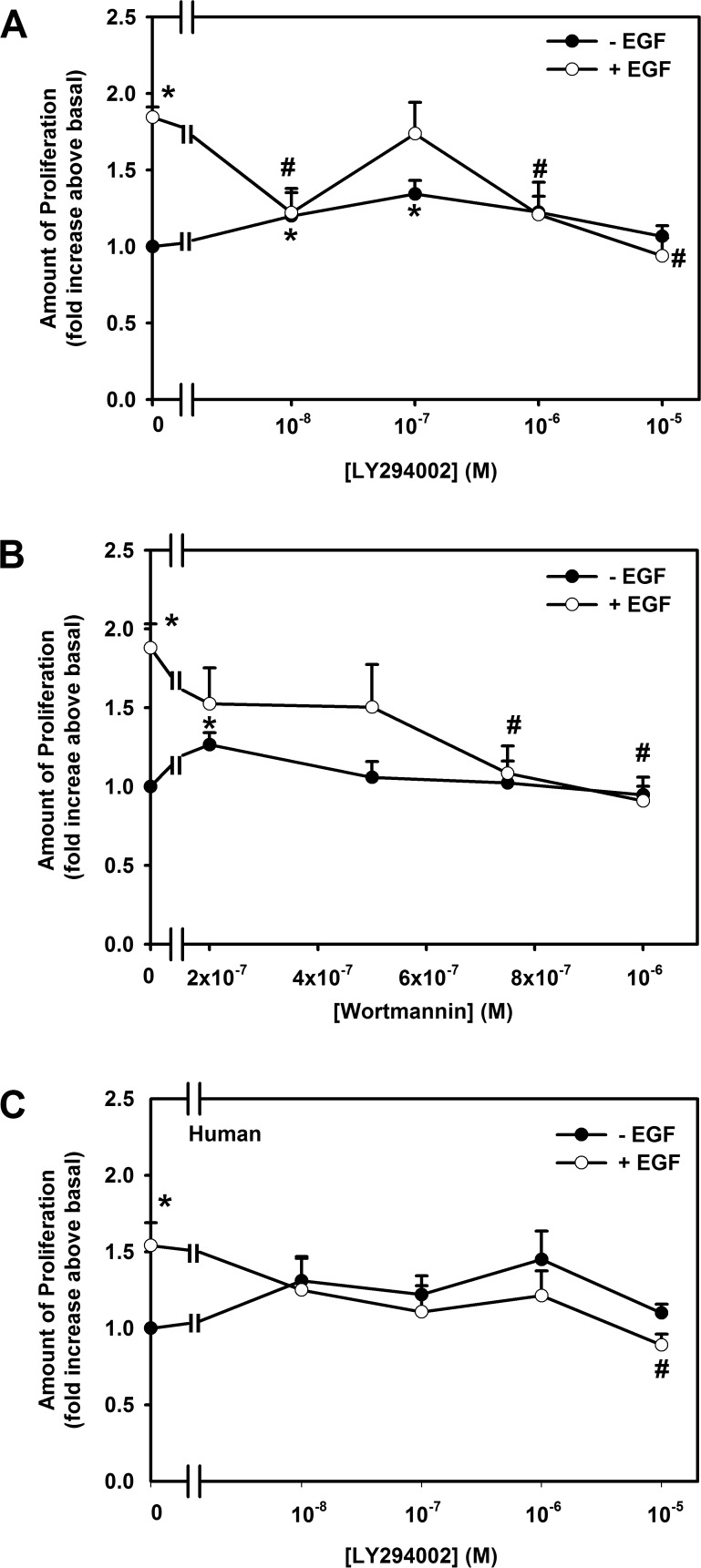
Rat goblet cells were preincubated with the PI-3K inhibitors LY294002 at 10−8 to 10−5 M or wortmannin at 2 × 10−7 to 10−6 M for 30 minutes and then stimulated with EGF at 10−7 M for 24 hours. EGF significantly stimulated proliferation 1.8 ± 0.1-fold above basal levels (Fig. 1A). LY294002 completely inhibited EGF-stimulated proliferation in a concentration-dependent manner, with a maximum inhibition obtained at 10−5 M. In the next set of experiments, EGF (10−7 M) significantly stimulated proliferation 1.9 ± 0.2-fold above basal (Fig. 1B). Wortmannin significantly decreased EGF-stimulated proliferation in a concentration-dependent manner, with complete inhibition obtained at 10−6 M (Fig. 1B). LY 294002 and wortmannin slightly increased basal goblet cell proliferation (Figs. 1A, 1B).
Figure 1.
Effect of PI-3K inhibitors on EGF-stimulated proliferation of cultured conjunctival goblet cells. Cultured rat conjunctival goblet cells were preincubated with LY294002 (10−8–10−5 M) (A) or wortmannin (0.2–1.0 μM) (B) for 30 minutes prior to stimulation with EGF (10−7 M) or with no addition for 24 hours. Cultured human conjunctival goblet cells were preincubated with LY294002 (10−7–10−5 M) (C) for 30 minutes prior to stimulation with EGF (10−7 M) or without EGF for 24 hours. The number of proliferating cells was determined by WST-8. Data are mean ± SEM from four independent experiments for (A) and (B) and three independent experiments for (C). *Statistically significant difference from 0. #Statistically significant difference from EGF.
The effect of LY 294002 was tested on human conjunctival goblet cells (Fig. 1C). EGF (10−7 M) significantly stimulated proliferation 1.5 ± 0.3-fold above basal. All concentrations of LY294002 blocked EGF-stimulated proliferation.
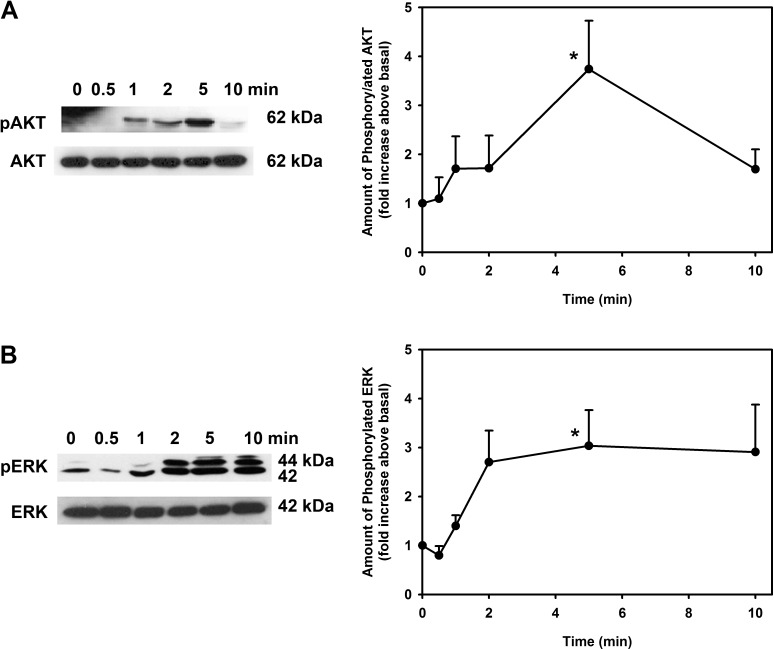
As these data suggest that EGF activates PI-3K to stimulate both human and rat goblet cell proliferation, we next determined whether EGF stimulates phosphorylation and thus activation of one of the main targets of PI-3K, AKT. Western blot analysis with antibodies to phosphorylated (active) and total AKT were used. Rat conjunctival goblet cells were incubated with EGF (10−7 M) for 0 to 10 minutes. EGF incubated for 5 minutes significantly increased phosphorylation of AKT by 3.7 ± 0.9-fold over basal level (Fig. 2A).
Figure 2.
Time course for AKT and ERK phosphorylation in EGF-stimulated rat goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours and then stimulated with EGF (10−7 M) for 0 to 10 minutes. Western blot analysis was performed using antibodies against phosphorylated and total AKT (A) and ERK (B). Representative blots from three experiments shown in figures on the left; data shown in the figures on the right represent mean ± SEM of three independent experiments. *Statistical significance compared with 0.
