Abstract
As climate change alters environmental conditions, the incidence and global patterns of human diseases are changing. These modifications to disease profiles and the effects upon human pharmaceutical usage are discussed. Climate-related environmental changes are associated with a rise in the incidence of chronic diseases already prevalent in the Northern Hemisphere, for example, cardiovascular disease and mental illness, leading to greater use of associated heavily used Western medications. Sufferers of respiratory diseases may exhibit exacerbated symptoms due to altered environmental conditions (e.g., pollen). Respiratory, water-borne, and food-borne toxicants and infections, including those that are vector borne, may become more common in Western countries, central and eastern Asia, and across North America. As new disease threats emerge, substantially higher pharmaceutical use appears inevitable, especially of pharmaceuticals not commonly employed at present (e.g., antiprotozoals). The use of medications for the treatment of general symptoms (e.g., analgesics) will also rise. These developments need to be viewed in the context of other major environmental changes (e.g., industrial chemical pollution, biodiversity loss, reduced water and food security) as well as marked shifts in human demographics, including aging of the population. To identify, prevent, mitigate, and adapt to potential threats, one needs to be aware of the major factors underlying changes in the use of pharmaceuticals and their subsequent release, deliberately or unintentionally, into the environment. This review explores the likely consequences of climate change upon the use of medical pharmaceuticals in the Northern Hemisphere.
CLIMATE CHANGE, DISEASE, AND HEALTH
Anthropogenic releases of greenhouse gases, such as carbon dioxide (CO2), methane (CH4), and the nitrous oxides (NOx), have played a significant role in the rise in global temperatures since the mid-20th century, with a mean surface temperature increase of 0.74°C between 1906 and 2005 (Intergovernmental Panel on Climate Change 2007). Projections of future greenhouse gas emissions indicate that atmospheric CO2 concentrations will reach 500 to 1100 ppm by 2100, resulting in mean surface warming of 1.1 to 6.4°C by the end of the 21st century (Intergovernmental Panel on Climate Change 2007). Such major changes in the earth's climate, and in the global environment more generally, have enormous significance for the human population (World Health Organization 2009). Indeed, in 2009, the Lancet Commission concluded that “climate change is the biggest global health threat in the 21st century” (Editorial 2009).
Climate models predict more rain in high latitudes leading to flooding, more forceful tropical cyclones with greater wind speeds, and a poleward shift in storm tracks. These changes alter wind, precipitation, and temperature patterns (Intergovernmental Panel on Climate Change 2007; 2012). Several influential papers discussed the effects that climate change might exert on pathogens (Mills et al. 2010; Relman et al. 2008), disease vectors (Bengis et al. 2004; Bohm et al. 2007), environmental health (Harvell et al. 2002; Campbell et al. 2011; Epstein 2001a), livestock (Boxall et al. 2009), human health (McMichael et al. 2006; Haines and Patz 2004; Cooney 2011; Kang 2011), disease mitigation and control strategies across various parts of the globe or regarding specific health threats (Beggs 2010; Chitsulo et al. 2000; Costello et al. 2011), and even environmental contamination by persistent organic pollutants (POPs; United Nations Environmental Programme and Arctic Monitoring and Assessment Programme 2010). However, it is striking that little attention has been paid to the influence that climate-related changes in disease patterns and incidence may exert upon medical pharmaceutical usage, both in terms of their economics implications and regarding their environmental impacts.
This review aims to provide a number of illustrative examples to stimulate further thought and debate, as opposed to conducting a full systematic review of the subject; therefore, the applied methodology consists of utilizing reviews of the impacts of climate change upon disease distribution to first establish the main areas for discussion. Selection of illustrative examples within the identified topics was based upon the availability of information, with respect to detailed predictions regarding potential range shifts of agents (and vectors and hosts, where applicable) and the presence of several studies with similar or related findings (as opposed to a single study in isolation, thus with a limited data set). Further information regarding pharmaceutical use was obtained by searching a number of medical literature resources (please see table legend) for the selected illustrative diseases/illnesses.
PHARMACEUTICAL DEMAND AND IMPACT
The transport, fate, and subsequent ecotoxicological effects of pharmaceuticals and their metabolites in the environment have been under examination for several decades and have been extensively documented (Ayscough et al. 2000; Halling-Sorensen et al. 1998; Heberer 2002; Ternes 1998; Daughton and Ternes 1999). As pharmaceuticals are neither completely removed by routine sewage treatment processes nor entirely degraded, their ubiquitous presence in the environment is perhaps unsurprising (Carballa et al. 2004; Golet et al. 2002; Heberer et al. 2001; Kümmerer 2010). Concern is growing, however, about the damaging consequences that these compounds currently exert on biota, or may in the future. This has been illustrated already by the deaths of millions of vultures on the Indian subcontinent following exposure to the nonsteroidal anti-inflammatory drug (NSAID) diclofenac, the well-known (and ongoing) cases of fish feminization associated with excreted components of women's contraceptive pills, and the global spread of antibiotic resistance in the environment (Taggart et al. 2007; Gilbert 2011; Hirsch et al. 1999). The latter recently led the UK's Chief Medical Officer to declare, “Antibiotics are losing their effectiveness at a rate that is both alarming and irreversible—similar to global warming” (Dame Sally Davis November 2012).
Changes in pharmaceutical use in the Northern Hemisphere because of climate change and the subsequent increased presence of drugs in the environment could have unintended and unexpected effects on the structure and functions of ecosystems. Impacts may be exacerbated by several factors, including our growing global population, the aging demographics of many Western and developing nations, obesity, the depression epidemic, and the rising availability of inexpensive generic drugs. These factors may result in elevated drug consumption and environmental release (Depledge 2011a; 2011b). Horizon scanning will be necessary to assess which pharmaceuticals could do most harm and identify potential mitigatory and preventative interventions.
The aim here is not to provide an exhaustive review of changes in disease distribution and related pharmaceutical use due to the complex and interactive nature of climate change and other factors affecting pharmaceutical demand (including public health mitigation and control measures, and socio-economic status). Instead, this review highlights specific climate change impacts that lead to disease: for example, those linked to temperature extremes, altered air quality, increased rainfall, and flooding. This review illustrates that changes in acute and chronic disease patterns (communicable and non-communicable diseases, vector, soil-, water- and food-borne diseases) are inevitable, and that associated changes in pharmaceutical use need to be managed.
The three linked diagrams presented in this review show how different pharmaceuticals are associated with various health conditions. Climate change could make these drugs more prevalent. A key to the diagrams is provided in Figure 1.
FIGURE 1.
Key to Link Diagrams (Figures 2–4) (color figure available online).
NON-COMMUNICABLE AND COMMUNICABLE DISEASES
Temperature Effects
Non-communicable diseases currently represent about two-thirds of all global diseases (Friel et al. 2011). The number of people affected will rise by several hundred thousand worldwide as the climate continues to change. The frequency, duration, and intensity of heat waves will exert an especially strong influence. An increasing number of vulnerable people have suffered heat stress during recent summers, causing thousands of deaths across Europe. The elderly and those with underlying cardiovascular and cerebrovascular diseases face particular risks (D'Ippoliti et al. 2010). Treatment of cardiovascular disease is often complex and involves a “cocktail” of drugs tailored to the patient's symptoms. This often includes diuretics to reduce blood pressure, statins to tackle high cholesterol, and salicylates and antiplatelets to reduce blood clot formation (Table 1). Prophylactic treatments for those prone to cerebrovascular disease are similar to treatments used in cardiovascular disease, although anticoagulants are most important after an ischemic stroke (Table 1).
TABLE 1.
Non-communicable and Communicable Diseases, Climate Change, and Recommended Pharmaceuticals
| Illness/disease | Climatic conditions/agent responsible | Medication/treatment: categories | Medication: examples |
|---|---|---|---|
| Sunburn | Sun exposure | Cortisone creams Antihistamine Analgesics |
Hydrocortisone Diphenhydramine Ibuprofen, acetaminophen, aspirin |
| Actinic keratosis leading to squamous-cell carcinoma | Sun exposure | Chemotherapeutic antimetabolite | Fluorouracil |
| Scabies | Poor personal hygiene due to water shortages leading to infestation bySarcoptes scabei | Insecticides Anti-itching agents Anthelmintic (sever cases) |
Permethrin, benzyl benzoate Crotamiton Ivermectin |
| Impetigo (secondary infection due to scabies) | Infection with Streptococcus or Staphylococcus, including MRSA | Antibiotics | Fusidic acid, mupirocin (topical). Flucloxacillin, erythromycin (oral). |
| Conjunctivitis | Poor personal hygiene due to water shortages leading to infestation by Chlamydia trachomatis | Fluroquinolone antibiotics (bacterial) Antihistamines (allergic and viral) |
Besifloxacin, gatifloxacin Epinastine, olopatadine, axelastine |
| Trachoma (a.k.a. quiet disease) | Long-term/repeated infestation by Chlamydia trachomatis | Antibiotics | Tetracycline, azithromycin |
| Cardiovascular disease | Cold temperatures leading to increased blood pressure | Diuretics (β-blockers, ACE inhibitors) Statins Statins Salicylates Antiplatelets Vasodilators |
Propranolol hydrochloride, captopril Atorvastatin, rosuvastatin Atorvastatin, rosuvastatin Aspirin Clopidogrel Nitroglycerine |
| Cerebrovascular disease | Cold temperatures leading to increased blood pressure | Prophylactic treatment—see cardiovascular disease above Anticoagulants |
See cardiovascular disease Warfarin, aspirin, clopidogeral |
| Respiratory infections: coughs, colds and influenza | Cold temperatures | Decongestants Expectorants Analgesics Antiviral |
Pseudoephedrine hydrochloride Ipecacuanha (as component of cough syrup) Acetaminophen, aspirin Oseltamivir, zanamivir |
| Respiratory infections: pneumonia, bronchitis | Cold temperatures increasing susceptibility to bacterial or viral infection | Antibiotics | Erythromycin, trimethoprim, levofloxacin, ampicillin, vancomycin |
| Respiratory illness: asthma | Aeroallergens (pollen, mold spores, pollution, harmful algal bloom toxins) | Corticosteroids β-Agonists Bronchodialators Leukotriene receptor antagonists |
Prednisolone, hydrocortisone (acute)Budesonide, fluticase propionate (chronic) Salbutamol, terbutaline (acute) Formoterol, salmeterol (chronic) Theophylline (acute) Montelukast, zafirlukast (chronic) |
| Respiratory illness: allergic rhinitis (a.k.a. hay fever) | Aeroallergens (pollen, mold spores, pollution, ozone) | Corticosteroids Decongestants Antihistamines Leukotriene receptor antagonists |
Fluticasone Pseudoephedrine Loratadine Montelukast |
| Atopic dermatitus (a.k.a. eczema) | Aeroallergens (pollen, mold spores, pollution) via association with asthma or allergic rhinitis | Antihistamines Corticosteroids Immunosuppressants, including calcineurin inhibitors Antibiotics (for co-occurring infections) |
Desloratadine, chlorphenamine maleate Hydrocortisone, flurandrenolone Pimecrolimis, tacrolimis, cyclosporin Fusidic acid, neomycin sulfate |
| Physical injury | Floods | Analgesics Antibiotics |
Morphine, codeine (opoid analgesics for severe pain) Cefuroxime, flucloxacillin, ciprofloxacin |
| Psychological distress and mental health impacts/disorders | Floods and other natural disasters | Sedatives (anxiolytics, hypnotics, and barbiturates) Antipsychotics (a.k.a. neuroleptics) Antimania Antidepressants (tricyclic, monoamine-oxidase inhibitors, selective serotonin reuptake inhibitors) |
Benzodiazepine compounds (e.g., diazepam), Chlorpromazine, pipotiazine, clozapine Carbamazepine, valproic acid, lithium carbonate Trazodone hydrochloride, phenelzine, fluoxetine |
Note. Sources are references cited in the main body of article; Centers for Disease Control and Prevention, Diseases and Conditions Online database; Infectious Diseases Society of America (IDSA) online factsheets; PubMed Health A.D.A.M. Medical Encyclopedia online; Medline Plus Medical Encyclopedia online; World Health Organization Disease Factsheets online; UK Health Protection Agency Infections online information sheets; UPMC Biosecurity Centre fact sheets online; and British National Formulary, Edition 58 (2009).
Personal behavior also alters during heat waves: Individuals spend more time outdoors, leading to more cases of sunburn and increased use of cortisone (steroidal) creams and pain relief medications (Table 1 and Figure 2). Longer term exposure to sunlight may also produce actinic keratoses of the skin. In severe cases, or cases in which squamous-cell carcinoma develops, treatment with chemotherapeutic antimetabolites may be necessary (Table 1 and Figure 2).
FIGURE 2.
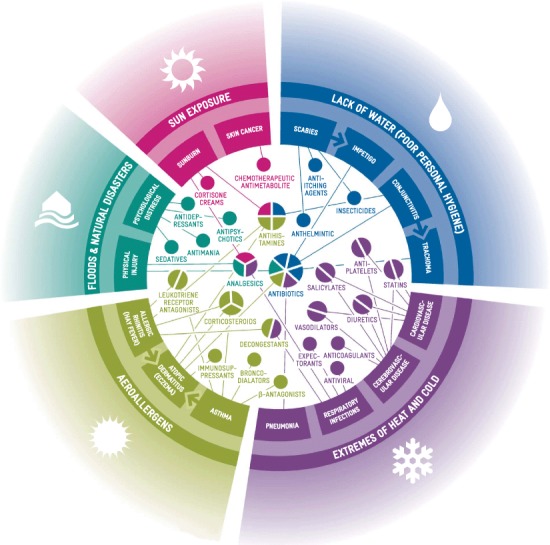
Climatic Conditions causing non-communicable Illnesses and Associated Pharmaceutical Treatments (color figure available online).
