Abstract
Objectives
Metabolic syndrome is a precursor of diabetes and cardiovascular disease (CVD). Walnut ingestion has been shown to reduce CVD risk indices in diabetes. This randomized controlled crossover trial was performed to investigate the effects of daily walnut consumption on endothelial function and other biomarkers of cardiac risk in a population of overweight individuals with visceral adiposity.
Methods
Forty-six overweight adults (average age, 57.4 years; 28 women, 18 men) with elevated waist circumference and 1 or more additional signs of metabolic syndrome were randomly assigned to two 8-week sequences of walnut-enriched ad libitum diet and ad libitum diet without walnuts, which were separated by a 4-week washout period. The primary outcome measure was the change in flow-mediated vasodilation (FMD) of the brachial artery. Secondary measures included serum lipid panel, fasting glucose and insulin, Homeostasis Model Assessment–Insulin Resistance values, blood pressure, and anthropometric measures.
Results
FMD improved significantly from baseline when subjects consumed a walnut-enriched diet as compared with the control diet (1.4% ± 2.4% versus 0.3% ± 1.5%; p = 0.019). Beneficial trends in systolic blood pressure reduction were seen, and maintenance of the baseline anthropometric values was also observed. Other measures were unaltered.
Conclusion
Daily ingestion of 56 g of walnuts improves endothelial function in overweight adults with visceral adiposity. The addition of walnuts to the diet does not lead to weight gain. Further study of the potential role of walnut intake in diabetes and CVD prevention is warranted.
Keywords: endothelial function, walnut, metabolic syndrome
INTRODUCTION
Obesity is hyperendemic in the United States and, increasingly, around the world. A significant percentage of overweight and obese individuals show signs of insulin resistance, up to and including the metabolic syndrome [1,2]. Overweight individuals with insulin resistance are in turn at increased risk of type 2 diabetes and cardiovascular disease (CVD). CVD has been the leading cause of morbidity and mortality in the United States for more than 80 years [3].
Impaired fasting glucose, hypertension, atherogenic dyslipidemia, obesity, and endothelial dysfunction have each been shown to increase the risk of type 2 diabetes mellitus and CVD [4]. In the EpiDREAM cohort study, a 1-mmol/l increase in fasting plasma glucose was associated with a 17% increase in the risk of future cardiovascular events and death [5]. In the IDEA study, the frequency of CVD and type 2 diabetes mellitus increased proportionally with waist circumference for both genders [6]. In the Framingham Heart study, the risk of major coronary heart disease events increased by nearly 25% for every 5-mg/dl decrease in high-density lipoprotein cholesterol (HDL-C) below the median values [7].
Therapeutic lifestyle change, with an emphasis on diet, is recommended as a first-line treatment for the prevention of diabetes and CVD in at-risk individuals [8,9]. Nuts in particular are gaining increasing attention in this area [10], with a recent meta-analysis indicating reduced risk of diabetes among those consuming more nuts and less red meat [11]. Emerging clinical trial and epidemiological evidence indicates that nuts can favorably alter levels of oxidative stress, inflammation, and lipids and can improve glucose metabolism and vascular reactivity [12–15].
Walnuts are a uniquely rich source of α-linolenic acid (ALA), and epidemiological studies suggest that plant-derived ALA may confer particular cardiovascular benefits [16,17]. A meta-analysis investigating the impact of walnut consumption on blood lipids showed that walnut-enriched diets significantly decreased total cholesterol and low-density lipoprotein cholesterol (LDL-C) when compared with control diets for the duration of the short-term trials [18]. Furthermore, walnuts are also rich in tocopherol, phenolic antioxidants, folic acid, and magnesium, nutrients that have been shown to impact endothelial function favorably [19].
Previously, we reported that a walnut-enriched ad libitum diet improves endothelium-dependent vasodilatation in type 2 diabetic individuals, suggesting a potential reduction in overall cardiac risk in this population [20]. To our knowledge, the effects of walnuts on endothelial function in adults with visceral adiposity and free of diabetes have not been examined further. We therefore conducted a randomized controlled crossover trial to investigate the effects of daily walnut ingestion in overweight adults with central obesity on endothelial function as well as body mass index (BMI), weight, waist circumference, lipid panel, insulin sensitivity, and blood pressure.