EGF Stimulates Phosphorylation of ERK1/2 in Rat Conjunctival Goblet Cells
By measuring the effect of ERK1/2 inhibitors on EGF-stimulated proliferation and EGF-induced translocation of ERK1/2 to the nucleus by immunofluorescence microscopy, we previously demonstrated that EGF uses ERK1/2 to cause goblet cell proliferation.6 To directly demonstrate the activation of ERK1/2 by EGF, we used Western blot analysis with antibodies to phosphorylated and total ERK1/2. We found that EGF (10−7 M) stimulated ERK1/2 phosphorylation in a time-dependent manner, with maximum activation of 3.0 ± 0.7-fold compared to basal occurring at 5 minutes of incubation (Fig. 2B).
EGF Induces AKT Activation to Cause Phosphorylation of ERK1/2 in Rat Conjunctival Goblet Cells
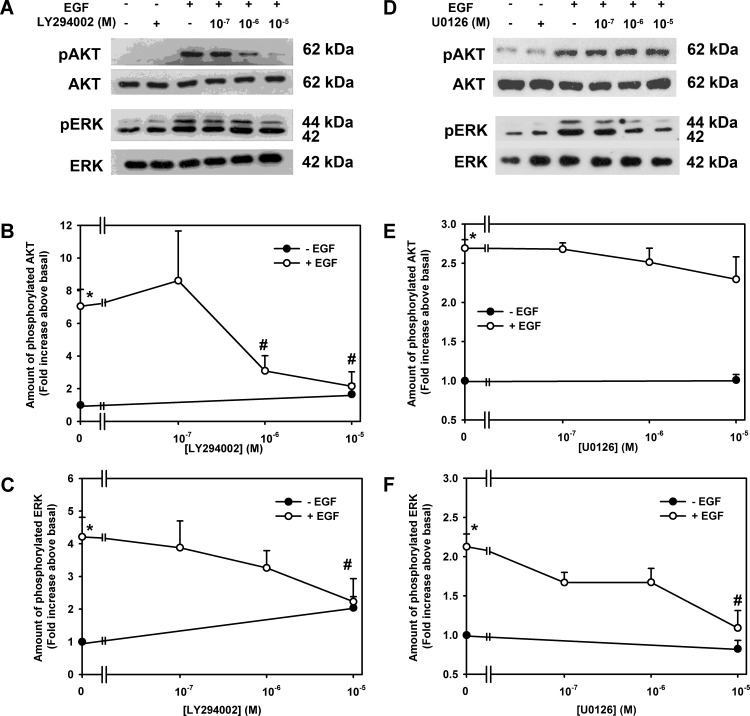
To determine if the PI-3K/AKT and PLCγ/ERK1/2 pathways are separate or if they interact, we measured the effect of the PI-3K inhibitor LY294002 and the inhibitor of MEK activation of ERK1/2 U0126 on phosphorylation of AKT and ERK1/2. EGF (10−7 M) stimulated AKT phosphorylation by 7.0 ± 1.0-fold compared to basal (Figs. 3A, 3B). LY294002 (10−5 M) almost completely blocked this phosphorylation. EGF caused a 4.2 ± 0.6-fold activation of ERK1/2 that was completely blocked by LY294002 at 10−5 M (Figs. 3A, 3C). Inhibition of PI-3K blocked activation of both AKT and ERK1/2.
Figure 3.
Effect of LY294002 and U0126 on EGF-stimulated AKT and ERK1/2 activation of rat goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours. The cells were preincubated with the PI-3K inhibitor LY294002 (10−7–10−5 M) (A–C) or MEK inhibitor U0126 (10−7–10−5 M) (D–F) for 30 minutes followed by stimulation with EGF (10−7 M) for 5 minutes. Western blot analysis was performed using antibodies against phosphorylated and total AKT (A, B, D, E) and ERK (A, C, D, F). A representative blot of three experiments is shown in (A, D), and the blots scanned and data shown in (B, C, E, F) represent mean ± SEM of three experiments. *Statistical significance compared with basal. #Statistical significance compared with EGF alone.
In another set of cells, EGF (10−7 M) stimulated AKT by 2.7 ± 0.1-fold compared to basal (Fig. 3D). U0126 did not alter EGF-induced phosphorylation of AKT at any concentration (Figs. 3D, 3E). In contrast, EGF (10−7 M) stimulated ERK1/2 activation of 2.1 ± 0.2-fold that was completely inhibited at 10−5 M U0126 (Figs. 3D, 3F). Inhibition ERK1/2 did not block activation of AKT.
We conclude that activation of AKT is upstream of the MEK activation of ERK1/2 in the EGF-dependent signaling pathway that can stimulate goblet cell proliferation.
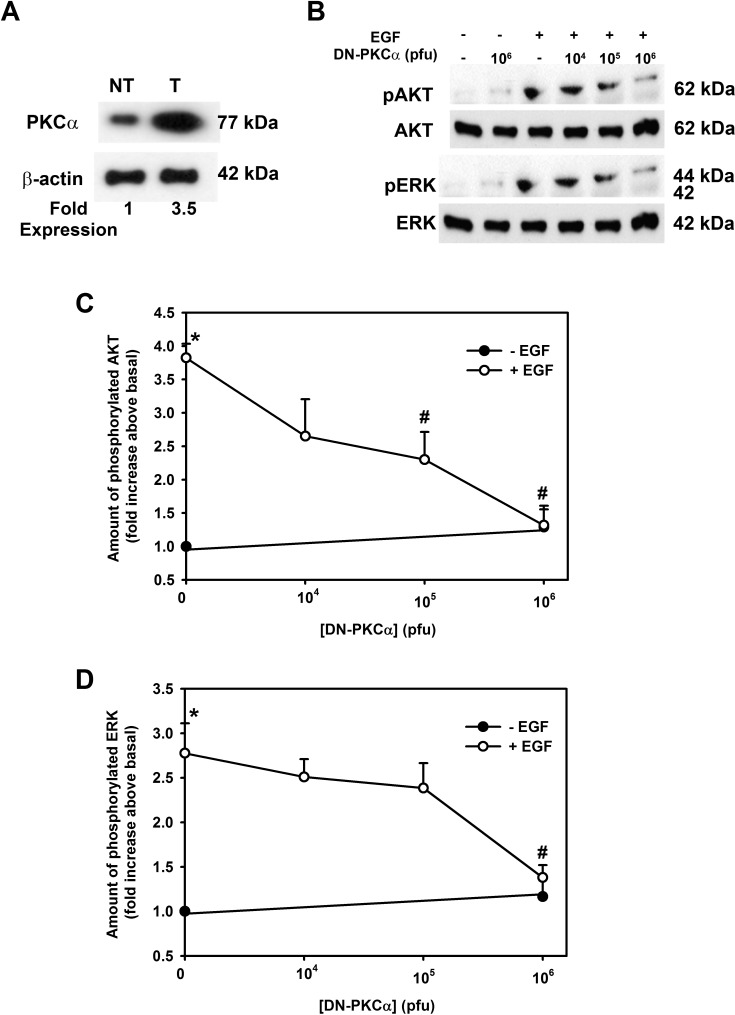
Inhibition of PKCα Blocks EGF-Stimulated AKT and ERK1/2 Activity in Rat Conjunctival Goblet Cells
We previously found that EGF activates PKCα to induce rat and human conjunctival goblet cell proliferation.5,7,9 To investigate if AKT and ERK1/2 phosphorylation were dependent on PKCα activation, we examined cells transfected with adenovirus for dominant negative PKCα that specifically blocks PKCα activity.5 Incubation of rat conjunctival goblet cells with Ad-DNPKCα increased the amount of PKCα in these cells (Fig. 4A). Transduction with 106 pfu of Ad-DNPKCα caused a 3.5-fold overexpression of PKCα compared to nontransduced cells (Fig. 4A). Inhibition of PKCα with DN-PKCα completely blocked activation of both AKT (Figs. 4B, 4C) and ERK1/2 by EGF (10−7 M) at 106 pfu (Figs. 4B, 4D). We suggest that PKCα is upstream of the AKT and ERK1/2 pathway induced by EGF in rat conjunctival goblet cells.