People tend to wash themselves and their clothing more often in hotter weather, increasing the use of deodorants and detergents and other personal care products. Climate change is also associated with more erratic rainfall patterns, which in some regions results in seasonal water shortages and less washing. Infections may occur if good standards of personal hygiene are not maintained. An infestation of the mite Sarcoptes scabei produces dermatitis, and an infection with the gram-negative bacteria Chlamydia trachomatis produces conjunctivitis and trachoma (World Health Organization 2001a; 2001b). Antibiotics are necessary in the treatment of trachoma (Table 1). Scabies infections are dealt with using medicated creams that commonly contain insecticides, and even with oral anthelmintics. Diseases that disrupt the skin surface increase the risk of secondary bacterial infection. In the case of scabies, the secondary illness, impetigo may develop after infection with Streptococcus or Staphylococcus (including methicillin-resistant Staphyloccocus aureaus [MRSA]). These diseases require additional drug treatment, but often leave the patient vulnerable to secondary infection, requiring additional antibiotics and other pharmaceuticals (Table 1 and Figure 2).
The term “global warming” is often used to describe the impact of greenhouse gas accumulation on the earth's climate. However, it is now clear that extreme climatic variations, involving both heating and cooling, take place. Heat waves and more severe winters are likely in the Northern Hemisphere. Overall winter temperatures may be higher, but more frequent and intense winter storms are predicted. Strong storm winds increase cold exposure for many vulnerable individuals, raising the incidence of illnesses directly related to cold temperatures (such as hypothermia and frostbite) and lowering resistance to respiratory infection. Complications are more likely to arise in those with underlying cardiovascular and respiratory diseases (Conlon et al. 2011). Decongestants, expectorants, pain relief medication, and, for susceptible individuals, antiviral medications are commonly used to treat common coughs, colds, and influenza; these drugs may be required in larger amounts in the future (Table 1 and Figure 2). Pneumonia and acute bronchitis usually have a viral and/or bacterial etiology. Because it can be difficult to ascertain the etiology of these diseases, antibiotics are typically administered, especially to those at greatest risk (Table 1). The preceding examples illustrate how more illness arising as the climate changes may lead to increased pharmaceutical use.
Precipitation and Flooding
Climate change is affecting rainfall patterns as well as temperature. The frequency and intensity of flooding are increasing worldwide (Lugeri et al. 2010; Kundzewicz et al. 2006; 2008). Health issues over a wide range are associated with floods, but many depend upon the socio-economic status of the population affected and the responses of local authorities. Extreme flood events, in particular flash floods, elevate the risk of drowning, physical injury such as broken bones, and hypothermia. However, in areas where the local authority response is inadequate, secondary consequences may follow and exert a significant influence on local pharmaceutical requirements. These include injury complications such as infections, detrimental outcomes for mental health, the emergence of communicable and vector-borne diseases, particularly with contaminated water, and in the worst cases, malnutrition (Du et al. 2010). Mental illnesses, including psychological distress following natural disasters and severe environmental degradation, are exacerbated by ill health and vice versa (Verger et al. 2003; Chae et al. 2005; Reacher et al. 2004; Speldewinde et al. 2009). Various pharmaceuticals are needed to treat the injuries and diseases linked with floods (Tables 1 and 2 and Figure 2 and 3). To date, however, detailed information about the extent of increased drug use and associated costs has not been assembled.
TABLE 2.
Vector-Borne Diseases, Climate Change, and Recommended Pharmaceuticals
| Illness/disease | Pathogen responsible | Vector | Vector host/pathogen reservoir | Medication/treatment: categories | Medication: examples |
|---|---|---|---|---|---|
| Dengue fever (a.k.a. breakbone fever), dengue hemorrhagic fever and dengue shock syndrome | Dengue fever virus (Flaviviridae) | Aedes spp., e.g., Asian tiger mosquito (A. albopictus Skuse) | Humans and other primates | No specific treatment; symptomatic treatment, analgesics | Acetaminophen |
| Yellow fever | Yellow fever virus (Flaviviridae) | Aedes spp., e.g. Asian Tiger Mosquito (A. albopictus Skuse) | Humans and other primates | No specific treatment; symptomatic treatment, analgesics | Acetaminophen |
| Chikungunya | Chikungunya virus (Togaviridae alphavirus sp.) | Aedes spp., e.g. Asian Tiger Mosquito (Aedes albopictus Skuse) and Aedes aegypti | Humans and other primates Possibly other wild animals | No specific treatment; symptomatic treatment. Analgesics and anti-inflammatory | Acetaminophen, ibuprofen |
| West Nile fever/encephalitis/meningitis and West Nile virus Poliomyelitis | West Nile virus (Flaviviridae) | Various mosquito species, from generas: Anopheles, Coquillettidia, Culex, Culiseta, Deinocerites, Mansonia, Orthopodomyia, Psorophora, Uranotaenia, Aedes (e.g. Asian Tiger Mosquito; A. albopictus Skuse) Rarely transmission has occurred via blood transfusion, organ transplant, transplacental and breast-feeding |
Predominantly birds; infected horses, cats, dogs, bats, chipmunks, skunks, squirrels, and rabbits have been reported | No specific treatment; symptomatic treatment. Analgesics Anti-inflammatory Antiviral Anticonvulsant (encephalitis) Corticosteroids (encephalitis) Sedative (encephalitis) |
Acetaminophen Diclofenac, meloxicam Ribavirin Lorazepam, diazepam Betamethasone, dexamethasone Diazepam |
| Antipyretics (fever) | Ibuprofen, aspirin | ||||
| Antiemetics (meningitis) | Dolasetron | ||||
| Eastern equine encephalitis (a.k.a. sleeping sickness) | Eastern equine encephalitis virus (Togaviridae alphavirus sp.) | Various mosquito species from the generas: Coquillettidi, Culex, Aedes (e.g., Asian tiger mosquito; A. albopictus Skuse). | Birds | No specific treatment; symptomatic treatment. See West Nile fever above | See West Nile fever |
| Usutu virus infection | Usutu virus (flaviviridae) | Culex mosquito spp., e.g., C. pipiensand C. neavei and Aedes albopictus Skuse (asian tiger mosquito) | Birds; infections of horses also known | Only few human cases confirmed worldwide, therefore treatment not established. Symptomatic treatment, see West Nile fever | See West Nile fever |
| Antibiotics (secondary infection) | Benzylpenicillin, cefotaxime, chloramphenicol | ||||
| H2 antagonists (fulminant hepatitis) | Cimetidine | ||||
| Cytoprotective agent (fulminant hepatitis) | Sucralfate | ||||
| Antifungal (pre-liver transplant, due to fulminant hepatitis) | Fluconazole | ||||
| Ross River fever (a.k.a. epidemic polyarthritis) | Ross River virus (Togaviridae Alphavirus sp.) | Mosquitos from the genus Aedes, including A. vigilax, A. notoscriptus, A. albopictus Skuse (Asian tiger mosquito) and Culex annulirostris | Various mammals, including kangaroos, wallabies, horses, rodents, and possums. Humans during epidemic events. | No specific treatment; symptomatic treatment. Analgesics Anti-inflammatory (arthritic symptoms) Antipyretics (fever) Antiparasitic agent (treatment) |
Paracetamol, codeine Diclofenac, meloxicam Aspirin, ibuprofen |
| Heartworm infection (a.k.a. dog heartworm). Human pulmonary dirofilariasis, intra-abdominal infection (human) | Nematodes Dirofilaria (Dirofilaria) immitis | Culex quinquefasciatus, C. pipiens (often infects cats), Anopheles maculipennis, Coquillettidia richiardii, Ochlerotatus notoscriptus, Aedestriseriatus, A. albopictus (Asian Tiger Mosquito), A. aegypti, A. taeniorhynchus, A. scapularis, A. trivittatus, Xenopsylla cheopis | Dogs (D. immitis) | Antiparasitic agent (treatment) | Ivermectin (humans;prophylactic and treatment for dogs and cats). |
| Subcutaneous dirofilariasis (human; conjunctiva, eyelid, scrotum, breast, arms, less) | Nematodes Dirofìlaria (Nochtiella) species repens, tenuis and striata are common agents of human infection; D. (Nochtiella) ursi and sybdermata rarely cause human infections. | Raccoon (D. tenuis), Dogs and Cats (D. repens), Bear (D. ursi) | Anthelmintic (treatment) | Diethylcarbamazine (dogs and humans). | |
| Antibiotics (treatment) | Deoxycycline (dogs; early stage infection). | ||||
| Arsenic based compounds (treatment) | Melarsomine dihydrochloride, thiacetarsamide sodium (adult nematodes in dogs). | ||||
| Antiparasitic agent (prophylactic) | Moxidectin, milbemycin, and selamectin (dogs and cats). | ||||
| Insecticide (prophylactic) | Imidacloprid (dogs and cats) | ||||
| Corticosteroids (treatment) | Prednisolone (cats) | ||||
| Malaria | Plasmodium vivax, P.falciparum, P. ovale and P. malariae | Anopheles spp. and Aedes spp. | Humans | Antimalarials (combination drug for prevention; as component of artemisinin-based combination therapies for treatment) | Chloroquine (occasionally used as monotherapy for nonresistant P. vivax), sulfadoxine-pyrimethamine, dapsone, pyrimethamine, doxycycline, mefloquine, atovaquone, proguanil, quinine bisulphate, lumefantrine, doxycycline |
| Artemisinin derivatives (as component of combination therapy for treatment only) | Artesunate, artemether, dihydroartemisinin, artemotil | ||||
| Lyme disease (a.k.a. borreliosis and Bannwarth syndrome) | Bacterium Borrelia burgdorferi (USA), B. afzelii and B. garinii (Europe). | Several tick species (Ixodes spp.; a.k.a. the blacklegged tick), including castor bean tick (a.k.a. sheep tick, Ixodes ricinus) | Sheep, deer, mice | Antibiotics | Doxycycline, amoxicillin, erythromycin, cefuroxime axetil. ceftriaxone, cefotamine, benzylpenicillin (neurological and cardiac complications). |
| Analgesics | Acetaminophen | ||||
| Tick-borne encephalitis | Tick-borne encephalitis virus (Flaviviridae); 3 subtypes European (a.k.a. Western), Siberian and Far eastern (a.k.a. Russian spring summer encephalitis virus). | Several tick species, dependent upon viral subtype (European, Ixodes ricinus; Siberian and Far Eastern, I. persulcatus). | Small mammals, livestock (goats, sheep, cows), and some bird species. | Vaccine available. | TicoVac |
| Consumption of unpasteurized diary products from goats, sheep, or cows, can also result in human infection. | No specific treatment; symptomatic treatment of meningitis, encephalitis or meningoencephalitis. See West Nile fever | ||||
| Antipsychotics, antidepressants, mood stabilizers, sedatives, etc. (treatment of neuropsychiatric sequelae, which occur in 10–20% of cases) | Chlorpromazine, pipotiazine, clozapine, trazodone hydrochloride, phenelzine, fluoxetine, lithium, benzodiazepine compounds (e.g., diazepam) | ||||
| Tularemia (a.k.a. deerfly fever, rabbit fever, Pahvant Valley plague, Ohara disease, Yato-byo, and lemming fever). Forms include ulceroglandular, glandular, oculoglandular, oropharyngeal, pneumonic, typhoidal, and septic (dependent upon pathogen subspecies, strain virulence, and infection route). | Bacterium Francisella tularensis, subspecies F. tularensis biovar tularensis (N. America) and F. tularensis biovar palaearctica (Europe and Asia) | Ticks (Ixodes spp.), fleas (Xenopsylla cheopis), horsefly (Tabanus sp.), deerfly (Chrysops sp.), and mosquitos. | Small mammals, predominantly rodents and lagomorphs. | Vaccine under development | Doxycycline, ciprofloxacin |
| Zoonotic transmission from vertebrate hosts to humans also occurs via contact with infected animals tissues/fluid, soil or water, and inhalation of aerosolized bacteria or infected soil/plant material. | Oral antibiotics (tetracyclines and fluoroquinolones) | ||||
| Intramuscular or intravenous antibiotics (aminoglycosides) | Streptomycin, gentamicin | ||||
| Plague (a.k.a. bubonicplague, pneumonic plague, septicemic plague) | Bacterium Yersinia pestits | Fleas (Xenopsylla cheopis). | Wild rodents, e.g., rats and prairie dogs, and domestic cats. | Antibiotics (treatment) | Streptomycin, gentamicin. Occasionally doxycycline, or ciprofloxacin |
| Transmission to humans can also occur via direct contact, inhalation and ingestion of contaminated materials. Pneumonic plague can be directly transmitted between humans. | Antibiotics (preventative e.g. for flea bites, contact with infected animal, contact with case of pneumonic plague) | Chloramphenicol or effective drugs from the tetracyclines or sulfonamides classes | |||
| Leishmaniasis (visceral and cutaneous forms most common, rarely mucocutaneous form) | Protozoan Leishmaniasis spp. (20+ species). L. donovani and L. infantum most common pathogens for visceral, L. tropica for cutaneous | Sandfly (Phlebotomus and Lutzomyia spp.) | Canines, marsupials, rodents | Antimonial (preventative) | Sodium stibogluconate, Meglumine antimoniate |
| Antifungal drugs (oral; treatment of cutaneous form) | Fluconazole (L. major, L. braziliensis), Ketoconazole (L. mexicana, L. panamensis and L. major). Itraconazole | ||||
| Antimicrobial (treatment of cutaneous form) | Pentamidine isethionate (L. guyanensis, limited efficacy with L. braziliensis) | ||||
| Antibiotic (topical treatment of cutaneous form) | Paromomycin | ||||
| Antiprotozoal (treatment of cutaneous and visceral forms) | Miltefosine | ||||
| Antimonial (intralesional; treatment of cutaneous form) | Sodium stibogluconate, Meglumine antimoniate | ||||
| Antifungal (treatment of visceral form, also used for post-treatment secondary prophylaxis in HIV co-infected individuals) | Liposomal amphotericin B | ||||
| Additional supportive treatment of anemia, nutritional status, hemorrhagic complications and secondary infections. | |||||
| Chagas disease (a.k.a. American trypanosomiasis) | Protozoan Trypanosoma cruzi | Triatomine bugs (a.k.a. assassin bugs; kissing bugs) | Wild and domesticated mammals including rodents | Antiparasitic agents | Benznidazole, nifurtimox. |
| Human to human transmission can also occur via blood transfusions and organ donation from infected individuals, and congenitally from mother to child | Symptomatic treatment of acute cases. Antipyretics, analgesics, anti-inflammatory. | Aspirin, ibuprofen, diclofenac | |||
| Symptomatic treatment of chronic cases depends upon systems affected i.e., cardiac, digestive, neurological or mixed |
Note. Sources are references cited in the main body of article; Centers for Disease Control and Prevention, Diseases and Conditions Online database; Infectious Diseases Society of America (IDSA) online factsheets; PubMed Health A.D.A.M. Medical Encyclopedia online; Medline Plus Medical Encyclopedia online; World Health Organization Disease Factsheets online; UK Health Protection Agency Infections online information sheets; UPMC Biosecurity Centre fact sheets online; and British National Formulary, Edition 58 (2009).