MATERIALS AND METHODS
Participants
Forty-six participants (18 men and 28 women) were recruited from the Lower Naugatuck Valley in Connecticut through flyers and newspaper advertisements. Those responding (n = 346) were prescreened using a semistructured telephone interview. Participants were required to be nonsmoking adults aged 30–75 years with a BMI greater than 25 and a waist circumference of more than 40 inches for men or more than 35 inches for women. All included participants also exhibited 1 or more additional risk factors for metabolic syndrome: blood pressure ≥130/≥85mmHg or taking antihypertensive medication, fasting serum glucose (FPG) ≥ 100 mg/dl, fasting serum triglyceride (TG) level ≥150 mg/dl, or fasting HDL-C ≤40 mg/dl in men or ≤50 mg/dl in women. All ethnic and minority groups were equally eligible to participate in the study. Participants were excluded if they were pregnant or had been diagnosed with atherosclerotic vascular disease, diabetes, severe hypertension, sleep apnea, tuberculosis, acquired immune deficiency syndrome, cancer, psychotic disorder, and/or eating disorder. Participants were also excluded if they had a prior history of substance abuse, consumed restricted diets by choice (i.e., vegan, carbohydrate-restricted, etc.), were allergic to walnuts or any other nuts, or were unwilling to refrain from taking medication for 12 hours prior to assessment. Individuals who regularly used nonsteroidal anti-inflammatory drugs or vasoactive medication, fiber supplements, aspirin, lipid-lowering medications, or antihypertensive medications and had been taking them at a stable dosage for less than 3 months were also excluded from participation.
Those passing the telephone screening (n = 92) underwent a clinical screening examination consisting of height, weight, BMI, blood pressure measurements, and laboratory testing including fasting serum lipids and FPG. Subject participation and study progression are shown in Fig. 1.
Fig. 1.
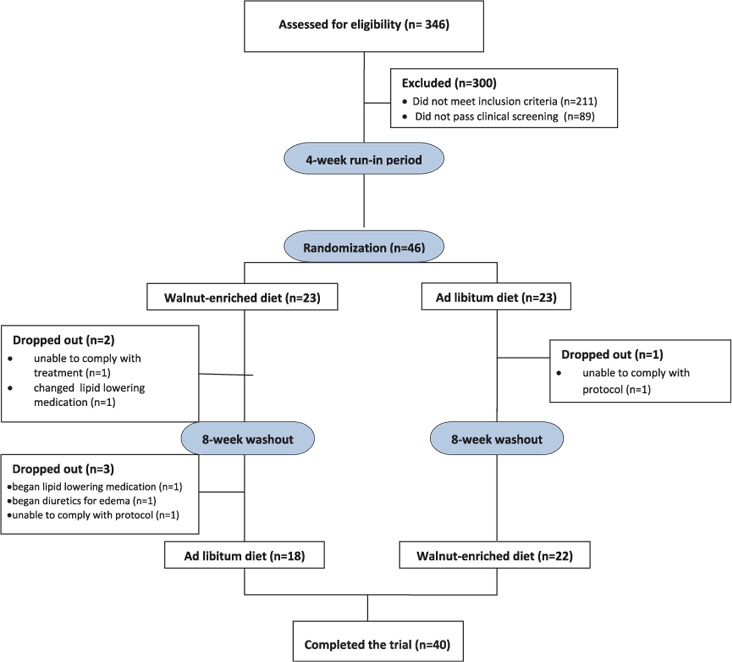
Subject participation and study progression.
Study Design
This study was a randomized, controlled, single-blind, crossover clinical trial. All participants first took part in a dietary group session that was led by a registered dietitian. This group session was the initiation of the 4-week run-in period to allow for diet and weight stabilization. Each group session consisted of about 10 participants. The registered dietitian provided detailed instructions of how to fill out a diet record. The 3-day diet records were done once during the original run-in period, once during each 8-week dietary period, and once during the 4-week washout period. At the end of the run-in period, participants (n = 46) were randomly assigned to one of two possible treatment sequences consisting of an 8-week walnut-enriched ad libitum diet and an ad libitum diet without walnuts for 8 weeks. Treatment assignments were separated by a 4-week washout period.
During the walnut-enriched diet treatment, participants were given an 8-week supply of walnuts. They were instructed to consume 56 g of shelled, unroasted English walnuts per day as a snack or with a meal. A consumption log sheet was provided for participants to keep a record of their walnut consumption. Participants were instructed to maintain their baseline medication and supplement use and physical activity level throughout the study. To ensure that weight remained stable during the walnut-enriched phase, participants were counseled by a registered dietitian during the group session about strategies for equivalently substituting calories from walnuts for calories from other foods in the diet but were instructed to otherwise continue with their usual dietary patterns. Compliance with treatment and dietary patterns was assessed from subjects’ diet records and consumption log sheets.
During each visit, participants underwent endothelial function testing in the morning after a minimum 8-hour fast. Height, weight, blood pressure, fasting serum lipids, FPG, and fasting serum insulin were measured.