Figure 4.
Expression of dominant negative PKCα inhibits EGF-stimulated AKT and ERK1/2 activation of rat goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours and transduced with adenovirus containing a gene for dominant negative PKCα (DN-PKCα) for 22 hours followed by EGF stimulation for 5 minutes. Western blot analysis was performed using antibody to PKCα (A) (β-actin was loading control) to ensure that transduction had occurred. The amount of total and phosphorylated AKT and total and phosphorylated ERK was determined by Western blot analysis (B). The blots are representative of three independent experiments. The blots were scanned, and data shown in (C) and (D) represent mean ± SEM of three experiments. *Statistical significance compared with 0. #Statistical significance compared with EGF alone.
Activation of PKCα Stimulates Conjunctival Goblet Cell Proliferation by Activating PKC, AKT, and ERK1/2
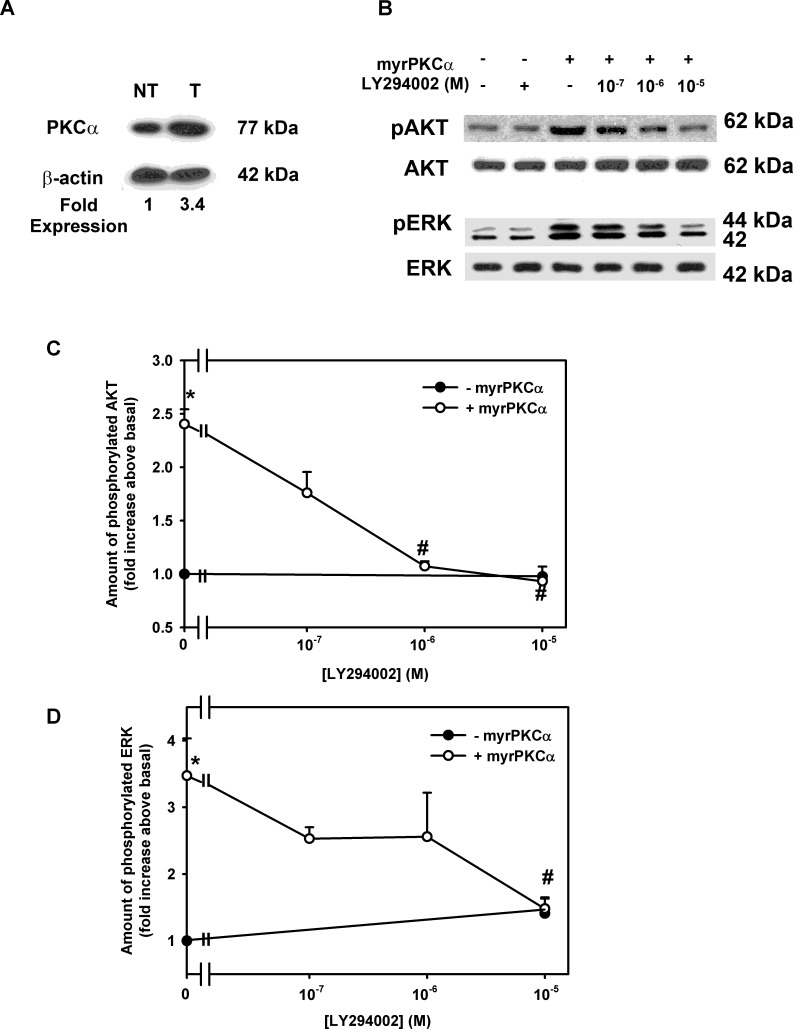
EGF activates multiple pathways when it interacts with its receptor, the EGF receptor. These pathways include PLCγ, Cbl and Gab1 (leading to PI-3K), and Shc/Grb2 (leading to the ERK1/2 and JNK cascades). Thus to determine if activation of PKCα itself in the absence of activation of other signaling pathways can increase AKT and ERK1/2 activity to induce proliferation, we stimulated conjunctival goblet cells with Ad-myrPKCα. Incubation with this Ad increased PKCα expression by 3.4-fold (Fig. 5A). We previously found that myrPKCα can induce ERK1/2 activation and stimulate conjunctival goblet cell proliferation.9 In the present experiments we investigated the role of PI-3K/AKT. Goblet cells were preincubated in LY294002 (10−7–10−5 M) before stimulation with Ad-myrPKCα (105 pfu). MyrPKCα stimulated AKT and ERK1/2 phosphorylation by 2.4 ± 0.1- and 3.5 ± 0.6-fold (Figs. 5B–D). LY294002 inhibited myrPKCα-stimulated AKT (Figs. 5B, 5C) and ERK1/2 phosphorylation (Figs. 5B, 5D) in a concentration-dependent manner, with 10−5 M completely preventing phosphorylation of both kinases. LY294002 did not alter basal phosphorylation of either AKT or ERK1/2. These results demonstrate that constitutively active PKCα alone can induce both AKT and ERK1/2 activation.
Figure 5.
Effect of inhibition of PI-3K on constitutively active PKCα-stimulated cultured rat conjunctival goblet cell phosphorylation of AKT and ERK. Cultured rat conjunctival goblet cells were serum starved for 24 hours. The cells were preincubated with LY294002 (10−7–10−5 M) for 30 minutes prior to stimulation with an adenovirus containing myristolyated (active) PKCα (myrPKCα) (1 × 107 pfu) or with no addition for 22 hours. Western blot analysis was performed using an antibody to PKCα (A) (β-actin was loading control). The amount of total and phosphorylated AKT and total and phosphorylated ERK was determined by Western blot analysis (B). The blots are representative of three independent experiments. The blots were scanned, and data shown in (C) and (D) represent mean ± SEM of three experiments. *Statistically significant difference from basal. #Statistical significant difference from myrPKCα alone.