FIGURE 3.
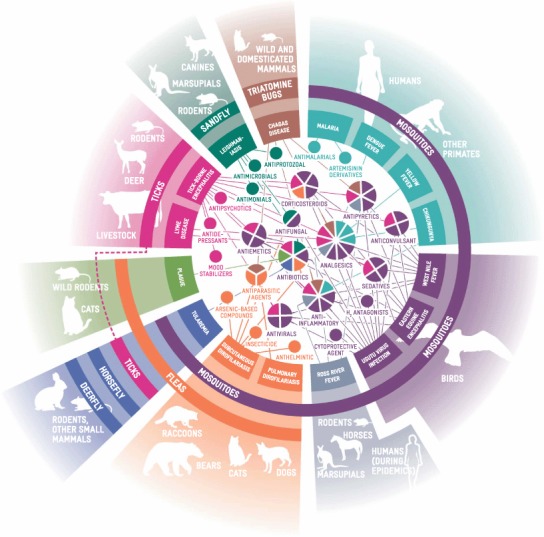
Vector-Borne Illnesses and Associated Pharmaceutical Treatments (color figure available online).
Climate and Air Quality
Climate change exacerbates the health impacts of aeroallergens such as pollen and mold spores, and increases the prevalence of algal toxins. Changing meteorological conditions and rising temperatures influence atmospheric pollen content; this occurs through changes in pollen production and shifts in, or even lengthening of, the pollen-production season. In addition, the geospatial distribution of pollen is changing: Plant ranges and long-distance atmospheric transport are moving poleward as new and predicted atmospheric circulation patterns emerge. Interestingly, the movement of plant ranges may also result in the disappearance of some pollen types, along with the emergence of other pollens more suited to the new climate. The health consequences of this have not been assessed. Although agricultural crops grown on large scales are one of the more important pollen sources to consider, shifting plant ranges, crop varieties, and densities make it difficult to predict whether pollen counts will rise overall.
Higher CO2 concentrations and temperatures associated with climate change may result in the production of pollen that is more allergenic in nature (defined as allergen concentration in the pollen grain) (Beggs 2010). Conversely, increasing amounts of ground level ozone globally may suppress photosynthesis and ultimately pollen production (Royal Society 2008). Climate change is also expected to affect the production of mold spores, particularly indoors. This may be especially relevant in developed countries, where adults and children spend more than 90% of their time indoors (U.S. Environmental Protection Agency Office of Air and Radiation 1989).
In the future, coastal communities may experience increased exposure to algal toxins in aerosols (Anderson et al. 2012). In Florida, for example, toxic algal blooms release aerosolized brevetoxins into the atmosphere, exacerbating asthma and other existing respiratory diseases in exposed, susceptible individuals (Fleming et al. 2011). Potential rises in the pharmaceutical treatment of these conditions mirror elevation in algal bloom events in marine ecosystems that are being driven by climate change (Intergovernmental Panel on Climate Change 2007).
It is a compelling hypothesis that a rise in aeroallergens and algal toxins influences rates of respiratory disease, including asthma and allergic rhinitis, while also exacerbating atopic dermatitis. This leads to greater pharmaceutical use. Prophylactic treatments for chronic asthma consist of aerosolized medications of beta-adrenergic agonists in combination with corticosteroids and the leukotriene receptor antagonists. For acute asthma attacks, beta-agonists, anticholinergics, and corticosteroids are used, while allergic rhinitis (“hay fever”) is treated with corticosteroids, decongestants, antihistamines, and leukotriene receptor antagonists. Antihistamines and corticosteroids are used to treat atopic dermatitis (eczema), which often occurs alongside asthma and in allergic rhinitis sufferers (Table 1 and Figure 2).
Respiratory conditions are made worse by deteriorating air quality associated with climate change. Kinney (2008) describes how more sunlight and higher temperatures lead to increased ozone formation at ground level. Further, long periods of drought elevate the risk of wildfires, which intensify fine particulate air pollution. This has been particularly apparent in Australia in recent years (Chen et al. 2006). Floods elevate the prevalence of molds and microbes, which also aggravate respiratory illnesses (Kunii et al. 2002; Biswas et al. 1999; Centers for Disease Control and Prevention 2006; Riggs et al. 2008). All of these factors worsen symptoms in those with respiratory conditions, and increase pharmaceutical use.
VECTOR-BORNE DISEASES
Changes in climate prompt changes in the abundance and distribution of populations of organisms in ecosystems. Since pathogens are transmitted between a variety of vertebrate animals and humans, changes in the incidence of vector-borne disease can be expected. These zoonotic infections represent approximately 60% of human pathogenic illnesses (Bengis et al. 2004). The impacts that climate change exerts upon the pathogen itself, pathogen vectors, and the hosts of vectors are important to take into consideration.
Mosquito Vectors
Various models have predicted the spread of mosquito species poleward in the Northern Hemisphere. Fischer et al. (2011) modeled the European distribution of the Asian tiger mosquito, Aedes albopictus Skuse, under climatic change using four different models. Areas suitable for A. albopictus are already increasing in Western and Central Europe, although their spread into Eastern Europe is expected to be delayed. Suitable areas in Southern Europe are likely to fall in number, suggesting the species range will shift. As a vector organism, A. albopictus can transmit at least 22 types of arboviruses (including all four dengue fever serotypes, yellow fever, chikungunua virus, West Nile virus, eastern equine encephalitis, Usutu virus, and Ross River virus), as well as the parasitic nematodes Dirofilaria immitis and D. repens (Gratz 2004; Mitchell et al. 1987; Cancrini, et al. 2003a; 2003b; Calzolari et al. 2010; Vázquez et al. 2011). Transmission of the Dirofilaria spp. to humans rarely produces health problems, although occasionally treatment with an antiparasitic agent and an anthelmintic is required (Table 2 and Figure 3).
Pharmaceuticals are also used in prophylactically and treatment for pets succumbing to Dirofilaria infections (Table 2). The spread of A. albopictus in Europe and the United States has already affected human health; endemic chikungunya virus and dengue fever have reappeared in Italy, France, Croatia, Hawaii, Texas, and Florida (Rezza et al. 2007; La Ruche et al. 2010; Queyriaux et al. 2008; Schmidt-Chanasit et al. 2010; Gjenero-Margan et al. 2011; Centers for Disease Control and Prevention 2010b; Adalja et al. 2012). The zoonotic flavivirus West Nile virus is now also widespread in the United States and Canada after its suspected introduction by migratory birds in 1999 (Epstein 2001b; Rappole et al. 2000; El Adlouni et al. 2007).
It is temperature that largely determines the development of the protists Plasmodium vivax and P. falciparum, which produce malaria. Temperature changes due to climate change may lead to a 12–27% increase in transmission (Martens et al. 1997), placing an additional 200 million individuals at risk of contracting malaria (Béguin et al. 2011) and requiring an enormous rise in drug therapy. Former breeding sites for the malaria mosquito vectors, Anopheles spp. and Aedes spp., are still present in many developed countries, including the United States and Southern and Central Europe, where malaria may return (Martens et al. 1997). However, since malaria was eradicated previously, it seems likely that managing this problem by land drainage and the use of pesticides will be feasible if the need arises. Further information about the range of medications required to treat mosquito-borne diseases are provided in Table 2 and Figure 3.
Tick Vectors
Mosquitoes are not the only disease vectors that are affected by climate change. The range of the castor bean/sheep tick, Ixodes ricinus, for example, is spreading northward (Lindgren et al. 1999). Lyme disease and tick-borne encephalitis are caused by several species of Borrelia bacteria and the tick-borne encephalitis virus, respectively. Both will be of huge concern for Europe (Gage et al. 2008). Lyme disease is treated with antibiotics (Table 2 and Figure 3). Although an inactivated vaccine is available for tick-borne encephalitis, there is not an established treatment program for sufferers (see Table 2 for associated medications).
Modeling indicates a range shift for transmission of the zoonotic bacterial pathogen Francisella tularensis, which produces tularemia (or “rabbit fever;” Nakazawa et al. 2007). With diseases of this type, in which complex pathogen–vector–host interactions occur, the effects of climate change on the vectors (ticks, fleas, and flies), their hosts (rodents and lagomorphs), and the pathogen itself (which may be directly transmitted between vertebrate hosts and humans) needs to be considered (Harvell et al. 2002; Relman et al. 2008). The F. tularensis subspecies responsible for the infection, strain virulence, and infection route play important roles in the symptomatic profile observed, although all cases are treated with antibiotics (Table 2 and Figure 3).
Flea Vectors
The flea Xenopsylla cheopis is the vector of the bacterial pathogen Yersinia pestis, which produces plague in humans. Prevailing climate is crucial in determining disease transmission, with warmer and wetter conditions benefitting both plague vectors and hosts. Once again, climate change is driving a poleward range shift in transmission of the disease (Enscore et al. 2002; Eisen et al. 2007; Nakazawa et al. 2007; Parmenter et al. 1999). The importance of climate in relation to plague transmission was further emphasized by studies exploring the relationship between climatic events (e.g., the Pacific decadal oscillation and El Niño southern oscillations) and human transmission rates, and with prairie dog colony extinction events (Ari et al. 2008; Stapp et al. 2004). The infection route for Y. pestis in humans determines which form of disease is contracted (bubonic plague, pneumonic plague, or septicemic plague), thus influencing the symptomatic response. More antibiotics will be used to treat plague cases—drugs that are similar to those used in tularemia (Table 2 and Figure 3). Where prophylactic measures are needed to limit further spread of the disease (e.g., when an individual has had close contact with someone suffering from pneumonic plague), antibiotics are also used.
Fly Vectors
Visceral and cutaneous leishmaniasis are produced by different subspecies of the protozoan Leishmania; both are now endemic in Europe. The impact of climatic change on the distribution of leishmaniasis vectors (i.e., the sandfly Phlebotomus and Lutzomyia spp.) is difficult to predict, as the presence of specific vegetative habitats correlates with transmission rates. Localized land use will therefore exert a significant impact (Ready 2010). However, in areas where leishmaniasis is endemic, climatic events such as El Niño southern oscillations may result in greater transmission rates in subsequent years (Franke et al. 2002). El Niño events are expected to increase in frequency as climate change proceeds, leading to an elevated worldwide risk of infection with Leishmania.
The presence and density of hosts (including canines, marsupials, and rodents), which serve as disease reservoirs (Diniz et al. 2008), is likely to be of even greater importance to leishmaniasis transmission. These factors affect disease incidence and need for drug treatment. The general health of individuals is also significant, including co-infections of leishmaniasis with HIV, which is well known in Southern Europe (Alvar et al. 1997; Rosenthal et al. 1995). Pentavalent antimony compound medications are used in preventative and treatment regimes, along with a number of antimicrobial/antifungal/antiprotozoal drugs (Table 2 and Figure 3). Supportive therapy is also required to tackle co-occurring anemia, hemorrhagic complications, secondary infections, and the nutritional status of the infected individual.