Vascular Reactivity Testing: Brachial Artery Reactivity Studies
Endothelial function was measured noninvasively in the right brachial artery by a high-frequency ultrasound scanning machine (Sonos 4500, Phillips Medical Systems, Andover, MA) in accordance with published guidelines [21] and our previous endothelial function studies [20, 22–24]. Subjects were required to rest in a quiet, temperature-controlled, softly lit room for 15 minutes before scanning was initiated. The right brachial artery was imaged longitudinally, 2–5 cm above the antecubital fossa, by an experienced registered vascular technologist (RVT) who was blinded to the treatment assignments. A resting scan was performed, and arterial flow velocity was measured. An occluding cuff placed on the upper arm was inflated to a pressure of 250 mmHg for 5 minutes and rapidly deflated to induce reactive hyperemia. Brachial artery scans were acquired on magnetic optical disk continuously between 30 and 180 seconds after cuff deflation, including a repeated flow-velocity measurement during the first 15 seconds after cuff release. Brachial artery diameters were analyzed by commercially available software (Brachial Analyzer, Medical Imaging Application, Iowa City, IA). Dilatation from baseline was measured at 50–80 seconds after cuff deflation to assess endothelium-dependent vasodilatation. To test intraobserver reliability, a random sample of 20 brachial artery reactivity studies was re-read by the same RVT to ensure consistency of measurement and interpretation. The result of the intraobserver reliability coefficient was 0.95.
Outcome Measures
Endothelial Function. Endothelial function was measured as flow-mediated vasodilation (FMD), the percentage change of brachial artery diameter from before cuff inflation to 60 seconds after cuff release. In addition to brachial diameter at 60 seconds after cuff release, flow after cuff deflation within the first 15 seconds was used as an indicator of stimulus strength, hyperemic flow being the stimulus for endothelial reactivity. To account for potential variability in stimulus strength, FMD was divided by flow at 15 seconds after cuff deflation to create a stimulus-adjusted response measure.
Cardiovascular Biomarkers. Fasting serum lipids (consisting of total cholesterol, HDL-C, LDL-C, TGs, and total cholesterol: HDL ratio), FPG, and fasting serum insulin were measured at the Griffin Hospital laboratory using standard procedures at each visit. Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) values were generated from FPG and fasting serum insulin levels (HOMA calculator version 2.2.1) to gauge the degree of insulin resistance.
Anthropometric Measures. Height, weight, BMI, and waist circumference were measured at each visit. To measure weight, participants were asked to remove their heavy outer garments (jacket, coat, etc.) and shoes and stand in the center of the platform with weight distributed evenly on both feet. Waist circumference was measured at the umbilicus with the measurement tape surrounding the abdomen horizontal to the floor. BMI was calculated using the weight and height measurements.
Blood Pressure. Blood pressure was determined with the use of the Datascope Accutorr Plus automatic digital blood pressure device (Data-scope Corp, Mahwah, NJ) with the subject supine after a 5-minute period of rest. Both systolic and diastolic pressures were calculated as the mean value of 2 readings 5 minutes apart.
Diet Record Analysis
Diet records were analyzed using The Food Processor II, ESHA Research's basic nutrition and diet analysis software (version 7.0, ESHA Research, Salem, OR).
Ethics
The study protocol and consent form were approved by the Griffin Hospital (Derby, CT) Institutional Review Board. Signed informed consent was obtained from all study participants, and all participants received monetary compensation for their participation.
Statistical Analysis
Repeated-measures analysis of variance (ANOVA) was used to assess differences in intraindividual responses across treatments. Paired t tests were also used to compare baseline mean values of all outcome measures among participants by group assignment. The combined effect of independent variables (age, race, BMI, hypertensive status, dyslipidemia, and treatment sequence) and treatment assignment was assessed with respect to all outcome measures using multivariable ANOVA models. All analyses of endpoints were based on the intention-to-treat principle. Statistical significance was set at 2-tailed alpha <0.05. Data were analyzed with the use of SAS software for Windows version 9.1. Results are expressed as means ± SD in text and tables. The sample size was determined to allow for ≈20% attrition and noncompliance and to provide >80% power to detect a minimal difference of 3% in FMD between treatments (21) with maximum allowable type I error of 5%. The sample size was calculated using the formula N = [(Zα + Zβ)2·SD2]/d2, where N = the number of participants needed, Zα = 1.96 for 2 sided at 5%, Zβ = 0.84 at 80% power, SD = 6.5%, and D = 3% (minimal detectable difference in change in FMD between intervention and control assignments); N = [(1.96 + 0.84)2 · 6.52]/32 = 37 participants are needed. Allowing for 20% withdrawal and loss to follow-up, 46 participants were needed for the trial.