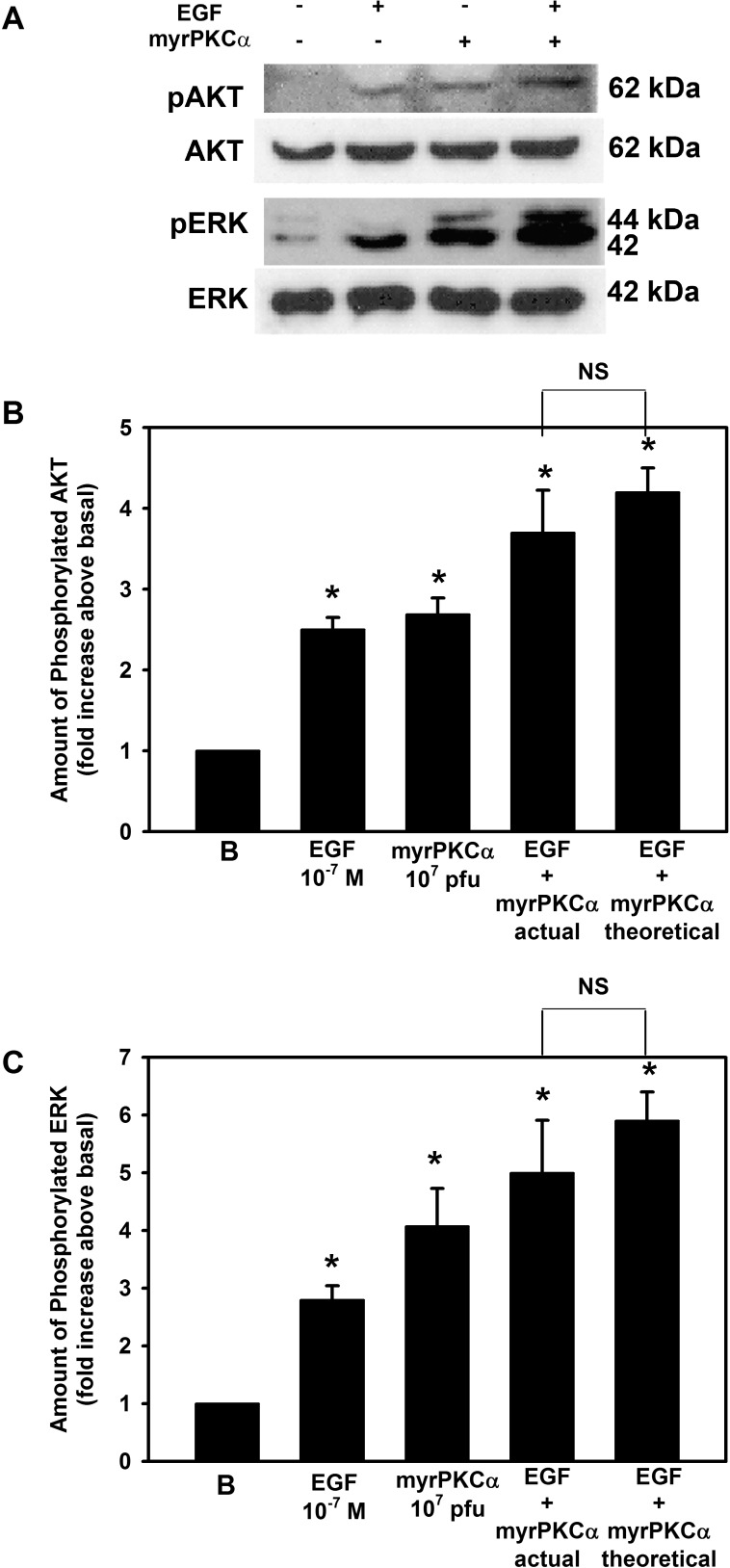
We next compared activation of AKT and ERK1/2 by constitutively active PKCα and EGF. Cultured conjunctival goblet cells were incubated with EGF (10−7 M), Ad-myrPKCα (107 pfu), or both (Figs. 6A–C). EGF and myrPKCα each significantly increased AKT phosphorylation by 2.5 ± 0.2- and 2.7 ± 0.2-fold, respectively (Figs. 6A, 6B), while EGF and Ad-myrPKCα each significantly increased ERK phosphorylation by 2.8 ± 0.3- and 4.1 ± 0.7-fold, respectively (Figs. 6A, 6C). When both agonists were added at the same time, AKT phosphorylation was significantly increased by 3.7 ± 0.5-fold and ERK1/2 by 5.0 ± 0.9. When calculated additivity (response to EGF alone plus myrPKCα alone minus basal) was determined and compared with experimental additivity, AKT phosphorylation was significantly increased by 4.2 ± 0.3-fold and ERK1/2 by 5.9 ± 0.5 above basal. As experimental additivity was not significantly different from calculated additivity, we conclude that EGF can activate AKT and ERK1/2 using different, additional signaling components other than those activated by constitutively active PKCα.
Figure 6.
The effect of myrPKCα on EGF-stimulated AKT and ERK activation in rat goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours and either stimulated with EGF (10−7 M) for 5 minutes, transduced with an adenovirus containing myristolyated (active) PKCα (myrPKCα) for 22 hours, or both. Western blot analysis was performed using antibodies against phosphorylated AKT, total AKT, phosphorylated ERK, and total ERK (A). The blots shown are representative of three independent experiments. The blots were scanned, and the data shown in (B) and (C) represent mean ± SEM of three experiments. *Statistical significance compared with basal. NS, not significant.
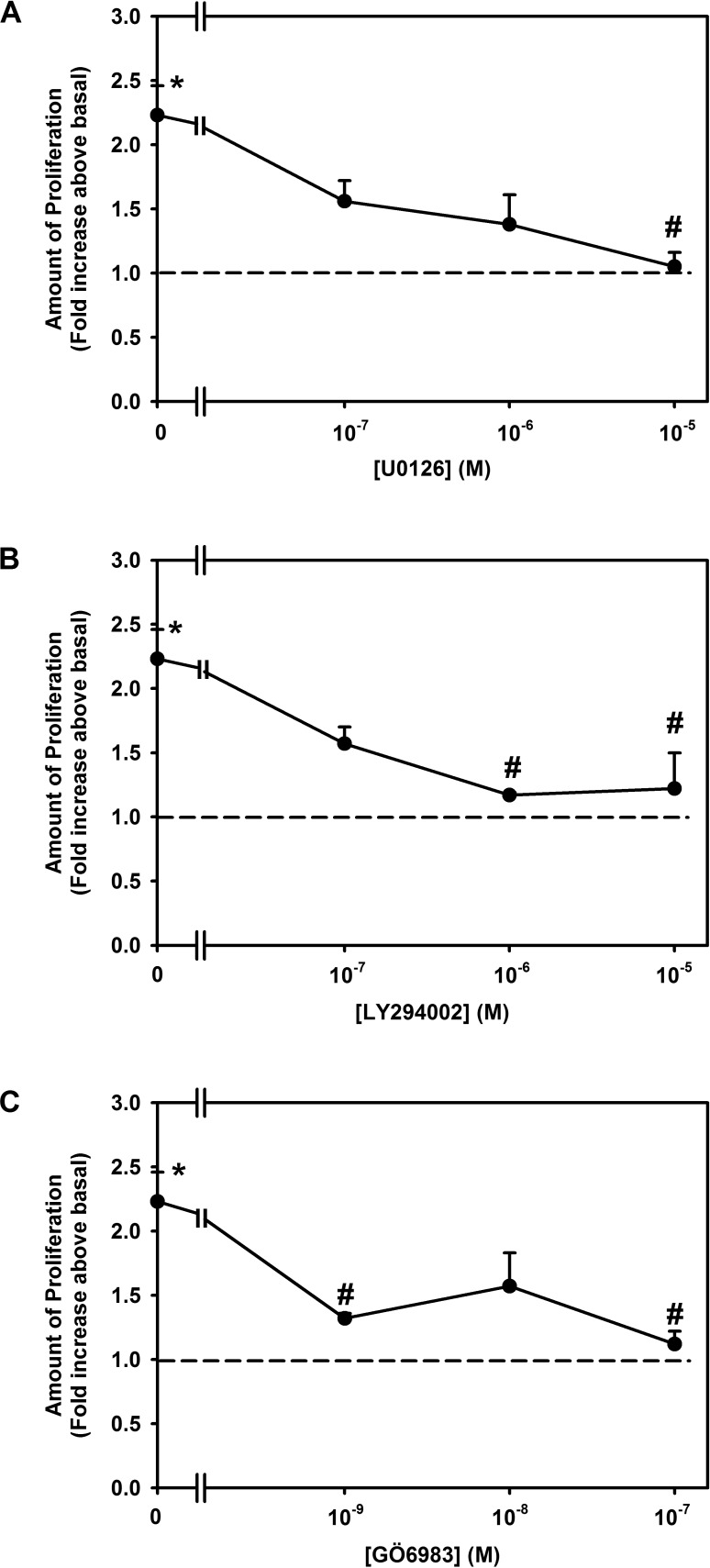
To evaluate whether PKC, AKT, and ERK activation is involved in the proliferation induced by constitutively active PKCα, we measured cell proliferation using inhibitors of MEK/ERK (U0126), PI3K/AKT (LY294002), or as a control PKC (GÖ6983). Rat goblet cells were preincubated with the MEK inhibitor U0126 at 10−7 to 10−5 M, AKT inhibitor LY294002 10−7 to 10−5 M, or the PKC inhibitor GÖ6983 10−9 to 10−7 M, then stimulated with Ad-myrPKCα at 107 pfu. In the absence of inhibitors, constitutively active PKCα significantly stimulated proliferation 2.2 ± 0.2-fold above basal (Figs. 7A–C). Increasing concentrations of U0126 inhibited myrPKCα-stimulated proliferation in a concentration-dependent manner, with complete inhibition obtained at 10−5 M U0126 (Fig. 7A), 88.8 ± 16.6% inhibition with 10−6 M LY294002 (Fig. 7B), and 92.0 ± 0.5% inhibition with 10−7 M GÖ6983, the control inhibitor (Fig. 7C). None of the inhibitors altered basal goblet cell proliferation (data not shown).