Other Vectors
Chagas disease (also known as American trypanosomiasis) is another vector-borne disease that may become increasingly important as climate change progresses. The pathogen responsible is the flagellate protozoan Trypanosoma cruzi, with Triatominae (assassin bugs) as the disease vector. Chagas disease starts with an acute infection, which, over years, develops into a chronic condition affecting the heart, digestive, and nervous systems. Antiparasitic drug use will increase if climate change enables the incidence of Chagas disease to rise. In turn, the application of treatments for cardiac, digestive, and neurological symptoms will also rise (Bern et al. 2007; Table 2 and Figure 3).
Wild and domesticated mammals are among the wide range of mammalian hosts carrying the disease's insect vector. Significant correlations between populations of Triatominae vectors and host rodents have been found (Peterson et al. 2002). The influence of climate change on the insect vector is difficult to predict, but the geographic distribution of wild vectors will probably widen due to higher temperatures (Carcavallo 1999). Lower humidity might also shorten life cycles.
Hosts and Disease Reservoirs
The prevalence of various vector-borne illnesses heavily depends on how climate change impacts host ranges and densities. Climate change might reduce disease transmission as species are driven out of areas that become too wet or unstable to support them. Nonetheless, range extensions have attracted most attention. For example, rodent populations are affected by precipitation: Heavy rainfall events that increase primary production subsequently result in a boom in rodent populations (Brown and Singleton 1999; Leirs et al. 1996; Lima et al. 1999; Brown and Zeng 1989). When drought precedes these rainfall events, rodent outbreaks are magnified by declining predator populations (Epstein 2001a). Regardless of population size, extreme climatic events raise the likelihood of disease transmission as rodent behavior is modified (e.g., entering human dwellings to seek food or refuge) (Gubler et al. 2001).
In more rural areas worldwide, the effects of climatic change on wild animals serving as disease reservoirs and vector hosts may be one of the most important factors influencing vector-borne diseases for humans and livestock. Bohm et al. (2007) illustrated the key role of wild vertebrates by studying the expanding range of all six UK deer species. Wild animals are not vaccinated so may act as a disease reservoir, transferring diseases to livestock, humans, or invertebrate vectors. Wild deer are commonly subject to at least 39 infestations, most of which are produced by contact with livestock. Regions where deer populations are extending their range or becoming denser, due to changing climatic conditions, may see a rise in tick- and flea-borne diseases.
The drugs required to treat zoonotic and vector-borne diseases originate from diverse classes within the pharmaceutical arsenal. The changing ranges of these types of diseases could lead to major shifts in drug use profiles (Table 2 and Figure 3). It may become necessary to treat more livestock, either as a control measure or to address infection. People spending time in natural areas through occupation or recreation will be at greater risk. Those living in “animal-loving” countries may also be more vulnerable to vector-borne and zoonotic diseases because pets can be vector hosts or reservoirs of disease. Further application of pharmaceuticals and insecticides on pets, either for treatment or as a prophylactic measure, may be required (Otranto and Wall 2008).
SOIL-, WATER-, AND FOOD-BORNE DISEASES
The World Health Organization and the United Nations Environment Programme are among those drawing attention to the threat climate change poses to soil and water quality, and food security. These changes have major implications for human health and pharmaceutical use. Outbreaks of food poisoning for example are expected rise by 10,000 cases per year in the United Kingdom as climate change proceeds.
Soil-Borne Pathogens
Human cryptococcal infections produce cryptococcal meningitis or pneumonia. The inhalation of fungal spores also leads to simultaneous cryptococcomas in the lung, brain, or muscle. These infections are primarily produced by the yeast species Cryptococcus neoformans and C. gattii, and historically have been seen in tropical and subtropical zones (Sorrell 2001). The last decade has seen escalating outbreaks of C. gatti in human and animal populations in Canada and the United States, some of which were severe enough to result in death (Datta et al. 2009; Centers for Disease Control and Prevention 2010a; MacDougall et al. 2007; Galanis and MacDougall 2010). Cryptococcus gatti emergence in temperate regions has been attributed to the creation of conditions suitable for spore survival and propagation because of climate change, or to the adaptation of C. gatti to a new climatic niche. Cryptococcus gatti is expected to spread to the Northern Hemisphere over the coming years (Dixit et al. 2009; Datta et al. 2009). Cryptococcal infections are treated with antifungal agents, and corticosteroids when the central nervous system has been affected (Perfect et al. 2010) (Table 3 and Figure 4).
TABLE 3.
Soil-, Water-, and Food-Borne Diseases, Climate Change, and Recommended Pharmaceuticals
| Illness/disease | Pathogen responsible | Pathogen environment/life cycle | Transmission route | Medication/treatment: categories | Medication: examples |
|---|---|---|---|---|---|
| Cryptococcal infection: meningitis or pneumonia | Fungus Cryptococcus neoformans and C. gatti | Soil. Often in association with bird droppings (C. neoformans) or specific tree species, e.g., eucalyptus (C. gatti) | Inhalation of spores | Antifungal | Amphotericin B (intravenous; lipid formulations if renal impairment present) combined with 5-flucytosine (oral). Fluconazole, itraconazole, voriconazole, or posaconazole (as suppressive regime). Polyene antimycotics (for meningoencephalitis). |
| Corticosteroids (for cases with sever central nervous system symptoms) | Betamethasone, dexamethasone | ||||
| Cryptosporidiosis (a.k.a. Cryptosporidium enteritis) | Protozoan Cryptosporidium | Inhabits human and animal intestines. Once shed in stool it is able to survive for long periods of time outside of the body. Therefore found in soil, food, water, or on fecal-contaminated surfaces. | Consumption of faecal contaminated water or food. Contact with fecal-contaminated surfaces. | Antiprotozoal | Nitazoxanide, atovaquone |
| Antibiotic | azithromycin, metronidazole, paromomycin, trimethoprim-sulfamethoxazole combination. | ||||
| Cholera | Bacterium Vibrio cholerae. Enterotoxins produced by V. cholerae cause the diarrhea. | Inhabits human and animal intestines. Water- and food-borne. Naturally occurring in brackish and coastal waters. | Consumption of fecal contaminated water or food. Contact with fecal-contaminated surfaces. Consumption of raw/undercooked shellfish. | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Vaccine Antibiotics (severe cases) | Dukoral, ShanChol (oral) Tetracycline, doxycycline, furazolidone, erythromycin, ciprofloxacin. | ||||
| E. coli enteritis (a.k.a. travelers’ diarrhea), can progress to hemorrhagic colitis (bloody diarrhea). Hemolytic uremic syndrome (severe case characterised by acute renal failure, hemolytic anemia and thrombocytopenia) | Bacterium Escherichia coli, certain strains are pathogenic (e.g. E. coli O157:H7). Toxins (verotoxins or Shiga-like toxins) produced by enterohemorhagic/enterotoxigenic) E. coli cause the diarrhea. | Inhabits human and animal intestines. Water-borne and food-borne | Consumption of contaminated water or food, such as undercooked meat or unpasteurized dairy products. Rarely transmitted between humans. | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Antibiotics (severe cases or high risk individuals e.g. pregnant, immunosuppressed) | Fluoroquinolones (e.g., ciprofloxacin), azithromycin | ||||
| Hemolytic uremic syndrome: No specific treatment; symptomatic treatment inclusive of anemia, dialysis, transplants, and maintenance of blood pressure. | |||||
| Shigellosis (a.k.a. shigella enteritis/gastroenteritis). Hemolytic uremic syndrome (severe case with acute renal failure, hemolytic anemia and thrombocytopenia due to S. dysenteríae). Post-infectious arthritis (a.k.a. Reither's syndrome, reactive arthritis, arthritis urethritica, venereal arthritis, polyarteritis enterica; 2% of S. flexneri cases) | Bacterium Shigella spp., e.g., S. sonnei (a.k.a. group D Shigella), S. flexneri (a.k.a. group B Shigella), S. dysenteriae type 1, S. boydii | Inhabits human and animal intestines. Water-borne and food-borne. Can be transmitted by flies | Consumption of faecal contaminated water or food. Direct transmission between humans (via contaminated fecal material) | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Antibiotics (severe cases, or to limit transmission in crowded living environments) | Sulfamethoxazole-trimethoprim combination, ampicillin, ciprofloxacin, azithromycin, ceftriaxone | ||||
| Nonsteroidal anti-inflammatory drugs and analgesics (postinfectious arthritis) | Diclofenac, meloxicam, aspirin, ibuprofen | ||||
| Corticosteroids (persistent inflammation; postinfectious arthritis) | Betamethasone, dexamethasone | ||||
| Hemolytic uremic syndrome: No specific treatment; symptomatic treatment inclusive of anemia, dialysis, transplants, and maintenance of blood pressure. | |||||
| Salmonella enterocolitis (a.k.a. salmonellosis, Salmonella gastroenteritis). Typhoid fever (a.k.a. enteric fever; caused by S. typhi). Can lead to the autoimmune postinfectious arthritis (a.k.a. Reiter's syndrome, reactive arthritis, arthritis urethritica, venereal arthritis, polyarteritis enterica) | Bacterium Salmonella spp., >2500 serotypes known, e.g., S. nteriditis, S. Newport and S. typhimurium (gastroenteritis), S. typhi (typhoid fever) | Inhabits human and animal intestines and can transport to lymph nodes, gallbladder, liver, and spleen via bloodstream. Water-borne and food-borne. | Consumption of contaminated raw animal products (e.g., poultry, eggs, beef) and occasionally unwashed fruit and vegetables (manure contaminated). Handling carrier animals, e.g., reptiles, birds, cats, dogs. Humans can become nonsymptomatic carriers. | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Antibiotics (severe adult cases, i.e., bloodstream infected; typhoid fever) | Fluoroquinolones, e.g., ciprofloxacin, chloramphenicol, ampicillin and amoxicillin and trimethoprim-sulfamethoxazole combination | ||||
| Antibiotics (severe childhood cases, i.e., bloodstream infected; typhoid fever) | Third-generation cephalosporins, e.g., ceftriaxone, chloramphenicol, ampicillin and amoxicillin and trimethoprim-sulfamethoxazole combination | ||||
| Vaccine (typhoid fever) | Ty21a (Vivotif Berna; oral), ViCPS (Typhim Vi; intravenous) | ||||
| For treatment of postinfectious arthritis, see shigellosis | See shigellosis | ||||
| Hepatitis A (a.k.a. viral hepatitis; infectious hepatitis). Rarely can become fulminant hepatitis. | Hepatitis A virus | Water-borne and food-borne. Inhabits human intestines | Consumption of food or water contaminated by stool from an infected person. Anal/oral sex with infected individual (via stool or blood) | Vaccine | Havrix (a.k.a. VAQTA) |
| Gastrointestinal illness. Septic shock (a.k.a. septicemia, blood stream infection; severe cases in immunocompromised individuals). | Bacterium Vibrio vulnificus | Food-borne and water-borne. Naturally occurring in marine waters. | Consumption of contaminated seafood (e.g., raw oysters) or exposure of open skin would to marine waters | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Surgical intervention (severe cases of infection via open wound) with removal of necrotic tissue; amputation may be necessary | |||||
| Antibiotics | Doxycycline combined with third-generation cephalosporins (e.g., ceftazidime), fluoroquinolones, trimethoprim-sulfamethoxazole combination with an aminoglycoside. | ||||
| Gastrointestinal illness. Septic shock (a.k.a. septicemia, blood stream infection; severe cases in immunocompromised individuals) | Vibrio parahaemolyticus | Food-borne and water-borne. Naturally occurring in marine waters. | Consumption of contaminated seafood (e.g., raw oysters, finfish and bloody clams) or exposure of open skin would to marine waters | Rehydration salts (key treatment) | Sugar and electrolyte solutions, e.g., Dioralyte, WHO formulation oral rehydration salts |
| Antibiotics (severe or prolonged cases) | Tetracycline, ciprofloxicin | ||||
| Acute Schistosomiasis (a.k.a. Katayama fever, swimmer's itch), chronic schistosomiasis (a.k.a. bilharzia) | Trematode flatworm Schistosoma spp. | Eggs are found in animal/human faecal or urine contaminated water. Freshwater aquatic snails act as intermediate hosts, and free-swimming trematode larvae (cercariae) are released into the water. Cercariae burrow into skin of definitive host, e.g., humans. | Water-borne larvae burrow into the skin. | Anthelmintic | Praziquantel |
| Corticosteroids (acute cases) | Prednisone, dexamethasone | ||||
| β-Blockers (chronic cases) | Labetalol, celiprolol | ||||
| Anticonvulsants (neuroschistosomiasis) | Diazepam | ||||
| Fascioliasis (a.k.a. liver fluke infection) | Trematode Fasciola hepatica (a.k.a. common liver fluke, sheep liver fluke), F. gigantica | Immature eggs are found in water contaminated with human/animal feces. Eggs hatch into miracidia, which infect freshwater snail intermediate hosts, e.g., Gajba, Fossaria and Pseudosuccinea spp. Free swimming larvae (cercariae) are released into the water after a number of developmental stages. Cercariae encysts as infective larvae (metacercariae) upon aquatic vegetation. After consumption by a definitive herbivore host, larvae mature into egg-laying adults in the bile ducts. Eggs are discharged in feces. Definitive host include cattle, goats, sheep, and zebra (F. gigantica); sheep and cattle (F hepactica); humans (both species). | Consumption of raw/unwashed aquatic plants contaminated with infective larvae, e.g., watercress. Transmission can also occur via ingestion of contaminated water. Few examples of infection after consumption of undercooked sheep or goat liver. | Anthelmintic | Triclabendazole |
| Clonorchiasis (a.k.a. liver fluke infection) | Trematode Clonorchis spp., e.g., Clonorchis sinensis (a.k.a. Chinese/oriental liver fluke). | Eggs are found in water contaminated with human/animal feces. Freshwater snails ingest the eggs and act as an intermediate host. After development, larvae are discharged into the water where they encyst in freshwater fish, some shrimp, crabs, and crayfish muscles and under scales. After consumption by a definitive animal host, cysts migrate to the small intestine and liver. After feeding upon bile and maturing, eggs are produced, which are subsequently released in feces. Definitive hosts include humans, cats, dogs, and pigs. | Transmission occurs via consumption of raw/undercooked fish, crabs, crayfish that are contaminated with parasite cysts. | Anthelmintic | Praziquantel, albendazole |
| Opistorchis infection (a.k.a. liver fluke infection) | Trematode Opisthorchis spp., e.g., O. viverrini (AKA Southeast Asian liver fluke), O. felineus (AKA cat liver fluke) | Eggs are found in human/animal faecal contaminated water. Intermediate snail host ingests eggs and the parasite becomes a miracidia. After development within the snail host, cercariae are released into freshwater. Cercariae encyst as metacercariae in the muscles or under scales offish. After consumption by a mammalian definitive host, parasites reside in the biliary and pancreatic ducts and develop into egg-laying adults. Eggs are excreted in the feces. | Consumption of undercooked/raw fish. | Anthelmintic | Praziquantel |
| Paragonimiasis (a.k.a. lung fluke infection) | Trematode > 30 Paragonimus spp., e.g., P. westermani (AKA oriental lung fluke; Asia), P. africanus (Africa), P. mexicanus (Central & South America), P. kellicotti (Midwestern & Southern United States) | Eggs enter the freshwater environment from the definitive host and hatch becoming miracidia. Miracidia invade intermediate host snails. After several developmental stages cercariae are released into water. Cercariae encyst in gills, muscles, viscera, and legs of crustacean intermediate host and become infectious metacercariae. After consumption by the definitive host, the parasites migrate to the lungs, where they mature into adult egg-laying flukes. Immature eggs are expelled in host bronchial secretions, or in feces (if sputum is swallowed). Occasionally parasites migrate to the central nervous system (instead of the lungs), which causes more serious paragonimiasis cases. Mammalian definitive hosts include pigs, dogs, cats and humans. | Consumption of undercooked/raw crustaceans, e.g., crayfish, crabs. | Anthelmintic | Praziquantel, triclabendazole, bithionol |
| Corticosteroids (parasites migrated to central nervous system) | Betamethasone, dexamethasone |
Note. Sources are references cited in the main body of article; Centers for Disease Control and Prevention, Diseases and Conditions Online database; Infectious Diseases Society of America (IDSA) online factsheets; PubMed Health A.D.A.M. Medical Encyclopedia online; Medline Plus Medical Encyclopedia online; World Health Organisation Disease Factsheets online; UK Health Protection Agency Infections online information sheets; UPMC Biosecurity Centre fact sheets online; and British National Formulary, Edition 58 (2009).