RESULTS
Demographic and Baseline
Forty-six overweight adults participated in the study. Sixty-one percent of the participants were female. The average age of the participants was 57 years. Their average baseline endothelial function (measured as FMD) was 8.8%. Other baseline values are shown in Table 1. Of the 46 adults enrolled, 40 completed the study. Three subjects dropped out of the study due to changes in medication, 1 dropped out due to inability to comply with the protocol, and 2 dropped out due to schedule conflicts.
Table 1.
Demographic Characteristics and Baseline Values
| Variable | Value |
|---|---|
| Gender | |
| Female | 28 (60.9%) |
| Male | 18 (39.1%) |
| Age (y) | 57.4 ± 11.9 |
| Endothelial function | |
| Flow-mediated dilatation (%) | 8.8 ± 2.4 |
| Stimulus-adjusted response measure | 0.08 ± 0.04 |
| Anthropometric measure | |
| Body mass index (kg/m2) | 33.2 ± 4.4 |
| Waist circumference (cm) | 111.6 ± 12.7 |
| Lipid profile | |
| Total cholesterol (mg/dl) | 205.1 ± 29.1 |
| Triglycerides (mg/dl) | 157.0 ± 60.3 |
| HDL-cholesterol (mg/dl) | 52.7 ± 15.1 |
| LDL-cholesterol (mg/dl) | 121.4 ± 24.5 |
| Total cholesterol/HDL-cholesterol | 4.5 ± 3.1 |
| Blood pressure | |
| Systolic blood pressure (mmHg) | 133.8 ± 13.2 |
| Diastolic blood pressure (mmHg) | 78.7 ± 8.4 |
| Other serum measures | |
| Fasting blood glucose (mg/dl) | 98.9 ± 9.5 |
| Insulin, serum (mIU/ml) | 19.0 ± 18.9 |
| HOMA-IR | 4.7 ± 5.3 |
| A-tocopherol, vitamin E (mg/l) | 12.6 ± 4.4 |
Values are mean ± SD except otherwise stated. HDL = high-density lipoprotein, LDL = low-density lipoprotein, HOMA-IR = Homeostasis Model Assessment–Insulin Resistance.
Dietary Intake. Dietary intakes of polyunsaturated fatty acids (PUFAs), n-3 fatty acids, n-6 fatty acids, and fat increased significantly during the walnut-enriched diet treatment compared with the control diet (PUFAs, p < 0.01; n-3 fatty acids, p < 0.01; n-6 fatty acids, p < 0.01; and fat, p = 0.01). Dietary intake of other nutrients remained stable throughout the study (Table 2).
Table 2.
Selected Nutrient Intake
| Variable | Walnut-Enriched Diet | Control Diet | p Value |
|---|---|---|---|
| Energy (kcal) | 2222.0 ± 1054.4 | 1926.6 ± 928.5 | 0.19 |
| Fat (kcal) | 945.0 ± 525.6 | 676.7 ± 386.7 | 0.01 |
| Fat (g) | 105.1 ± 58.5 | 75.3 ± 43.0 | 0.01 |
| % Kcal fat | 41.4 ± 8.8 | 33.9 ± 7.5 | <0.01 |
| Saturated fatty acids (kcal) | 274.7 ± 180.5 | 231.8 ± 144.1 | 0.24 |
| % Kcal saturated fatty acids | 11.8 ± 3.5 | 11.6 ± 3.6 | 0.84 |
| MUFAs (g) | 27.7 ± 17.6 | 24.0 ± 14.1 | 0.29 |
| % Kcal MUFA | 10.8 ± 3.0 | 10.7 ± 3.2 | <0.01 |
| PUFAs (g) | 32.1 ± 16.6 | 12.7 ± 8.1 | <0.01 |
| % Kcal PUFA | 13.2 ± 6.0 | 6.0 ± 2.8 | <0.01 |
| Omega-3 fatty acids (g) | 5.0 ± 2.4 | 1.2 ± 0.8 | <0.01 |
| % Kcal omega-3 fatty acids | 2.1 ± 0.8 | 0.6 ± 1.1 | <0.01 |
| Omega-6 fatty acids (g) | 25.9 ± 14.0 | 10.4 ± 7.1 | <0.01 |
| % Kcal omega-6 fatty acids | 10.7 ± 5.1 | 4.8 ± 2.5 | <0.01 |
| Protein (g/d) | 91.8 ± 46.2 | 90.6 ± 43.7 | 0.91 |
| % Kcal protein | 16.8 ± 4.1 | 20.1 ± 7.4 | 0.02 |
| Carbohydrates (g) | 221.3 ± 105.8 | 218.4 ± 112.6 | 0.91 |
| % Kcal carbohydrate | 40.5 ± 7.5 | 45.3 ± 8.8 | 0.01 |
| Fiber (g/d) | 19.1 ± 10.4 | 16.9 ± 8.9 | 0.16 |
| % Kcal fiber | 3.5 ± 1.1 | 3.4 ± 1.0 | 0.58 |
| Cholesterol (mg/d) | 297.1 ± 176.5 | 321.9 ± 172.0 | 0.53 |
Values are mean ± SD; p values were obtained from 1-way analysis of variance. MUFA = monounsaturated fatty acid, PUFA = polyunsaturated fatty acid.