Figure 7.
Effect of inhibition of MEK, PI-3K, and PKC on constitutively active PKCα-stimulated cultured rat conjunctival goblet cell proliferation. Cultured rat conjunctival goblet cells were serum starved for 24 hours. The cells were preincubated with U0126 ([A], 10−7–10−5 M), LY294002 ([B], 10−7–10−5 M), or GÖ6983 ([C], 10−9–10−7 M) for 30 minutes prior to addition of an adenovirus containing myristolyated (active) PKCα (myrPKCα) (1 × 107 pfu) or with no addition for 22 hours. The number of proliferating cells was determined by WST-8. Data are mean ± SEM from three independent experiments. Dotted line indicates basal value, set to 1.0. *Statistically significant difference from basal. #Statistically significant difference from myrPKCα.
Thus, we suggest that constitutively active PKCα induces phosphorylation of AKT and ERK1/2 to cause conjunctival goblet cell proliferation and that AKT and ERK1/2 activation are downstream of PKCα activation.
EGF Increases Src Activity to Activate AKT and ERK1/2 to Stimulate Conjunctival Goblet Cell Proliferation
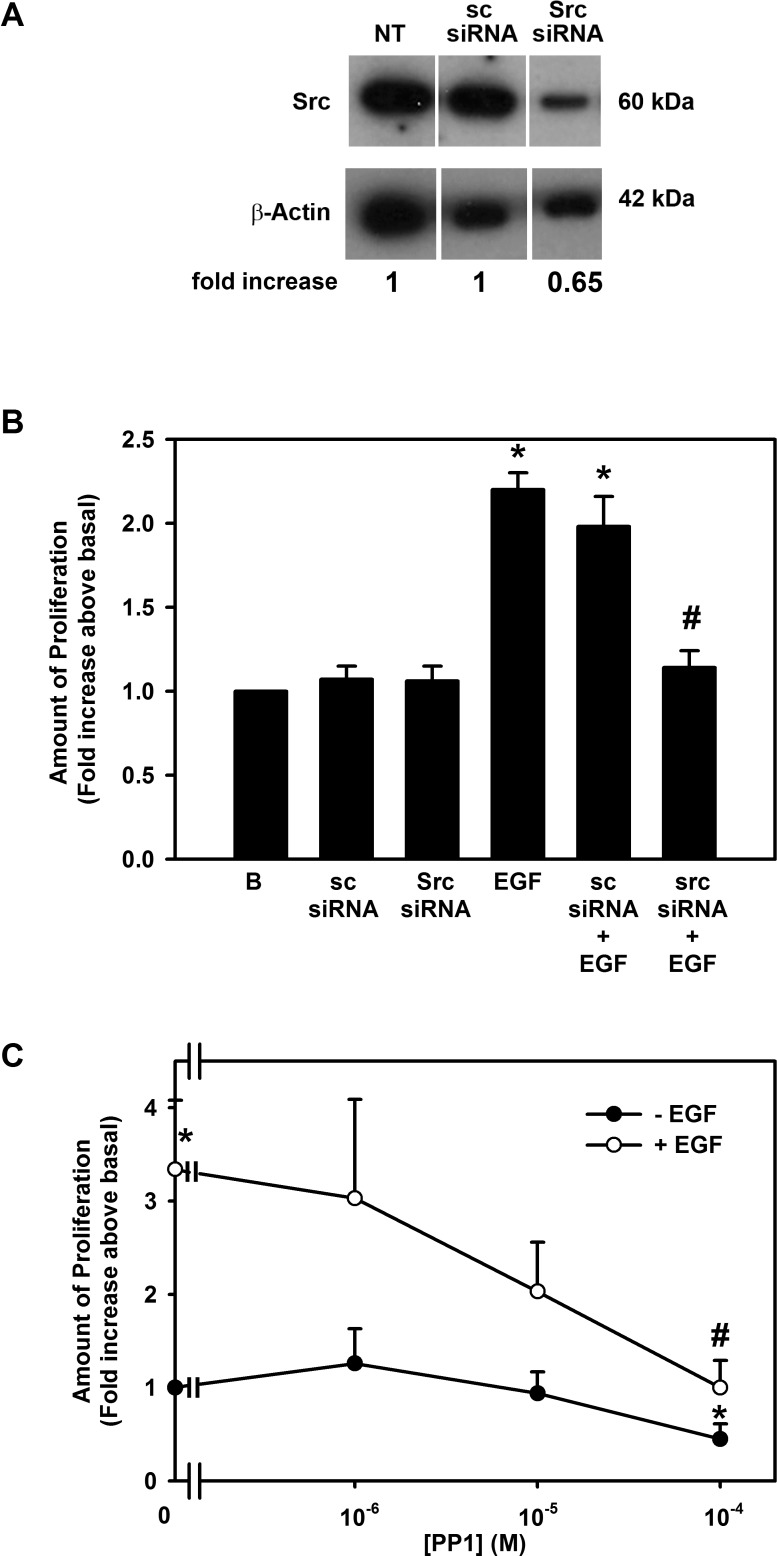
Src and PYK2 are nonreceptor tyrosine kinases that are known to activate PI-3K.16,17 To investigate whether EGF activates Src, two different inhibitors were used: siRNA specific for Src and the chemical inhibitor PP1. siRNA was first used to determine the effect of EGF on cellular proliferation. Conjunctival goblet cells were preincubated in buffer alone, scrambled siRNA (sc siRNA), or Src siRNA. Incubation with scrambled siRNA had no effect on the expression of Src, while Src siRNA substantially reduced its expression (Fig. 8A). The same conditions were used to deplete goblet cells of siRNA before stimulation with EGF (10−7 M). Neither sc siRNA nor Src siRNA altered basal proliferation (Fig. 8B). EGF stimulated proliferation 2.2 ± 0.1-fold. sc siRNA did not alter the EGF increase in proliferation, but Src siRNA blocked proliferation by 91.5 ± 7.2%.
Figure 8.
Effect of inhibition of Src on EGF-stimulated proliferation in rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours and incubated with scrambled sequence siRNA (sc siRNA) or Src siRNA for 48 hours (A, B) or PP1 for 30 minutes (C) followed by EGF for 24 hours. Western blot analysis demonstrating that Src siRNA reduced the amount of Src is shown in (A) and is representative of three independent experiments. The number of proliferating cells determined by WST-8 is shown in (B, C). Data are mean ± SEM from three independent experiments. *Statistically significant difference from basal, set to 1.0. #Statistically significant difference from EGF.
As a second method to determine if EGF uses Src to stimulate conjunctival goblet cell proliferation, rat goblet cells were incubated with the Src inhibitor PP1. EGF (10−7 M) stimulated proliferation by 3.3 ± 0.7-fold (Fig. 8C). Pre-exposure of cells to PP1 (10−6–10−4 M) blocked EGF-stimulated proliferation in a concentration-dependent manner, with almost complete inhibition occurring with 10−4 M PP1 (Fig. 8C).