FIGURE 4.
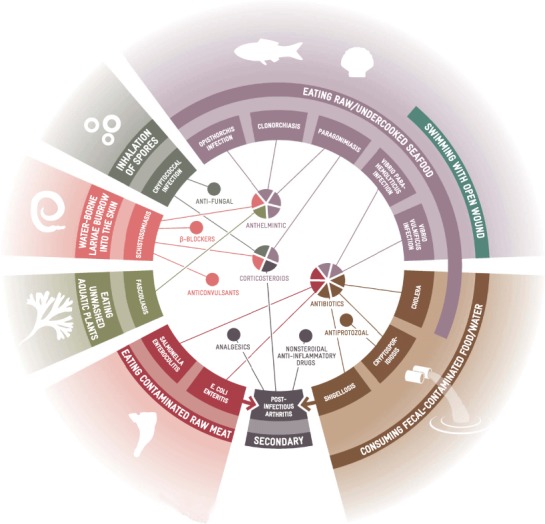
Soil, Water and Food-Borne Illnesses and Associated Pharmaceutical Treatments (color figure available online).
FRESHWATER- AND FOOD-BORNE PATHOGENS
It is known that climate change is likely to produce more floods and heavy rain due to warming of the oceans (Haines and Patz 2004; Diaz 2004). Indirect health consequences include a surge in freshwater diarrheal diseases, such as cryptosporidiosis and cholera, which are correlated to sewer overflows, wastewater treatment plant overflows, and contamination of water supplies by storm water runoff (O'Shea and Field 1992; Lipp et al. 2001a; 2001b; 2002; Rose et al. 2001; 2002; Checkley et al. 2000; Colwell 1996; Pascual et al. 2000; Portier et al. 2010). The transmission of pathogens such as E. coli, Shigella, Salmonella, and viral hepatitis A is also likely to increase as a result of water contamination. The transmission dynamics of these diseases are further complicated if livestock or crops are contaminated with feces, posing a health risk to farm and outdoor workers (Casteel et al. 2006; Tanabe et al. 1999; Pianetti et al. 2004). In severe cases, or situations in which there is a high risk of human-to-human transmission, the use of antibiotics is required. Where complications occur, such as the development of hemolytic uremic syndrome or postinfectious arthritis, a number of supportive medical therapies are needed (Table 3 and Figure 4).
Schistosomiasis, also known as bilharzia, is a zoonotic disease produced by digenetic trematode flatworms (genus Schistosoma). Three species are responsible for most human infections including cases observed in Northern Hemisphere endemic countries (Turkey and areas of the eastern Mediterranean) (Gryseels et al. 2006; Chitsulo et al. 2000). Human infection occurs when host freshwater snails release free-swimming larval parasites (cercaria) into the water. These parasites burrow into human skin upon contact. Infestations develop from acute cases (Katayama fever) into chronic schistosomiasis with varying levels of severity, and lead to life-threatening hepatosplenic disease and associated hepatic, periportal, and portal complications (Pearce and MacDonald 2002). The anthelmintic Praziquantel is effective against all Schistosoma species, and is often administered in conjunction with corticosteroids to suppress hypersensitivity (Gryseels et al. 2006); see Table 3 and Figure 4.
The influences of climate change on the zoonotic helminthiase (parasitic worm) diseases have not received as much attention as on the arboviruses, zoonotic microbial or viral pathogens. The effects on helminthiases and the other zoonotic pathogens differ because of the substantially longer life cycles of the helminths. It subsequently takes longer for organismal responses to changing environmental conditions to become apparent (Mas-Coma et al. 2009). As snails are essential to the life cycle of Schistosoma, the presence of appropriate snail species in the Northern Hemisphere is a key determinant in the emergence of this disease. Recent range expansions of 4 of the 34 known Biomphalaria species into areas such as Egypt, Hong Kong, and southern states of the United States have been observed (Pointier et al. 2005). The presence of B. tenagophila within Eastern Europe (Romania) has also been confirmed (Majoros et al. 2008). This study did not detect the presence of trematode larvae inside these snails, but the presence of established snail populations means schistosomiasis could be introduced, and become endemic, via tourist or immigrant feces contaminated with flukes or eggs (Majoros et al. 2008). An investigation in Brazil suggested that the duration of drought periods and snail population density were vital determinants for schistomiasis prevalence, and that coastal regions were at greatest risk (Bavia et al. 1999).
Despite extensive control programs, schistomiasis is common in China, leading researchers such as Zhou et al. (2008) to investigate the impact of climate change upon transmission rates via risk mapping. Schistosomiasis ranges are expanding into the northern (currently) nonendemic regions, placing a total of 8.1% of China's surface area at risk by 2050. The recent range expansion of the snail, and the impact of drought and rising temperatures upon schistosomiasis transmission, imply that climatic change will increase bilharzia prevalence and the associated use of pharmaceuticals (Gajadhar and Allen 2004). Other trematodes (such as the liver and lung parasites Fasciola, Clonorchis, Opisthorchis, and Paragonimus) are also likely to be affected by climate change in a manner similar to effects on Schistosoma, through the effects on their Biomphalaria hosts (Morgan et al. 2001). Infections by these liver and lung flukes are typically treated with anthelmintics and corticosteroids when there is a risk of damage to the central nervous system (Table 3 and Figure 4).
ESTUARINE/MARINE WATER- AND FOOD-BORNE PATHOGENS
The study of Vibrio spp. provides an insight into the influence of climatic change upon estuarine and marine-borne illnesses because of its effects on ecology and pathogen abundance. As Vibrio spp. have a symbiotic relationship with plankton (some of which are associated with harmful algal blooms), modifications to plankton or other host populations affect Vibrio spp. abundance. Vibrio vulnificus infects humans during recreational and occupational activities (such as fishing and swimming) when open wounds are present, while V. parahaemolyticus is spreading its range with rising temperatures and is responsible for an increasing number of infections occurring when contaminated shellfish are eaten (Lipp and Rose 1997; Wittman and Flick 1995). Gastrointestinal illnesses produced by Vibrio infections are usually treated with rehydration salts. However, if infection of the bloodstream occurs and/or there is underlying liver disease and immunosuppression, life-threatening septicemia arises and use of antibiotics is required (Table 3 and Figure 4).
Vibrio cholera, the best known of the Vibrio spp., is associated with zooplankton and also found in the marine system. Phytoplankton is the primary food source of zooplankton, and when phytoplankton bloom in warmer periods, V. cholera populations also increase (Lobitz et al. 2000; Barbieri et al. 1999). Warmer water temperatures associated with climate change are expected to expand the prevalence and range of V. cholera on both geospatial and temporal scales. This is because of ecological impacts upon phytoplankton, intrusion of salt water into inland systems due to sea-level rise, and the increasing frequency of El Niño southern oscillation events (Colwell and Huq 1998; Colwell 1996; Lipp et al. 2002). With rising V. cholera populations, the risk of cholera cases in humans increases due to the consumption of raw or undercooked contaminated shellfish, which produce diarrhea and septicemia (Hlady and Klontz 1996). This inevitably results in a rise in the use of antibiotics and other pharmaceuticals. It is worth pointing out, however, that factors other than climate change may be more significant in increasing cholera outbreaks: Evidence suggests that other anthropogenic factors (e.g., declining medical infrastructure, persistent insanitary conditions, and unintentional transmission of cholera via aid workers) are much more likely to foster increasing numbers of cholera outbreaks.
AGRI-/AQUACULTURE
Boxall et al. (2009) highlighted how changing climatic conditions might increase the level of contact between farm workers and livestock. If grazing land is waterlogged or animals suffer heat stress, it may be necessary to house livestock indoors. This elevates human-to-animal contact and the necessity for farm workers to dispose of manure. In turn, the risk of transmitting zoonotic pathogens such as Cryptosporidium rises (Reif et al. 1989; Boxall et al. 2009). Altered weather conditions driven by climate change may impair the health of livestock and increase the susceptibility of animals to infection. In the aquaculture sector, similar environmental conditions enable new diseases, pests, and parasites to emerge, producing changes in biodiversity, and temperature-related physiological stresses. These changes ultimately bring about range shifts and potential extinctions and/or mass mortalities of certain populations (Cochrane et al. 2009; Harvell et al. 1999; Hofmann et al. 2001; Occhipinti-Ambrogi 2007; Barange and Perry 2009; Rahel and Olden 2008; United Nations Environmental Programme 2009). To maintain meat and fish production under difficult and variable climatic conditions, the usage of veterinary pharmaceuticals (such as growth hormones, antifungal or antiparasitic agents, and antibiotics) will rise.
This important issue cannot be considered in detail here, but it is clear that climate change is more generally affecting practices in agriculture and aquaculture, leading to increased use of veterinary pharmaceuticals. This has major implications for human health. For example, the extensive employment of antibiotics within veterinary contexts is exerting significant adverse consequences for drug efficacy. The rising resistance of Salmonella to fluroquinolone antibiotics is an example. This resistance occurred only when fluroquinolones became licensed for veterinary use. For more extensive discussion regarding antimicrobial resistance, see available reviews in the literature (Taylor et al. 2011; Wright 2010; Kümmerer 2009a; 2009b).
Climate change will enable new weeds, pests, and diseases to thrive and will allow indigenous pests to proliferate. This poses challenges for arable crop production and is likely to result in higher pesticide use (Reidsma et al. 2007; Baker et al. 2000; Bale et al. 2002; Olesen et al. 2011). There is already evidence that warmer temperatures are leading to outbreaks of infestation, and a poleward spread of pests such as the European corn borer (Ostrinia nubilalis, Hubner) (Trnka et al. 2007). Exposure to numerous biocides was shown to harm human health. Epidemiological studies associated the incidence of Parkinson's disease with pesticide exposure (Ascherio et al. 2006). Repeated exposure to low doses of organophosphate biocides is thought to play an important role in neurotoxicity and development of cancers such as leukemia and lymphoma (López et al. 2007; Alavanja et al. 2004). Chlorphenoxy herbicide exposure has been linked with a range of human health impacts, including congenital, urogenital, and muscuolskeletal abnormalities, malformation of respiratory and circulatory systems, and possibly cancer (Stillerman et al. 2008).