Primary Endpoint
Endothelial Function. In this overweight population with at least 1 risk factor for metabolic syndrome, consumption of a walnut-enriched diet for 8 weeks improved FMD significantly from baseline as compared with the control diet (p = 0.019; see Table 3).
Table 3.
Change in Outcome Measures
| Variable | Walnut-Enriched Diet | Control Diet | Treatment Effect‡ (95% CI) | %Change† | p Value |
|---|---|---|---|---|---|
| Endothelial function | |||||
| Flow-mediated dilatation (%) | 1.4 ± 2.4∗ | 0.3 ± 1.5 | 1.1 (0.2, 2.0) | 367 | 0.019 |
| Stimulus-adjusted response measure | 0.02 ± 0.06∗ | 0.00 ± 0.03 | 0.2 (−0.0, 0.4) | 400 | 0.136 |
| Anthropometric measure | |||||
| Body mass index (kg/m2) | 0.1 ± 0.6 | −0.3 ± 0.8∗ | 0.4 (0.1, 0.6) | 133 | 0.016 |
| Weight (lb) | 0.4 ± 3.7 | −2.0 ± 5.4∗ | 2.3 (0.3, 4.3) | 120 | 0.019 |
| Waist circumference (cm) | −0.7 ± 3.7 | −0.3 ± 2.4 | −0.2 (−1.6, 1.2) | −133 | 0.570 |
| Lipid profile | |||||
| Total cholesterol (mg/dl) | −0.5 ± 23.2 | 0.3 ± 21.6 | −0.1 (−9.8, 9.6) | −267 | 0.696 |
| Triglycerides (mg/dl) | −4.5 ± 42.0 | 4.3 ± 44.9 | −4.6 (−23.4, 14.3) | −205 | 0.325 |
| HDL-cholesterol (mg/dl) | −0.1 ± 6.5 | −0.2 ± 6.2 | 0.2 (−2.5, 3.0) | 50 | 0.889 |
| LDL-cholesterol (mg/dl) | 0.4 ± 22.9 | −0.4 ± 20.0 | 0.5 (−8.8, 9.8) | 200 | 0.981 |
| Total cholesterol/HDL-cholesterol | 0.03 ± 0.59 | 0.11 ± 0.74 | −0.06 (−0.3, 0.2) | −73 | 0.609 |
| Other serum measures | |||||
| Fasting blood glucose (mg/dl) | −0.2 ± 8.8 | −1.5 ± 6.8 | 0.07 (−3.4, 3.5) | 87 | 0.457 |
| Insulin, serum (mIU/ml) | −0.3 ± 19.6 | −1.7 ± 6.6 | 0.1 (−3.4, 3.5) | 82 | 0.626 |
| HOMA-IR | −0.5 ± 5.7 | −0.3 ± 1.8 | −0.2 (−2.1, 1.6) | −67 | 0.81 |
| Blood pressure | |||||
| Systolic blood pressure (mmHg) | −2.6 ± 11.0 | 1.2 ± 10.7 | −3.5 (−8.4, 1.4) | −117 | 0.070 |
| Diastolic blood pressure (mmHg) | −3.6 ± 18.8 | −0.6 ± 7.7 | −2.8 (−9.2, 3.6) | −500 | 0.352 |
Values are mean ± SD. HDL = high-density lipoprotein, LDL = low-density lipoprotein, HOMA-IR, Homeostasis Model Assessment–Insulin Resistance.
The average differences between the walnut and control diets.
Percentage change with walnut diet compared with control diet.
Significant (p < 0.05) from baseline; p values were obtained from repeated-measures analysis of variance.∗ p < 0.05 from paired Student t test.
Secondary Endpoints
Anthropometric Measures. Daily consumption of the walnut-enriched diet for an 8-week period did not change anthropometric measures from baseline (BMI: p = 0.481; weight: p = 0.439; waist circumference: p = 0.344). The control diet was associated with a reduction in BMI (p = 0.016) and body weight (p = 0.019) relative to the walnut-enriched diet.