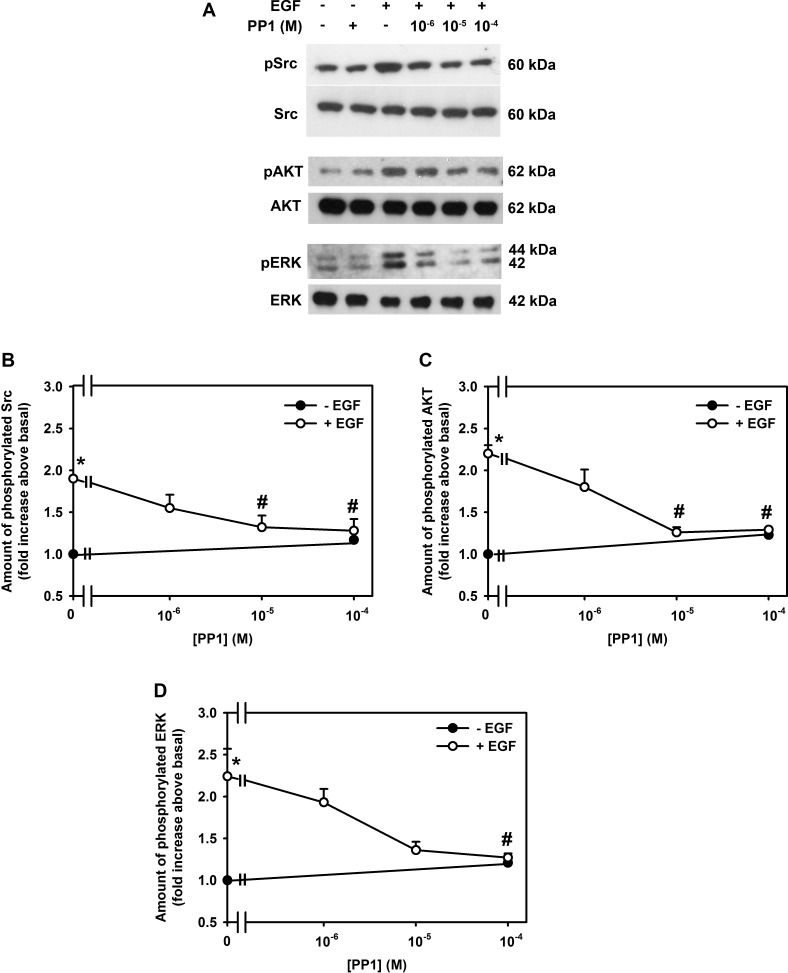
To determine if EGF activation of Src stimulated an increase in AKT and ERK1/2 activity, the effect of PP1 on EGF stimulation of AKT and ERK1/2 phosphorylation was measured using Western blot analysis. EGF (10−7 M) induced phosphorylation of Src, AKT, and ERK1/2 by 1.9 ± 0.0 (Figs. 9A, 9B)-, 2.2 ± 0.1 (Figs. 9A, 9C), and 2.2 ± 0.3 (Figs. 9A, 9D)-fold, respectively. As expected, PP1 completely inhibited Src activity (Figs. 9A, 9B). Preincubation with PP1 also completely inhibited EGF increase in AKT (Figs. 9A, 9C) and ERK1/2 phosphorylation (Figs. 9A, 9D). PP1 alone did not have any effect on basal activity (Figs. 9B–D).
Figure 9.
Effect of inhibition of Src on EGF-stimulated activation of Src, AKT, and ERK in rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 24 hours and preincubated with PP1 (10−6–10−4 M) for 30 minutes prior to addition of EGF for 5 minutes. Western blot analysis was performed using antibodies against phosphorylated and total Src (A, B), phosphorylated and total AKT (A, C), and phosphorylated ERK and total ERK (A, D). The blots shown in (A) are representative of three independent experiments. The blots were scanned, and the data shown in (B–D) represent mean ± SEM of three experiments. *Statistical significance compared with basal, set to 1.0. #Statistical significance compared with EGF alone.
We suggest that EGF activates Src and that this action causes subsequent stimulation of PI-3K to activate AKT. Based on Figure 3B, we suggest that phosphorylated AKT at an unidentified step in the ERK1/2 kinase cascade induces ERK1/2 phosphorylation that leads to an increase in cell proliferation.
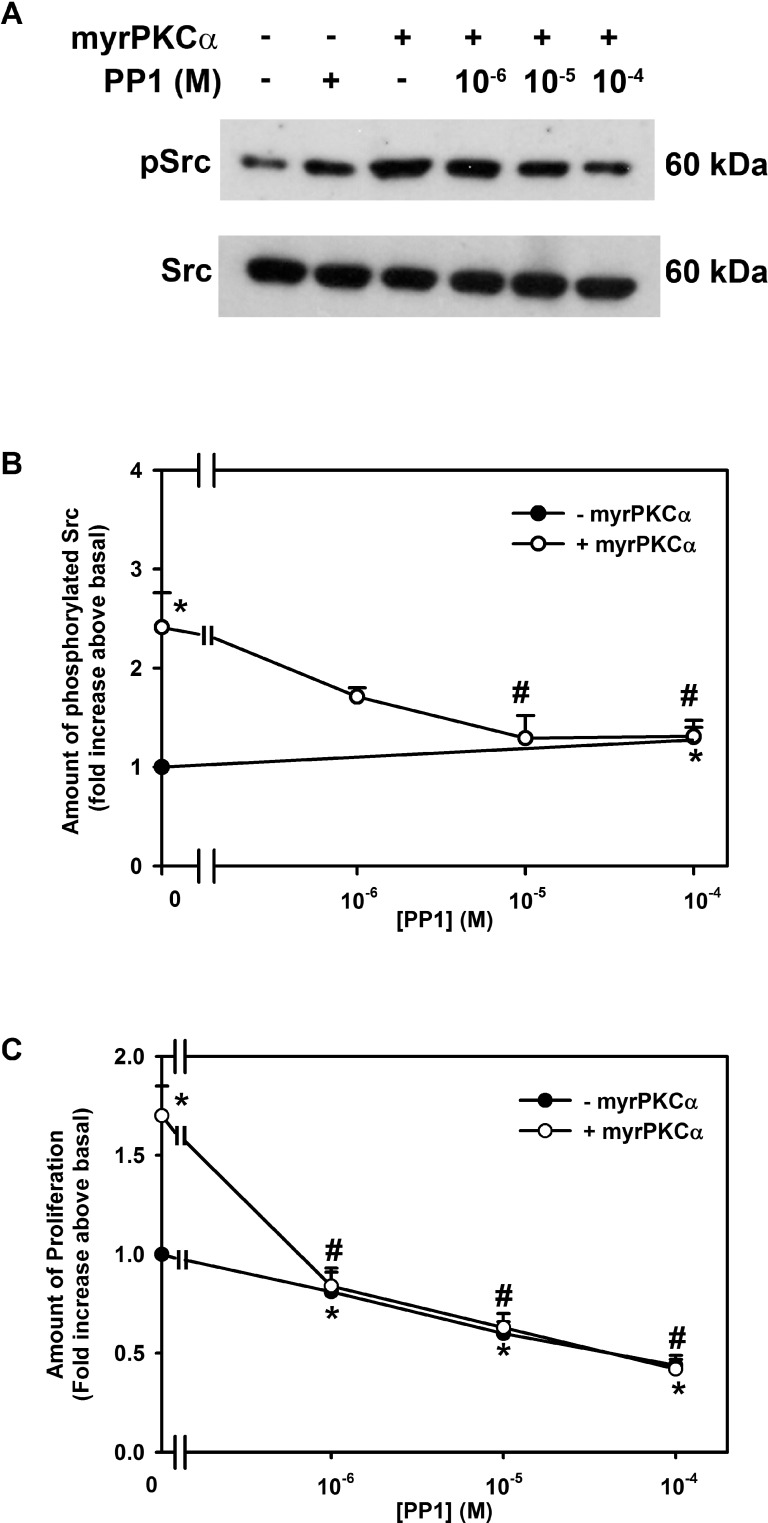
Activation of PKCα Stimulates Conjunctival Goblet Cell Proliferation by Activating Src
Since EGF used Src to activate PI-3K, we investigated if constitutively active PKCα also uses Src to stimulate goblet cell proliferation. Phosphorylation of Src was measured by Western blot analysis (Fig. 10A). Incubation with Ad-myrPKCα increased Src activity 2.4 ± 0.3-fold compared to basal. This increase was blocked by PPI (10−6–10−4 M), with a complete inhibition occurring at 10−4 M (Fig. 10B). The effect of PP1 also was tested on proliferation. Incubation with Ad-myrPKCα stimulated proliferation 1.7 ± 0.2-fold (Fig. 10C). PPI completely blocked this proliferation at all concentrations used.
Figure 10.