Treatment of these serious and often debilitating diseases requires long-term employment of polypharmacy (including psychoactive drugs, dopamine agonists, monoamine oxidase inhibitors, and catechol O-methyltransferase inhibitors for Parkinson's disease). For the treatment of cancers such as leukemia, induction chemotherapy is required, which typically involves the employment of corticosteroids, mitotic inhibitors, and anthracycline drugs, followed by consolidation therapy with antimetabolite drugs. In short, the increasing use of pharmaceuticals and other chemicals in agri-/aquaculture due to climate change is leading to environmental contamination and a range of acute and chronic diseases in humans and other animals. Ironically, this is necessitating an even greater use of pharmaceuticals.
CONCLUDING REMARKS
Over the next few decades, climate change in the Northern Hemisphere is likely to exacerbate diseases, some of which are already common, especially non-communicable diseases (e.g., cardiovascular disease and mental illness). Evidence is presented here that these changes will be associated with altered patterns of pharmaceutical use, notably the use of medications now classified as “heavy usage Western drugs” (e.g., beta-blockers, ACE inhibitors, statins, salicylates, antiplatelets, vasodilators, anticoagulants, and a myriad of antidepressants, antipsychotics, and sedatives; Figure 2). As the weather changes, those suffering from respiratory diseases such as asthma and allergic rhinitis may, in many locations, experience more severe symptoms. An increase in the number of sensitive individuals also seems plausible. The incidence of infectious disease is likely to rise (e.g., cryptococcal infection), and substantial growth in human and animal diarrheal disease is predicted as the transmission of Cryptosporidium, Vibrio spp., Escherichia coli, Salmonella, and other pathogens is promoted. Bacterial infections in general are likely to proliferate with the changing climate, including both primary (e.g., scabies, conjunctivitis) and secondary (e.g., impetigo, Staphylococcus, Streptococcus) infections (see Figure 2).
The emergence of parasitic and infectious diseases, some of which are already proliferating, will accelerate the use of drugs now used only irregularly in the Northern Hemisphere. These pharmaceuticals will undoubtedly gain entry into the environment but have to date received little attention from environmental researchers and ecotoxicologists. Vector-borne diseases that are likely to occur more widely as the climate changes include insect-borne and tick-borne infections. Among the new parasites now being seen more often are liver and lung helminths, including Schistosoma spp. To treat these emerging illnesses, medications for parasitic diseases (e.g., insecticides, anthelminitics, antimalarials, antimonials, antiprotozoals) and the symptomatic treatment of encephalitis and cryptoccocal meningitis (e.g., anticonvulsants, antipyretics, antiemetics) will be required in ever larger volumes (Figure 3 and 4). In addition, the increased use of pharmaceuticals used to treat common symptoms, or to prevent further risks to health, can be expected (e.g., analgesics, anti-inflammatory, and antibacterial or antifungal agents) (Figure 2 to 4).
The potential impact of climate change on disease incidence in the future remains uncertain and is subject to controversy, with opposing views represented in the scientific literature. Recent demands for greater rigor and a deeper appreciation of the complexity of climate change and disease interactions merit urgent consideration (Lafferty 2009; Rohr et al. 2008; Hay et al. 2002). A balanced assessment of available literature does, however, suggest that climate change has the potential to influence human health and diseases on a global scale, as shifts in climatic zones occur. Certain diseases, such as those discussed herein, have the potential to increase in incidence in a number of regions. There is also likely to be a concurrent decrease in the incidence of other diseases due to climate change; however, available literature does not generally focus upon decreased morbidity due to climate change. Such studies would be particularly beneficial when considering future provision of medical/health resources. The reality is that scientists are currently unable to predict with a high level of certainty when and where specific changes will occur.
It is known that geospatial disease distributions are being (and will be further) modified by changing climatic conditions. As a result, pharmaceutical use will alter, together with the release of these drugs into the environment. Whether new diseases will emerge in specific regions, become endemic, or pose a significant risk to human health will depend largely on local control and mitigation strategies, including vector control programs (World Health Organization 2010). This in turn will depend on local policies (Stern 2006). A number of other factors, such as regional poverty, human migration, land use, water quality, air quality, and ecological adaptation, will play fundamental roles in determining the proliferation of diseases. Due to uncertainty on the impact of climate change (in isolation) upon disease distribution, it is not possible to accurately discuss the influence of other factors without significant speculation, particularly as these are likely to be highly geographically localized and consist of complex networks of influencing factors, thereby complicating current predictions of future risks to human health.
With different disease profiles, the localized requirements for pharmaceuticals will alter in both quantity and type, regardless of whether control or mitigation strategies are put in place. This is because pharmaceuticals might act as the foundation of these programs, especially where a strategic prophylactic approach is being taken (Costello et al. 2011). Scientists investigating the environmental transport, fate, and ecotoxicology of drugs in ecosystems need to be prepared to reassess which pharmaceuticals are targeted for investigation.
The information presented here highlights some of the types of drugs that are likely to be used more extensively in the Northern Hemisphere. Further investigations addressing the interactions between pharmaceuticals and climate change are urgently needed to quantify pharmaceutical use, determine the economic implications (both in terms of drug provision and environmental consequences), and explore the medical and veterinary implications (in terms of training, prevention, and treatment). Research is also needed to develop a more systematic approach to future global disease management in a rapidly changing world.
REFERENCES
- Adalja A. A., Sell T. K., Bouri N., Franco C. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg. Infect. Dis. 2012;18:608–614. doi: 10.3201/eid1804.110968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavanja M. C. R., Hoppin J. A., Kamel F. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annu. Rev. Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Alvar J., Cañavate C., Gutiérrez-Solar B., Jiménez M., Laguna F., López-Vélez R., Molina R., Moreno J. J. Leishmania and human immunodeficiency virus coinfection: The first 10 years. Clin. Microbiol. Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. M., Cembella A. D., Hallegraeff G. M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012;4:143–176. doi: 10.1146/annurev-marine-120308-081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ari T. B., Gershunov A., Gage K. L., Snäll T., Ettestad P., Kausrud K. L., Stenseth N. C. Human plague in the USA: The importance of regional and local climate. Biol. Lett. 2008;4:737–740. doi: 10.1098/rsbl.2008.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A., Chen H., Weisskopf M. G., O'Reilly E., McCullough M. L., Calle E. E., Schwarzschild M. A., Thun M. J. Pesticide exposure and risk for Parkinson's disease. Ann. Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Ayscough N. J., Fawell J., Franklin G., Young W. Review of human pharmaceuticals in the environment. 2000. R & D Technical Report P390. UK Environmental Agency, Bristol.
- Baker R. H. A., Sansford C. E., Jarvis C. H., Cannon R. J. C., MacLeod A., Walters K. F. A. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agric. Ecosyst. Environ. 2000;82:57–71. [Google Scholar]
- Bale J. S., Masters G. J., Hodkinson I. D., Awmack C., Bezemer T. M., Brown V. K., Butterfield J., Buse A., Coulson J. C., Farrar J., Good J. E. G., Harrington R., Hartley S., Jones T. H., Lindroth R. L., Press M. C., Symrnioudis I., Watt A. D., Whittaker J. B. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biol. 2002;8:1–16. [Google Scholar]
- Barange M., Perry R. I. Physical and ecological impacts of climate change relevant to marine and inland capture fisheries and aquaculture. In: Cochrane K., De Young C., Soto D., Bahri T., editors. Climate change implications for fisheries and aquaculture: overview of current scientific knowledge, FAO Fisheries and Aquaculture technical paper no. 530. Rome, Italy: Food and Agriculture Organization of the United Nations; 2009. [Google Scholar]
- Barbieri E., Falzano L., Fiorentini C., Pianetti A., Baffone W., Fabbri A., Matarrese P., Casiere A., Katouli M., Kuhn I., Mollby R., Bruscolini F., Donelli G. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ. Microbiol. 1999;65:2748–2753. doi: 10.1128/aem.65.6.2748-2753.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavia M. E., Hale L. F., Malone J. B., Braud D. H., Shane S. M. Geographic information systems and the environmental risk of schistosomiasis in Bahia, Brazil. Am. J. Trop. Med. Hyg. 1999;60:566–572. doi: 10.4269/ajtmh.1999.60.566. [DOI] [PubMed] [Google Scholar]
- Beggs P. J. Adaptation to impacts of climate change on aeroallergens and allergic respiratory diseases. Int. J. Environ. Res. Public Health. 2010;7:3006–3021. doi: 10.3390/ijerph7083006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin A., Hales S., Rocklöv J., Åström C., Louis V. R., Sauerborn R. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Global Environ. Change. 2011;21:1209–1214. [Google Scholar]
- Bengis R. G., Leighton F. A., Fischer J. R., Artois M., Morner T., Tate C. M. The role of wildlife in emerging and re-emerging zoonoses. Rev. Sci. Technol. Oie. 2004;23:497–511. [PubMed] [Google Scholar]
- Bern C., Montgomery S. P., Herwaldt B. L., Rassi A., Marin-Neto J. A., Dantas R. O., Maguire J. H., Acquatella H., Morillo C., Kirchhoff L. V., Gilman R. H., Reyes P. A., Salvatella R., Moore A. C. Evaluation and treatment of Chagas disease in the United States. J. Am. Med. Assoc. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- Biswas R., Pal D., Mukhopadhyay S.P. A community based study on health impact of flood in a vulnerable district of West Bengal. Indian J. Public Health. 1999;43:89–90. [PubMed] [Google Scholar]
- Bohm M., White P. C. L., Chambers J., Smith L., Hutchings M. R. Wild deer as a source of infection for livestock and humans in the UK. Vet. J. 2007;174:260–276. doi: 10.1016/j.tvjl.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Boxall A. B. A., Hardy A., Beulke S., Boucard T., Burgin L., Falloon P. D., Haygarth P. M., Hutchinson T., Kovats R. S., Leonardi G., Levy L. S., Nichols G., Parsons S. A., Potts L., Stone D., Topp E., Turley D. B., Walsh K., Wellington E. M. H., Williams R. J. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ. Health Perspect. 2009;117:508–514. doi: 10.1289/ehp.0800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Zeng Z. Comparative population ecology of eleven species of rodents in the Chihuahuan Desert. Ecology. 1989;70:1507–1525. [Google Scholar]
- Brown P. R., Singleton G. R. Rate of increase as a function of rainfall for house mouse Mus domesticus populations in a cereal-growing region in southern Australia. J. Appl. Ecol. 1999;36:484–493. [Google Scholar]
- Calzolari M., Bonilauri P., Bellini R., Albieri A., Defilippo F., Maioli G., Galletti G., Gelati A., Barbieri I., Tamba M., Lelli D., Carra E., Cordioli P., Angelini P., Dottori M. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS One. 2010;5:e14324. doi: 10.1371/journal.pone.0014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. H., Harder T., Nielsen S., Kjelleberg S., Steinberg P. D. Climate change and disease: bleaching of a chemically defended seaweed. Global Change Biol. 2011;17:2958–2970. [Google Scholar]