Lipid Panel. After consumption of a walnut-enriched diet for 8 weeks, participants’ mean measures of total cholesterol, LDL-C, HDL-C, and TG did not differ significantly from baseline as compared with the mean measures of control diet (total cholesterol: p = 0.69; LDL-C: p = 0.98; HDL-C: p = 0.89; TG: p = 0.32).
Blood Pressure. After consumption of a walnut-enriched diet for 8 weeks, participants’ blood pressure values decreased nonsignificantly from baseline as compared with the control diet without walnut supplementation (systolic: p = 0.07; diastolic: p = 0.352).
Fasting Plasma Glucose, Fasting Insulin, and HOMA-IR. Fasting plasma glucose and fasting insulin did not change significantly in participants consuming a walnut-enriched diet from baseline as compared with control group participants.
Our findings are unchanged, controlling for age, race, gender, BMI, hypertensive status, dyslipidemia, and treatment sequence, using multivariable ANOVA models.
DISCUSSION
The daily addition of 56 g of walnuts to the diet for 8 weeks significantly improved endothelial function in overweight adults with visceral obesity as compared with an ad libitum diet not supplemented with walnuts. A beneficial trend in systolic blood pressure reduction was observed that did not quite reach statistical significance. Despite the walnut dose representing more than 350 kcal, weight gain was not observed in the walnut treatment arm of the study, and the addition of walnuts was even associated with a decline in waist circumference. Lipid panel measures, fasting insulin, insulin sensitivity, and fasting glucose levels did not change.
One of the most important observations in this study involves the improved brachial artery vasoactivity results. In general, these findings confirm and extend the results of most previous walnut consumption studies [20, 25–28], respective of the methodological differences that exist among these trials. Lopez-Uriarte et al. [29] also showed improvement in endothelial function after nut consumption by assessing by peripheral artery tonometry in metabolic syndrome participants. There is much evidence on the cholesterol-lowering effect of walnuts [18,25,30,31]. It has been hypothesized that one mechanism through which nut consumption improves endothelial function occurs via improvements in lipid panel indices [32]. However, in this study, the lipid panel measures were unaffected by walnut intake. The overall evidence for potential cardioprotective effects of walnut intake is robust. In a pooled analysis of 25 nut intervention trials spanning 7 countries, Sabate et al. showed that nuts produce favorable lipid-regulating effects in individuals with BMI <30 [31]. It has been argued that this effect could be due to overweight individuals’ displaying reduced lipid responsiveness [33]. The results of this trial are in agreement with those observed by Sabate et al. The average BMI of participants in the present trial is 33.2 ± 4.4, and lipid-regulating effects were not observed as a result of a walnut-supplemented ad libitum diet.
The lipid panel is only one traditional CVD risk factor—albeit an important one—among a cluster of traditional and nontraditional risk factors within the current models of CVD etiology [34]. Endothelial function is increasingly being viewed as a prognostic marker of susceptibility to future cardiac events, and changes in risk factors other than serum lipids may account for part of the strong inverse association between nut consumption and CVD risk [35].
Population blood pressure improvements are instrumental in diminishing overall CVD risk. We observed a beneficial trend in blood pressure reduction at the end of the walnut-supplemented ad libitum diet phase when compared with the ad libitum diet alone. Our observations correspond with other nut and walnut consumption trials that have observed either a positive or a neutral influence on blood pressure responsiveness with increased consumption [9,26,33,36]. Mechanisms through which walnuts may elicit a blood pressure-lowering response could involve their high content of monounsaturated fatty acids, PUFAs, magnesium, and fiber and their low levels of sodium and saturated fatty acids [37].
Participants’ anthropometric measures did not change significantly from baseline during the walnut-enriched diet phase of the intervention. Given walnuts’ rich fatty acid composition and high metabolic energy profile, there was initial apprehension that walnut consumption (and nut consumption in general) would lead to undesired weight gain. Such concerns have been attenuated by evidence from epidemiological trials and randomized clinical trials, which have shown that walnut consumption does not tend to elevate weight, waist circumference, or BMI [10].
Unexpectedly, participants in the placebo phase lost weight. Given that participants’ physical activity levels remained unaltered throughout the study, the reduction in weight seen here could be due to a placebo effect, with participation in the trial encouraging subjects to eat more healthfully and/or to consume less food. The lack of weight increase seen during the walnut-enriched phase of the trial could be explained by participants’ partially substituting walnuts for other foods in the diet and by walnuts’ ability to increase energy expenditures while providing a high satiety and low metabolizable energy source [38]. Of note, waist circumference declined during the walnut study phase, despite a higher calorie intake. In a study by Salas-Salvado et al. [39], the prevalence of metabolic syndrome was reduced as a result of decreased waist circumference after a Mediterranean diet supplemented with mixed nuts. The effects of walnuts on appetite, satiety, weight, and body composition clearly warrant further study.