Effect of inhibition of Src on constitutively active PKCα-stimulated cultured rat conjunctival goblet cell Src phosphorylation and proliferation. Cultured rat conjunctival goblet cells were serum starved for 24 hours and preincubated with PP1 (10−6–10−4 M) for 30 minutes prior to addition of an adenovirus containing myristolyated (active) PKCα (myrPKCα) (1 × 107 pfu) or with no addition for 22 hours. The amount of total and phosphorylated Src was determined by Western blot analysis, and a representative blot of three independent experiments is shown in (A). The blots were scanned, and the data shown in (B) represent mean ± SEM of three experiments. The number of proliferating cells was determined by WST-8 and is shown in (C). Data are mean ± SEM from three independent experiments. *Statistically significant difference from basal, set to 1.0. #Statistically significant difference from myrPKCα.
Similarly to EGF, constitutively active PKCα uses Src to stimulate conjunctival goblet cell proliferation.
Discussion
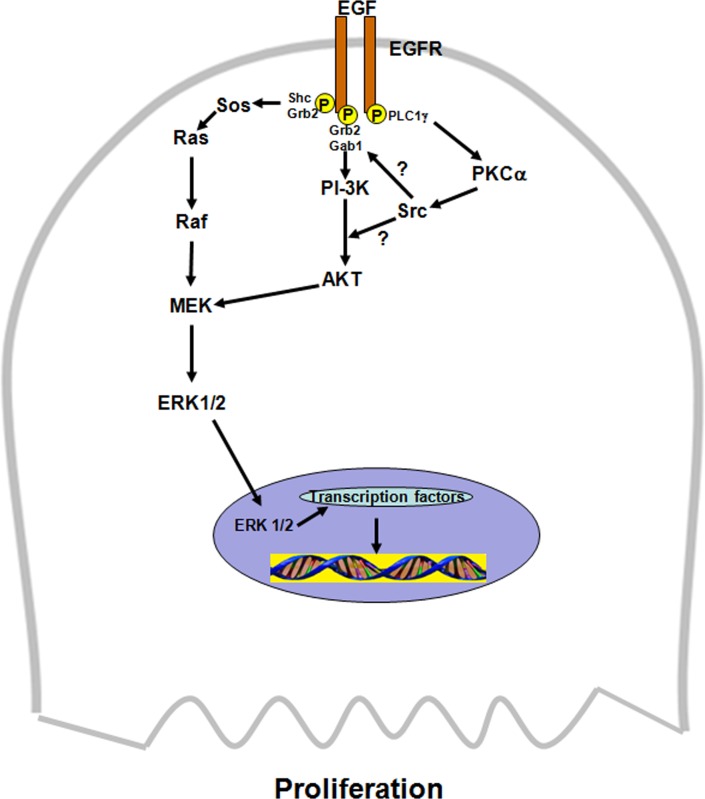
Results herein demonstrate that EGF uses a complex signaling pathway to stimulate conjunctival goblet cell proliferation. Use of a variety of signaling protein inhibitors allowed the order of the signaling components to be assigned. EGF interacts with the EGF receptor to stimulate PLCγ to activate PKCα that in turn uses Src, probably along with nonreceptor tyrosine kinase Pyk2,18,19 to stimulate PI-3K activity (Fig. 11). Active PI-3K then phosphorylates AKT that interacts with the ERK1/2 cascade to phosphorylate ERK1/2. As AKT is known to stimulate the GTPase Rac and p-21 activated protein kinase (PAK) to activate Raf in the ERK1/2 cascade, it is possible that this mechanism is responsible for activation of ERK1/2 in the present study. As shown previously,6 phosphorylated ERK1/2 then translocates to the nucleus where it likely activates transcription factors and immediate early genes to cause proliferation. Our conclusion is supported by the findings that each of the inhibitors (PKC, AKT, and ERK1/2) completely inhibited proliferation stimulated by EGF and myrPKCα but that ERK1/2 inhibitors do not block activation of AKT.
Figure 11.
Schematic diagram of signaling pathways used by EGF to stimulate goblet cell proliferation. Src could interact with PI-3K before or after its activation. AKT interacts with the ERK1/2 pathway at an unknown step. EGF, epidermal growth factor; EGF receptor, epidermal growth factor receptor; PLCγ1, phospholipase C gamma1; PKCα, protein kinase C alpha; Pyk2, a nonreceptor tyrosine kinase; Src, a nonreceptor tyrosine kinase; PI-3K, phosphoinositide 3-kinase; AKT, serine/threonine-specific protein kinase; Shc, adaptor protein; Grb2, adaptor protein; Ras, a GTPase; Raf, mitogen-activated protein kinase kinase kinase; MEK, mitogen-activated protein kinase kinase; ERK1/2, extracellular regulated kinase 1/2, also known as mitogen-activated protein kinase.
Results with the use of constitutively active PKCα and protein kinase inhibitors in the present study demonstrate that this kinase uses the same signaling constituents and pathways in the same order as EGF to cause proliferation. We suggest that there is a single pathway of EGF, EGF receptor, PLCγ1, PKCα, Src, PI-3K, AKT, and ERK1/2 that induces goblet cell proliferation (Fig. 11). Further evidence of this single pathway comes from the result showing that proliferation stimulated by both EGF and PKCα is not additive.9 If different pathways were used, the effect of the two agonists would be additive.
We found that constitutively active PKCα stimulates Src activity. In the present study, both EGF and constitutively active PKCα increased phosphorylation of Src on tyrosine 416. This suggests that Src was not the direct target of PKCα but that an additional kinase was activated. One possibility is Pyk2. Pyk2 can be activated by PKC and can bind to and activate Src.20 In conjunctival goblet cells, both Pyk2 and Src are activated by muscarinic receptors.18,19
Phosphorylation of EGF receptor can recruit PI-3K complexes to the plasma membrane by the SH2 domains of PI-3K. Once at the cell membrane, PI-3K produces D3-phosphorylated lipid products that activate their downstream signaling components phosphoinositide-dependent kinase-1 (PDK1) and AKT.21 In the present study, using inhibitors, we found that the PI-3K activity associated with proliferation was increased by constitutively active PKCα and by phosphorylated Src. This is possible because the myristoylate tag on PKCα activates PKCα by moving it to the membrane. If PI-3K and PKCα are present together in a scaffold, both PKC and PI-3K could move to the membrane where they would both be activated. However, we do not know if Src acts before PI-3K or afterward by directly phosphorylating AKT, as we did not measure PI-3K activity.22 Src activation of AKT independent of PI-3K can occur in the dysregulation of proliferation associated with cancer. Src can phosphorylate AKT at tyrosine (Y) 315/326, whereas PDK1 phosphorylates AKT at tyrosine 308. Given the proximity of these residues in the AKT protein, it is possible that in intact cells, EGF and constitutively active PKCα phosphorylation of these residues could interact and affect each other, changing the net effect on cell proliferation. Src also can activate additional signaling molecules or interact with the EGF receptor, and this interaction modifies the activation of PI-3K by the EGF receptor. In PDGF-stimulated mesangial cells, Src stimulates PI-3K activity.23 Those authors suggest that Src phosphorylates residues in the platelet-derived growth factor (PDGF) receptor that are different from those that activate PI-3K, but by some mechanism Src can regulate activation of PI-3K by the PDGF receptor. Thus Src could act in the goblet cells by activating PI-3K or by activating AKT or both.
After AKT is activated, it interacts with the ERK1/2 kinase cascade that consists of Shc, Grb2, Ras, Raf, MEK, and ERK1/2. In Ba/F3 cells, addition of a growth factor recruits Gab2, which brings together PI-3K and Grb2 where they can be activated.24 Another possible site of interaction of AKT with the ERK1/2 pathway is with Raf.17 In mouse fibroblast cells, AKT activates Raf by using the small GTPase Rac1.25 Thus there are several different mechanisms by which AKT can either directly or indirectly activate the ERK1/2 cascade.
The interactions of the signaling components described in the present experiments suggest that there is a large multiprotein scaffold in conjunctival goblet cells that allows signaling molecules to interact with each other in a specific manner so as to culminate in the translocation of ERK1/2 to the nucleus where it activates proliferation. The EGF receptor is a well-known scaffold that contains phosphorylation sites for multiple signaling proteins. We suggest that PLCγ, PI-3K, and Grb2/Shc are present in the scaffold. The substrates of these kinases, along with other signaling proteins not investigated in the present study such as Gab1, Pyk2, and Rac1, could also be present in the complex or could be recruited upon activation. This scaffold assembly would ensure the participation of the three pathways in a specific order to cause goblet cell proliferation in the presence of EGF.