- Cancrini G., Frangipane di Regalbono A., Ricci I., Tessarin C., Gabrielli S., Pietrobelli M. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet. Parasitol. 2003a;118:195–202. doi: 10.1016/j.vetpar.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Cancrini G., Romi R., Gabrielli S., Toma L., Di Paolo M., Scaramozzino P. First findings of Dirofilaria repens in natural populations of Aedes albopictus. Med. Vet. Entomol. 2003b;17:448–451. doi: 10.1111/j.1365-2915.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Carballa M., Omil F., Lema M. J., Llompart M., Garcia-Jares C., Rodriguez I., Gomez M., Ternes T. Behaviour of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004;38:2918–2926. doi: 10.1016/j.watres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Carcavallo R. U. Climatic factors related to Chagas disease transmission. Mem. Inst Oswaldo Cruz. 1999;94:367–369. doi: 10.1590/s0074-02761999000700071. [DOI] [PubMed] [Google Scholar]
- Casteel M. J., Sobsey M. D., Mueller J. P. Fecal contamination of agricultural soils before and after hurricane-associated flooding in North Carolina. J. Environ. Sci. Health A. 2006;41:173–184. doi: 10.1080/10934520500351884. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Mold prevention strategies and possible health effects in the aftermath of hurricanes and major floods. Morbid. Mortal. Weekly Rep. 2006;55(RR8, suppl. S):1–27. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Emergence of Cryptococcus gattii: Pacific Northwest, 2004–2010. Morbid. Mortal. Weekly Rep. 2010a;59:865–868. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Locally acquired dengue—Key West, Florida, 2009–2010. Morbid. Mortal. Weekly Rep. 2010b;59:577–581. [PubMed] [Google Scholar]
- Chae E.-H., Tong W. K., Rhee S.-J., Henderson T. D. The impact of flooding on the mental health of affected people in South Korea. Commun. Mental Health J. 2005;41:633–645. doi: 10.1007/s10597-005-8845-6. [DOI] [PubMed] [Google Scholar]
- Checkley W., Epstein L. D., Gilman R. H., Figueroa D., Cama R. I., Patz J. A., Black R. E. Effects of EI Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet. 2000;355:442–450. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- Chen L., Verrall K., Tong S. Air particulate pollution due to bushfires and respiratory hospital admissions in Brisbane, Australia. Int. J. Environ. Health Res. 2006;16:181–191. doi: 10.1080/09603120600641334. [DOI] [PubMed] [Google Scholar]
- Chitsulo L., Engels D., Montresor A., Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane K. L., De Young C., Soto D., Bahri T. Climate change implications for fisheries and aquaculture: Overview of current scientific knowledge. Rome, Italy: Food and Agriculture Organization of the United Nations; 2009. [Google Scholar]
- Colwell R. R. Global climate and infectious disease: The cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Colwell R. R., Huq A. Global microbial ecology: Biogeography and diversity of Vibrios as a model. J. Appl. Microbiol. 1998;85:134S–137S. doi: 10.1111/j.1365-2672.1998.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Conlon K. C., Rajkovich N. B., White-Newsome J. L., Larsen L., O'Neill M. S. Preventing cold-related morbidity and mortality in a changing climate. Maturitas. 2011;69:197–202. doi: 10.1016/j.maturitas.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C. M. Climate change and infectious disease: Is the future here? Environ. Health Perspect. 2011;119:A394–A397. doi: 10.1289/ehp.119-a394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A., Maslin M., Montgomery H., Johnson A. M., Ekins P. Global health and climate change: moving from denial and catastrophic fatalism to positive action. Philos. Trans. R. Soc. A. 2011;369:1866–1882. doi: 10.1098/rsta.2011.0007. [DOI] [PubMed] [Google Scholar]
- D'Ippoliti D., Michelozzi P., Marino C., de'Donato F., Menne B., Katsouyanni K., Kirchmayer U., Analitis A., Medina-Ramon M., Paldy A., Atkinson R., Kovats S., Bisanti L., Schneider A., Lefranc A., Iniguez C., Perucci C. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ. Health. 2010;9:37. doi: 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K., Bartlett K. H., Baer R., Byrnes E., Galanis E., Heitman J., Hoang L., Leslie M. J., MacDougall L., Magill S. S., Morshed M. G., Marr K. A. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg. Infect. Dis. 2009;15:1185–1191. doi: 10.3201/eid1508.081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. G., Ternes T. A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999;107:907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depledge M. Does the pharmaceutical industry need a new prescription? Sci. Parliment. 2011a;68:44–45. [Google Scholar]
- Depledge M. Reduce drug waste in the environment. Nature. 2011b;478:36. doi: 10.1038/478036a. [DOI] [PubMed] [Google Scholar]
- Diaz J. H. The public health impact of hurricanes and major flooding. J. Louisiana State Med. Soc. 2004;156:145–150. [PubMed] [Google Scholar]
- Diniz S. A., Silva F. L., Neta A. V. C., Bueno R., Guerra R. M. S. N. C., Abreu-Silva A. L., Santos R. L. Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J. Infect. Dev. Countries. 2008;2:24–33. doi: 10.3855/jidc.318. [DOI] [PubMed] [Google Scholar]
- Dixit A., Carroll S. F., Qureshi S. T. Cryptococcus gattii: An emerging cause of fungal disease in North America. Interdiscip. Perspect. Infect. Dis. 2009;2009:840452. doi: 10.1155/2009/840452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., FitzGerald G. J., Clark M., Hou X.-Y. Health impacts of floods. Prehosp. Disaster Med. 2010;25:265–272. doi: 10.1017/s1049023x00008141. [DOI] [PubMed] [Google Scholar]
- A commission on climate change. Lancet. 2009;373:1659. doi: 10.1016/S0140-6736(09)60922-3. [DOI] [PubMed] [Google Scholar]
- Eisen R. J., Glass G. E., Eisen L., Cheek J., Enscore R. E., Ettestad P., Gage K. L. A spatial model of shared risk for plague and hantavirus pulmonary syndrome in the southwestern United States. Am. J. Trop. Med. Hyg. 2007;77:999–1004. [PubMed] [Google Scholar]
- El Adlouni S., Beaulieu C., Ouarda T. B. M. J., Gosselin P. L., Saint-Hilaire A. Effects of climate on West Nile virus transmission risk used for public health decision-making in Quebec. Int. J. Health Geogr. 2007;6:1–7. doi: 10.1186/1476-072X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enscore R. E., Biggerstaff B. J., Brown T. L., Fulgham R. E., Reynolds P. J., Engelthaler D. M., Levy C.E., Parmenter R. R., Montenieri J. A., Cheek J. E., Grinnell R. K., Ettestad P. J., Gage K. L. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960–1997. Am. J. Trop. Med. Hyg. 2002;66:186–196. doi: 10.4269/ajtmh.2002.66.186. [DOI] [PubMed] [Google Scholar]
- Epstein P. R. Climate change and emerging infectious diseases. Microbes Infect. 2001a;3:747–754. doi: 10.1016/s1286-4579(01)01429-0. [DOI] [PubMed] [Google Scholar]
- Epstein P. R. West Nile virus and the climate. J. Urban Health. 2001b;78:367–371. doi: 10.1093/jurban/78.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D., Thomas S. M., Niemitz F., Reineking B., Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Global Planet Change. 2011;78:54–64. [Google Scholar]
- Fleming L. E., Kirkpatrick B., Backer L. C., Walsh C. J., Nierenberg K., Clark J., Reich A., Hollenbeck J., Benson J., Cheng Y. S. Review of Florida red tide and human health effects. Harmful Algae. 2011;10:224–233. doi: 10.1016/j.hal.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C. R., Ziller M., Staubach C., Latiff M. Impact of the El Niño/southern oscillation on visceral leishmaniasis, Brazil. Emerg. Infect. Dis. 2002;8:914–917. doi: 10.3201/eid0809.010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel S., Bowen K., Campbell-Lendrum D., Frumkin H., McMichael A. J., Rasanathan K. Climate change, noncommunicable diseases, and development: The relationships and common policy opportunities. Annu. Rev. Public Health. 2011;32:133. doi: 10.1146/annurev-publhealth-071910-140612. [DOI] [PubMed] [Google Scholar]
- Gage K.L., Burkot T.R., Eisen R.J., Hayes E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Gajadhar A. A., Allen J. R. Factors contributing to the public health and economic importance of waterborne zoonotic parasites. Vet. Parasitol. 2004;126:3–14. doi: 10.1016/j.vetpar.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Galanis E., MacDougall L. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 2010;16:251–257. doi: 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N. Drug waste harms fish. Nature. 2011;476:265. doi: 10.1038/476265a. [DOI] [PubMed] [Google Scholar]
- Gjenero-Margan I., Aleraj B., Krajcar D., Lesnikar V., Klobučar A., Pem-Novosel I., Kurečić-Filipović S., Komparak S., Martić R., Duričić S., Betica-Radić L., Okmadžić J., Vilibić-Čavlek T., Babić-Erceg A., Turković B., Avsić-Županc T., Radić I., Ljubić M., Sarac K., Benić N., Mlinarić-Galinović G. Autochthonous dengue fever in Croatia, August-September 2010. Eurosurveillance. 2011;16:19805. [PubMed] [Google Scholar]
- Golet E. M., Strehler A., Alder A. C., Giger W. Determination of fluoro-quinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002;74:5455–5462. doi: 10.1021/ac025762m. [DOI] [PubMed] [Google Scholar]
- Gratz N. G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Gubler D. J., Reiter P., Ebi K. L., Yap W., Nasci R., Patz J. A. Climate variability and change in the United States: Potential impacts on vector and rodent-borne diseases. Environ. Health Perspect. 2001;109:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines A., Patz J. A. Health effects of climate change. J. Am. Med. Assoc. 2004;291:99–103. doi: 10.1001/jama.291.1.99. [DOI] [PubMed] [Google Scholar]
- Halling-Sorensen B., Nielsen S. N., Lanzky P. F., Ingerslev F., Lutzhoft H. C. H., Jorgensen S. E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere. 1998;36:357–394. doi: 10.1016/s0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- Harvell C. D., Kim K., Burkholder J. M., Colwell R. R., Epstein P. R., Grimes D. J., Hofmann E. E., Lipp E. K., Osterhaus A. D. M. E., Overstreet R. M., Porter J. W., Smith G. W., Vasta G. R. Emerging marine diseases—Climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Harvell C. D., Mitchell C. E., Ward J. R., Altizer S., Dobson A. P., Ostfeld R. S., Samuel M. D. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hay S. I., Rogers D. J., Randolph S. E., Stern D. I., Cox J., Shanks G. D., Snow R. W. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–534. doi: 10.1016/s1471-4922(02)02374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002;131:5–17. doi: 10.1016/s0378-4274(02)00041-3. [DOI] [PubMed] [Google Scholar]
- Heberer T., Fuhrmann B., Schmidt-Baumler K., Tsipi D., Koutsouba V., Hiskia A. Occurrence of pharmaceutical residues in sewage, river, ground, and drinking water in Greece and Berlin (Germany) In: Daughton G., Jones-Lepp T., editors. Pharmaceuticals and personal care products in the environment: Scientific and regulatory issues. ACS symposium series 791. Washington, DC: American Chemical Society; 2001. pp. 70–83. [Google Scholar]
- Hirsch R., Ternes T. A., Haberer K., Kratz K.-L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999;225:109–118. doi: 10.1016/s0048-9697(98)00337-4. [DOI] [PubMed] [Google Scholar]
- Hlady W. G., Klontz K. C. The epidemiology of Vibrio infections in Florida, 1981–1993. J. Infect. Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- Hofmann E., Ford S., Powell E., Klinck J. Modeling studies of the effect of climate variability on MSX disease in eastern oyster (Crassostrea virginica) populations. Hydrobiologia. 2001;460:195–212. [Google Scholar]
- Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L., editors. Intergovernmental Panel on Climate Change. Climate change 2007: The Physical science basis. Contribution of Working Group I to the Fourth assessment report of the Intergovernmental Panel on Climate Change. 2007. http://www.ipcc.ch/publications_and_data/ar4/wg1/en/contents.html.