Lastly, this trial showed that a walnut-enriched ad libitum diet does not change fasting plasma glucose, fasting insulin, and HOMA-IR levels when compared with the control diet. These findings are similar to the ones observed in our previous study [20]. Overall, nut consumption studies provide varied results when it comes to glucose and insulin homeostasis [33,40]. Such findings are difficult to interpret given that these studies employ participants of varied health status, nuts of various sorts and differing amounts, and variable intervention durations and number of participants. Longer-term effects have generally been associated with observational studies, whereas intervention (feeding) studies have typically been of short duration, as is true in this case.
Limitations
We acknowledge that our study has several limitations, the most important being the absence of a prescribed standardized diet, which would then allow us to attribute the findings to the only independent variable under investigation, walnut consumption. Nevertheless, the ad libitum crossover design employed here compensates for this concern and strengthens the real-life applicability of our findings. The practical amount of walnuts consumed by the participants in this trial (56 g) adds further real-world applicability of our findings. The external validity of our trial is limited by the racial and gender homogeneity of the participating population. Compliance assessment in this trial was carried out via 3-day food records and walnut consumption log sheets, which may not provide a perfectly accurate record of subject compliance. Even so, given the positive results in the primary outcome measure, endothelial function, such concerns are of reduced importance. Since the present study was a trial of short length, it is uncertain whether our findings could be extrapolated to trials of longer duration.
CONCLUSION
Daily intake of 56 g of walnuts improves endothelial function in overweight adults with at least 1 sign of metabolic syndrome. Despite adding calories to an ad libitum diet, walnut intake was not associated with weight gain and was actually associated with a nonsignificant decline in waist circumference. This study provides suggestive evidence of a role for walnuts in protecting against diabetes and heart disease in at-risk individuals. The study also indicates that walnuts may be added to an ad libitum diet without weight gain, at least in the short term. Further study of walnuts in diabetes prevention and weight control is clearly warranted.
ACKNOWLEDGMENT
The authors wish to acknowledge the technical assistance of Mrs Michelle Pinto-Evans.
References
- 1.National Diabetes Information Clearinghouse. Insulin resistance and pre-diabetes. 2008. Accessed at: http://diabetes.niddk.nih.gov/DM/pubs/insulinresistance/
- 2.DiabetesLife: Insulin resistance symptoms. 2011. Accessed at: http://www.diabitieslife.com/diabetes/diabetes-care/tips/insulin-resistance-symptoms.htm.
- 3.Centers for Disease Control and Prevention. Prevalence of heart disease—United States. MMWR Morb Mortal Wkly Rep. 2007, 2005;56:113–118. [PubMed] [Google Scholar]
- 4.Bayturan O, Tuzcu EM, Lavoie A, Hu T, Wolski K, Schoenhagen P, Kapadia S, Nissen SE, Nicholls SJ. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch Intern Med. 2010;170:478–484. doi: 10.1001/archinternmed.2009.551. [DOI] [PubMed] [Google Scholar]
- 5.Anand SS, Dagenais GR, Mohan V, Diaz R, Probstfield J, Freeman R, Shaw J, Lanas F, Avezum A, Budaj A, Jung H, Desai D, Bosch J, Yusuf S, Gerstein HC. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol. 2012;19:755–764. doi: 10.1177/1741826711409327. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Deanfield JE, Despres JP, Bassand JP, Fox KA, Smith SC, Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castelli WP. Cardiovascular disease and multifactorial risk: challenge of the 1980s. Am Heart J. 1983;106:1191–1200. doi: 10.1016/0002-8703(83)90174-6. [DOI] [PubMed] [Google Scholar]
- 8.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 9.Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69:333–340. doi: 10.1017/S0029665110001539. [DOI] [PubMed] [Google Scholar]
- 10.Ros E, Tapsell LC, Sabate J. Nuts and berries for heart health. Curr Atheroscler Rep. 2010;12:397–406. doi: 10.1007/s11883-010-0132-5. [DOI] [PubMed] [Google Scholar]
- 11.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall CW, Esfahani A, Truan J, Srichaikul K, Jenkins DJ. Health benefits of nuts in prevention and management of diabetes. Asia Pac J Clin Nutr. 2010;19:110–116. [PubMed] [Google Scholar]
- 13.Bullo M, Lamuela-Raventos R, Salas-Salvado J. Mediterranean diet and oxidation: nuts and olive oil as important sources of fat and antioxidants. Curr Top Med Chem. 2011;11:1797–1810. doi: 10.2174/156802611796235062. [DOI] [PubMed] [Google Scholar]