EGF and constitutively active PKCα use the same pathway to stimulate conjunctival goblet cell proliferation.9 In contrast to what occurs with cell proliferation, EGF and constitutively active PKCα do not use the same signaling pathways to activate AKT or ERK1/2. When calculated additivity was compared with experimental additivity (Fig. 6) for phosphorylation of AKT and ERK1/2, experimental additivity was not significantly different from calculated. Thus activation of AKT and ERK1/2 by EGF compared to constitutively active PKCα could stimulate AKT- and ERK1/2-dependent cellular functions in addition to proliferation. Activation of different AKT and ERK1/2 substrates could lead to mucin secretion, cell survival, cell migration, or cell attachment. EGF does stimulate conjunctival goblet cell secretion that is ERK1/2-dependent.8 Compared to proliferation, secretion is a fast process, suggesting that different signaling mechanisms are used to cause the two processes.
Due to the limited availability of human conjunctiva, only experiments investigating the effect of the inhibitor LY294002 (a more specific PI-3K inhibitor than wortmannin26) on EGF-stimulated proliferation were performed in cultured goblet cells from both rat and human conjunctiva (Fig. 1C). In both species, LY294002 inhibited EGF-stimulated goblet cell proliferation with a similar concentration dependency. Previous studies have also demonstrated the similarities between cultured rat and human goblet cells. Rat and human goblet cells respond similarly to cholinergic agonists,19 EGF,27 leukotrienes,28 and resolvins.29 Thus we feel that rat goblet cells are an excellent model for human goblet cells.
Regulation of goblet cell proliferation plays a critical role in the health of the ocular surface and in ocular surface diseases. For the ocular surface to be healthy, an optimum amount of the goblet cell mucin MUC5AC needs to be present. Goblet cell mucin production is regulated by controlling goblet cell secretion, MUC5AC synthesis, and goblet cell proliferation. Little is known about the regulation of MUC5AC synthesis; however, much is known about the regulation of conjunctival goblet cell secretion and proliferation.5–9,15,19 Of all the compounds tested to date, only EGF stimulates both secretion and proliferation. There is little published on conjunctival goblet cells comparing the pathways used by EGF to cause secretion, a rapid event happening within seconds and continuing over hours, compared to proliferation, a slower event that occurs over days. EGF is known in conjunctival goblet cells to cause a rapid increase in intracellular [Ca2+]i as well as activation of ERK1/2 that causes secretion8 and a slower increase in ERK1/2 that leads to proliferation.6 There is, however, no published evidence in conjunctival goblet cells regarding whether PKC isoforms differentially regulate secretion and proliferation or whether activation of the PI-3K/AKT pathway plays a role in secretion. Comparison of these pathways could be useful in designing treatments to stimulate conjunctival goblet cell mucin production, by increasing secretion or proliferation, in ocular surface disease in which MUC5AC mucin levels are depleted. These diseases include dry eye, chemical and thermal burns, vitamin A deficiency, and ocular cicatricial pemphigoid.
We conclude that EGF stimulates conjunctival goblet cell proliferation by a pathway that contains PLCγ, PKCα, Src, PI-3K/AKT, and ERK1/2. Any of these signaling components are targets for development of treatments to regulate conjunctival goblet cell proliferation in inflammatory ocular surface diseases.
Acknowledgments
The authors thank Kameran Lashkari for providing human conjunctival tissue.
Supported by NIH EY019470. The authors alone are responsible for the content and writing of the paper.
Disclosure: D. Li, None; M.A. Shatos, None; R.R. Hodges, None; D.A. Dartt, None
References
- 1. Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001; 411: 355–365 [DOI] [PubMed] [Google Scholar]
- 2. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005; 5: 341–354 [DOI] [PubMed] [Google Scholar]
- 3. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000; 103: 211–225 [DOI] [PubMed] [Google Scholar]
- 4. Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009; 315: 683–696 [DOI] [PubMed] [Google Scholar]
- 5. Shatos MA, Hodges RR, Oshi Y, et al. Role of cPKCalpha and nPKCepsilon in EGF-stimulated goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009; 50: 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shatos MA, Gu J, Hodges RR, Lashkari K, Dartt DA. ERK/p44p42 mitogen-activated protein kinase mediates EGF-stimulated proliferation of conjunctival goblet cells in culture. Invest Ophthalmol Vis Sci. 2008; 49: 3351–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gu J, Chen L, Shatos MA, et al. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res. 2008; 86: 322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res. 2012; 103: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shatos MA, Hodges RR, Bair JA, Lashkari K, Dartt DA. Stimulatory role of PKCalpha in extracellular regulated kinase 1/2 pathway in conjunctival goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009; 50: 1619–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008; 27: 6473–6488 [DOI] [PubMed] [Google Scholar]
- 11. Yue Y, Lypowy J, Hedhli N, Abdellatif M. Ras GTPase-activating protein binds to Akt and is required for its activation. J Biol Chem. 2004; 279: 12883–12889 [DOI] [PubMed] [Google Scholar]
- 12. Roskoski R Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005; 331: 1–14 [DOI] [PubMed] [Google Scholar]
- 13. Shatos MA, Kano H, Rubin P, Garza G, Dartt DA. Isolation and characterization of human goblet cells in vitro: regulation of proliferation and activation of mitogen-activated protein kinase by EGF and carbachol. Adv Exp Med Biol. 2002; 506: 301–305 [DOI] [PubMed] [Google Scholar]
- 14. Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001; 42: 1455–1464 [PubMed] [Google Scholar]
- 15. Hodges RR, Raddassi I, Zoukhri D, Toker A, Kazlauskas A, Dartt DA. Effect of overexpression of constitutively active PKCalpha on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 2004; 45: 3974–3981 [DOI] [PubMed] [Google Scholar]
- 16. Kawanabe Y, Hashimoto N, Masaki T. Effects of nonselective cation channels and PI3K on endothelin-1-induced PYK2 tyrosine phosphorylation in C6 glioma cells. Am J Physiol Cell Physiol. 2003; 285: C539–C545 [DOI] [PubMed] [Google Scholar]
- 17. Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012; 40: 139–146 [DOI] [PubMed] [Google Scholar]
- 18. Hodges RR, Horikawa Y, Rios JD, Shatos MA, Dartt DA. Effect of protein kinase C and Ca(2+) on p42/p44 MAPK, Pyk2, and Src activation in rat conjunctival goblet cells. Exp Eye Res. 2007; 85: 836–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanno H, Horikawa Y, Hodges RR, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003; 284: C988–C998 [DOI] [PubMed] [Google Scholar]
- 20. Lu WY, Xiong ZG, Lei S, et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999; 2: 331–338 [DOI] [PubMed] [Google Scholar]
- 21. Sasaki T, Suzuki A, Sasaki J, Penninger JM. Phosphoinositide 3-kinases in immunity: lessons from knockout mice. J Biochem. 2002; 131: 495–501 [DOI] [PubMed] [Google Scholar]
- 22. Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J Cell Physiol. 2012; 227: 3178–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhury GG, Mahimainathan L, Das F, Venkatesan B. Ghosh-Choudhury N. c-Src couples PI 3 kinase/Akt and MAPK signaling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal. 2006; 18: 1854–1864 [DOI] [PubMed] [Google Scholar]
- 24. Nyga R, Pecquet C, Harir N, et al. Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J. 2005; 390: 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zugasti O, Rul W, Roux P, et al. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol Cell Biol. 2001; 21: 6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fry MJ. Phosphoinositide (PI) 3-kinase assays. Methods Mol Biol. 2009; 462: 345–362 [DOI] [PubMed] [Google Scholar]
- 27. Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003; 44: 2535–2544 [DOI] [PubMed] [Google Scholar]
- 28. Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011; 186: 4455–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li D, Hodges RR, Jiao J, et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]