- Field C. B., Barros V., Stocker T. F., Qin D., Dokken D. J., Ebi K. L., Mastrandrea M. D., Mach K. J., Plattner G.-K., Allen S. K., Tignor M., Midgley P. M., editors. Intergovernmental Panel on Climate Change. Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of Working Groups I and II of the Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press; 2012. [Google Scholar]
- Kang J. X. Omega-3: A link between global climate change and human health. Biotechnol. Adv. 2011;29:388–390. doi: 10.1016/j.biotechadv.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney P. L. Climate change, air quality, and human health. Am. J. Prev. Med. 2008;35:459–467. doi: 10.1016/j.amepre.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Kümmerer K. Pharmaceuticals in the environment. Annu. Rev. Environ. Resources. 2010;35:57–75. [Google Scholar]
- Kümmerer K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere. 2009a;75:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Kümmerer K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere. 2009b;75:435–441. doi: 10.1016/j.chemosphere.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kundzewicz Z. W., Mata L. J., Arnell N. W., Döll P., Jimenez B., Miller K., Oki T., Şen Z., Shiklomanov I. The implications of projected climate change for freshwater resources and their management. Hydrol. Sci. J. 2008;53:3–10. [Google Scholar]
- Kundzewicz Z. W., Radziejewski M., Pinskwar I. Precipitation extremes in the changing climate of Europe. Climate Res. 2006;31:51. [Google Scholar]
- Kunii O., Nakamura S., Abdur R., Wakai S. The impact on health and risk factors of the diarrhoea epidemics in the 1998 Bangladesh floods. Public Health. 2002;116:68–74. doi: 10.1038/sj.ph.1900828. [DOI] [PubMed] [Google Scholar]
- La Ruche G., Souarès Y., Armengaud A., Peloux-Petiot F., Delaunay P., Desprès P., Lenglet A., Jourdain F., Leparc-Goffart I., Charlet F., Ollier L., Mantey K., Mollet T., Fournier J.P., Torrents R., Leitmeyer K., Hilairet P., Zeller H., Van Bortel W., Dejour-Salamanca D., Grandadam M., Gastellu-Etchegorry M. First two autochthonous dengue virus infections in metropolitan France. Eurosurveillance. 2010;15:19676. [PubMed] [Google Scholar]
- Lafferty K. D. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Leirs H., Verhagen R., Verheyen W, Mwanjabe P., Mbise T. Forecasting rodent outbreaks in Africa: An ecological basis for Mastomys control in Tanzania. J. Appl. Ecol. 1996;33:937–943. [Google Scholar]
- Lima M., Marquet P. A., Iaksic F. M. El Niño events, precipitation patterns, and rodent outbreaks are statistically associated in semiarid Chile. Ecography. 1999;22:213–218. [Google Scholar]
- Lindgren E., Tälleklint L., Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 1999;108:119–123. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp E., Kurz R., Vincent R., Rodriguez-Palacios C., Farrah S., Rose J. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries Coasts. 2001a;24:266–276. [Google Scholar]
- Lipp E., Schmidt N., Luther M., Rose J. Determing the effects of El Niño-Southern Oscillation events on coastal water quality. Estuaries Coasts. 2001b;24:491–497. [Google Scholar]
- Lipp E. K., Huq A., Colwell R. R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp E. K., Rose H. The role of seafood in foodborne diseases in the United States of America. Rev. Sci. Technol. Off. Int. Epiz. 1997;16:620–640. doi: 10.20506/rst.16.2.1048. [DOI] [PubMed] [Google Scholar]
- Lobitz B., Beck L., Huq A., Wood B., Fuchs G., Faruque A. S. G., Colwell R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López O., Hernández A. F., Rodrigo L., Gil F., Pena G., Serrano J. L., Parrón T., Villanueva E., Pla A. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol. Lett. 2007;171:146–153. doi: 10.1016/j.toxlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Lugeri N., Kundzewicz Z., Genovese E., Hochrainer S., Radziejewski M. River flood risk and adaptation in Europe—Assessment of the present status. Mitig. Adapt. Strat. Global Change. 2010;15:621–639. [Google Scholar]
- MacDougall L., Kidd S. E., Galanis E., Mak S., Leslie M. J., Cieslak P. R., Kronstad J. W., Morshed M. G., Bartlett K. H. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg. Infect. Dis. 2007;13:42–50. doi: 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros G., Fehér Z., Deli T., Földvári G. Establishment of Biomphalaria tenagophila snails in Europe. Emerg. Infect. Dis. 2008;14:1812–1814. doi: 10.3201/eid1411.080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens W. J. M., Jetten T. H., Focks D. A. Sensitivty of malaria, schistosomiasis and dengue to global warming. Climatic Change. 1997;35:145–156. [Google Scholar]
- Mas-Coma S., Valero M. A., Bargues M. D. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009;163:264–280. doi: 10.1016/j.vetpar.2009.03.024. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Woodruff R. E., Hales S. Climate change and human health: Present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- Mills J. N., Gage K. L., Khan A. S. Potential influence of climate change on vector-borne and zoonotic diseases: A review and proposed research plan. Environ. Health Perspect. 2010;118:1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. J., Miller B. R., Gubler D. J. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J. Am. Mosq. Control. Assoc. 1987;3:460–465. [PubMed] [Google Scholar]
- Morgan J. A. T., Dejong R. J., Snyder S. D., Mkoji G. M., Loker E. S. Schistosoma mansoni and Biomphalaria: Past history and future trends. Parasitology. 2001;123:211–228. doi: 10.1017/s0031182001007703. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y., Williams R., Peterson A. T., Mead P., Staples E., Gage K. L. Climate change effects on plague and tularemia in the United States. Vector Borne Zoonotic Dis. 2007;7:529–540. doi: 10.1089/vbz.2007.0125. [DOI] [PubMed] [Google Scholar]
- O'Shea M. L., Field R. Detection and disinfection of pathogens in storm-generated flows. Can. J. Microbiol. 1992;38:267–276. doi: 10.1139/m92-045. [DOI] [PubMed] [Google Scholar]
- Occhipinti-Ambrogi A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007;55:342–352. doi: 10.1016/j.marpolbul.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Olesen J. E., Trnka M., Kersebaum K. C., Skjelv A. O.åg, Seguin B., Peltonen-Sainio P., Rossi F., Kozyra J., Micale F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011;34:96–112. [Google Scholar]
- Otranto D., Wall R. New strategies for the control of arthropod vectors of disease in dogs and cats. Med. Vet. Entomol. 2008;22:291–302. doi: 10.1111/j.1365-2915.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Parmenter R. R., Yadav E. P., Parmenter C. A., Ettestad P., Gage K. L. Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am. J. Trop. Med. Hyg. 1999;61:814–821. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- Pascual M., Rodó X., Ellner S.P., Colwell R., Bouma M. J. Cholera dynamics and El Niño-Southern oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., MacDonald A. S. The immunobiology of schistosomiasis. Nature Rev. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Dismukes W. E., Dromer F., Goldman D.L., Graybill J. R., Hamill R. J., Harrison T. S., Larsen R. A., Lortholary O., Nguyen M.-H., Pappas P. G., Powderly W. G., Singh N., Sobel J. D., Sorrell T. C. Clinical practice guidelines for the management of cryptococcal disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. T., Sanchez-Cordero V., Beard C. B., Ramsey J. M. Ecological niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg. Infect. Dis. 2002;8:662. doi: 10.3201/eid0807.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianetti A., Sabatini L., Bruscolini F., Chiaverini F., Cecchetti G. Faecal contamination indicators, Salmonella Vibrio and Aeromonas in water used for the irrigation of agricultural products. Epidemiol. Infect. 2004;132:231–238. doi: 10.1017/s095026880300181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointier J. P., David P., Jarne P. Biological invasions: The case of planorbid snails. J. Helminthol. 2005;79:249–256. doi: 10.1079/joh2005292. [DOI] [PubMed] [Google Scholar]
- Portier C. J., Thigpen T. K., Carter S. R., Dilworth C. H., Grambsch A. E., Gohlke J., Hess J., Howard S. N., Luber G., Lutz J. T., Maslak T., Prudent N., Radtke M., Rosenthal J. P., Rowles T., Sandifer P. A., Scheraga J., Schramm P. J., Strickman D., Trtanj J. M., Whung P.-Y. A human health perspective on climate change: A report outlining the research needs on the human health effects of climate change. Research Triangle Park, NC: Environmental Health Perspectives/National Institute of Environmental Health Sciences; 2010. [Google Scholar]
- Queyriaux B., Armengaud A., Jeannin C., Couturier E., Peloux-Petiot F. Chikungunya in Europe. Lancet. 2008;371:723–724. doi: 10.1016/S0140-6736(08)60337-2. [DOI] [PubMed] [Google Scholar]
- Rahel F. J., Olden J. D. Assessing the effects of climate change on aquatic invasive species (Evaluación de los efectos del cambio climático sobre especies acuáticas invasoras) Conserv. Biol. 2008;22:521–533. doi: 10.1111/j.1523-1739.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- Rappole J. H., Derrickson S. R., Hubálek Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg. Infect. Dis. 2000;6:319–328. doi: 10.3201/eid0604.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reacher M., McKenzie K., Lane C., Nichols T., Kedge I., Iversen A., Hepple P., Walter T., Laxton C., Simpson J. Lewes Flood Action Recovery Team. Health impacts of flooding in Lewes: A comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. Commun. Dis. Public Health. 2004;7:56–63. [PubMed] [Google Scholar]
- Ready P. D. Leishmaniasis emergence in Europe. Eurosurveillance. 2010;15:19505. [PubMed] [Google Scholar]
- Reidsma P., Ewert F., Lansink A. O. Analysis of farm performance in Europe under different climatic and management conditions to improve understanding of adaptive capacity. Climatic Change. 2007;84:403–422. [Google Scholar]
- Reif J. S., Wimmer L., Smith J. A., Dargatz D. A., Cheney J. M. Human cryptosporidiosis associated with an epizootic in calves. Am. J. Public Health. 1989;79:1528–1530. doi: 10.2105/ajph.79.11.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Hamburg M. A., Choffnes E. R., Mack A. Global climate change and extreme weather events: Understanding the contributions to infectious disease emergence: Workshop summary. Institute of Medicine Forum on Microbial Threats. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A. C., Panning M., Cordioli P., Fortuna C., Boros S., Magurano F., Silvi G., Angelini P., Dottori M., Ciufolini M. G., Majori G. C., Cassone A. Infection with Chikungunya virus in Italy: An outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Riggs M. A., Rao C. Y., Brown C. M., Van Sickle D., Cummings K. J., Dunn K. H., Deddens J. A., Ferdinands J., Callahan D., Moolenaar R. L., Pinkerton L. E. Resident cleanup activities, characteristics of flood-damaged homes and airborne microbial concentrations in New Orleans, Louisiana, October 2005. Environ. Res. 2008;106:401–409. doi: 10.1016/j.envres.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Rohr J. R., Raffel T. R., Romansic J. M., McCallum H., Hudson P. J. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl. Acad. Sci. USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. B., Epstein P. R., Lipp E. K., Sherman B. H., Bernard S. M., Patz J. A. Climate variability and change in the United States: Potential impacts on water- and foodborne diseases caused by microbiological agents. Environ. Health Perspect. 2001;109:211–222. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. B., Huffman D. E., Gennaccaro A. Risk and control of waterborne cryptosporidiosis. FEMS Microbiol. Rev. 2002;26:113–123. doi: 10.1111/j.1574-6976.2002.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal E., Marty P., Poizot-Martin I., Reynes J., Pratlong F., Lafeuillade A., Jaubert D., Boulat O., Dereure J., Gambarelli F., Gastaut J.-A., Dujardin P., Dellamonica P., Cassuto J.-P. Visceral leishmaniasis and HIV-1 coinfection in southern France. R. Soc. Trop. Med. Health. 1995;89:159–162. doi: 10.1016/0035-9203(95)90476-x. [DOI] [PubMed] [Google Scholar]
- Royal Society. Ground-level ozone in the 21st century: Future trends, impacts and policy implications. 2008. Royal Society Policy Document 15/08. London, UK.
- Schmidt-Chanasit J., Haditsch M., Günter S., Stark K., Frank C. Dengue virus infection in a traveller returning from Croatia to Germany. Eurosurveillance. 2010;15:19667. doi: 10.2807/ese.15.40.19677-en. [DOI] [PubMed] [Google Scholar]
- Sorrell T. C. Cryptococcus neoformans variety gattii. Med. Mycol. 2001;39:155–168. [PubMed] [Google Scholar]
- Speldewinde P. C., Cook A., Davies P., Weinstein P. A relationship between environmental degradation and mental health in rural Western Australia. Health Place. 2009;15:880–887. doi: 10.1016/j.healthplace.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Stapp P., Antolin M. F., Ball M. Patterns of extinction in prairie dog metapopulations: Plague outbreaks follow El Nino events. Front Ecol. Environ. 2004;2:235–240. [Google Scholar]
- Stern N. H. Stern review: The economics of climate change. Vol. 30. London, UK: HM Treasury; 2006. [Google Scholar]
- Stillerman K. P., Mattison D. R., Giudice L. C., Woodruff T. J. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008;15:631–650. doi: 10.1177/1933719108322436. [DOI] [PubMed] [Google Scholar]
- Taggart M. A., Senacha K. R., Green R. E., Jhala Y. V., Raghavan B., Rahmani A. R., Cuthbert R., Pain D. J., Meharg A. A. Diclofenac residues in carcasses of domestic ungulates available to vultures in India. Environ. Int. 2007;33:759–765. doi: 10.1016/j.envint.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Nakamura S., Kunii O. Bacteriological survey of diarrhoea epidemics in the 1998 Bangladesh floods. Kansenshogaku Zasshi. 1999;73:91–922. doi: 10.11150/kansenshogakuzasshi1970.73.918. [DOI] [PubMed] [Google Scholar]
- Taylor N. G. H., Verner-Jeffreys D. W., Baker-Austin C. Aquatic systems: Maintaining, mixing and mobilising antimicrobial resistance? Trends Ecol. Evolution. 2011;26:278–284. doi: 10.1016/j.tree.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Ternes T. A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998;32:3245–3260. [Google Scholar]
- Trnka M., Muška F., Semerádová D., Dubrovský M., Kocmánková E., Žalud Z. European corn borer life stage model: Regional estimates of pest development and spatial distribution under present and future climate. Ecol. Model. 2007;207:61–84. [Google Scholar]
- McMullen C. P., Jabbour J., editors. United Nations Environmental Programme. Climate change science compendium. 2009. http://www.unep.org/pdf/ccScienceCompendium2009/cc_ScienceCompendium2009_full_highres_en.pdf.
- United Nations Environmental Programme and Artic Monitoring and Assessment Programme. Climate Change and POPs: Predicting the impact. 2010. Report of the United Nations Environmental Programme/Artic Monitoring and Assessment Programme Expert Group: Secretariats of the Stockholm Convention in Geneva, Switzerland, and the Arctic Monitoring and Assessment Programme, Oslo, Norway.
- U.S. Environmental Protection Agency Office of Air and Radiation. Report to Congress on indoor air quality, Vol. II: Assessment and control of indoor air pollution. 1989. http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=9100LMBU.txt.
- Vázquez A., Jiménez-Clavero M. A., Franco L., Donoso-Mantke O., Sambri V., Niedrig M., Zeller H., Tenorio A. Usutu virus—Potential risk of human disease in Europe. Eurosurveillance. 2011;16:19935. [PubMed] [Google Scholar]
- Verger P., Rotily M., Hunault C., Brenot J., Baruffol E., Bard D. Assessment of exposure to a flood disaster in a mental-health study. J. Expos. Anal. Environ. Epidemiol. 2003;13:436–442. doi: 10.1038/sj.jea.7500290. [DOI] [PubMed] [Google Scholar]
- Wittman R. J., Flick G. J. Microbial contamination of shellfish: Prevalence, risk to human health, and control strategies. Annu. Rev. Public Health. 1995;16:123–140. doi: 10.1146/annurev.pu.16.050195.001011. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Water-related diseases. Scabies. World Health Organization; 2011a. http://www.who.int/water_sanitation_health/diseases/scabies/en/ (accessed September 9, 2011). [Google Scholar]
- World Health Organization. Water-related DIseases. Trachoma. World Health Organization; 2001 2011b. http://www.who.int/water_sanitation_health/diseases/trachoma/en/ (accessed September 9, 2011). [Google Scholar]
- World Health Organization. Protecting health from climate change: Connecting science, policy and people. 2009. http://www.who.int/globalchange/publications/reports/9789241598880/en/index.html.
- World Health Organization. World malaria report 2010. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- Wright G. D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010;13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Zhou X.-N., Yang G.-J., Yang K., Wang X.-H., Hong Q.-B., Sun L.-P., Malone J. B., Kristensen T. K., Bergquist N. R., Utzinger J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008;78:188–194. [PubMed] [Google Scholar]