- 14.Hudthagosol C, Haddad EH, McCarthy K, Wang P, Oda K, Sabate J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J Nutr. 2011;141:56–62. doi: 10.3945/jn.110.121269. [DOI] [PubMed] [Google Scholar]
- 15.Maranhao PA, Kraemer-Aguiar LG, de Oliveira CL, Kuschnir MC, Vieira YR, Souza MG, Koury JC, Bouskela E. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: a randomized controlled trial. Nutr Metab (Lond) 2011;8:32. doi: 10.1186/1743-7075-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holy EW, Forestier M, Richter EK, Akhmedov A, Leiber F, Camici GG, Mocharla P, Luscher TF, Beer JH, Tanner FC. Dietary {alpha}-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol. 2011;31:1772–1780. doi: 10.1161/ATVBAHA.111.226118. [DOI] [PubMed] [Google Scholar]
- 17.Damasceno NR, Perez-Heras A, Serra M, Cofan M, Sala-Vila A, Salas-Salvado J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds: effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21(suppl 1):S14–S20. doi: 10.1016/j.numecd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr. 2009;89:1649S–1656S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33:227–232. doi: 10.2337/dc09-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;16:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 22.Ali A, Yazaki Y, Njike VY, Ma Y, Katz DL. Effect of fruit and vegetable concentrates on endothelial function in metabolic syndrome: a randomized controlled trial. Nutr J. 2011;10:72. doi: 10.1186/1475-2891-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Njike V, Faridi Z, Dutta S, Gonzalez-Simon A, Katz DL. Daily egg consumption in hyperlipidemic adults: effects on endothelial function and cardiovascular risk. Nutr J. 2010;9:28. doi: 10.1186/1475-2891-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Njike VY, Faridi Z, Shuval K, Dutta S, Kay CD, West SG, Kris-Etherton PM, Katz DL. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2011;149:83–88. doi: 10.1016/j.ijcard.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Fitschen PJ, Rolfhus KR, Winfrey MR, Allen BK, Manzy M, Maher MA. Cardiovascular effects of consumption of black versus English walnuts. J Med Food. 2011;14:890–898. doi: 10.1089/jmf.2010.0169. [DOI] [PubMed] [Google Scholar]
- 26.West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Bagshaw DM, Wagner P, Ceballos RM, Holub BJ, Kris-Etherton PM. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr. 2010;29:595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2006;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 28.Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Uriarte P, Nogues R, Saez G, Bullo M, Romeu M, Masana L, Tormos C, Casas-Agustench P, Salas-Salvado J. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clin Nutr. 2010;29:373–380. doi: 10.1016/j.clnu.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Torabian S, Haddad E, Cordero-MacIntyre Z, Tanzman J, Fernandez ML, Sabate J. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur J Clin Nutr. 2010;64:274–279. doi: 10.1038/ejcn.2009.152. [DOI] [PubMed] [Google Scholar]
- 31.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 32.Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9:830–834. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21:126–135. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 34.vel Szic KS, Ndlovu MN, Haegeman G, Vanden Berghe W. Nature or nurture: let food be your epigenetic medicine in chronic inflammatory disorders. Biochem Pharmacol. 2010;80:1816–1832. doi: 10.1016/j.bcp.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Casas-Agustench P, Lopez-Uriarte P, Ros E, Bullo M, Salas-Salvado J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis. 2011;21(suppl 1):S21–S33. doi: 10.1016/j.numecd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, Yu D, Zong G, Zhou Y, Chen X, Tang L, Feng Y, Zhou H, Chen X, Li H, Demark-Wahnefried W, Hu FB, Lin X. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr. 2010;140:1937–1942. doi: 10.3945/jn.110.126300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr. 2010;104:465–473. doi: 10.1017/S0007114510001546. [DOI] [PubMed] [Google Scholar]
- 38.Mattes RD, Dreher ML. Nuts and healthy body weight maintenance mechanisms. Asia Pac J Clin Nutr. 2010;19:137–141. [PubMed] [Google Scholar]
- 39.Salas-Salvado J, Fernandez-Ballart J, Ros E, Martinez-Gonzalez MA, Fito M, Estruch R, Corella D, Fiol M, Gomez-Gracia E, Aros F, Flores G, Lapetra J, Lamuela-Raventos R, Ruiz-Gutierrez V, Bullo M, Basora J, Covas MI. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168:2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 40.Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63:1008–1015. doi: 10.1038/ejcn.2009.19. [DOI] [PubMed] [Google Scholar]


