Abstract
Growth hormone (GH) is a protein secreted by the anterior pituitary and circulates throughout the body to exert important actions on growth and metabolism. GH stimulates the secretion of insulin-like growth factor-I (IGF-I) which mediates some of the growth promoting actions of GH. The GH/IGF-I axis has recently been recognized as important in terms of longevity in organisms ranging from C. elegans to mice. For example, GH transgenic mice possess short lifespans while GH receptor null (GHR−/−) mice have extended longevity. Thus, the actions of GH (or IGF-I) or lack thereof impacts the aging process. In this review, we summarize the proteomic analyses of plasma and white adipose tissue in these two mouse models of GH action, i.e., GH transgenic and GHR−/− mice. At the protein level, we wanted to establish novel plasma biomarkers of GH action as a function of age and to determine differences in adipose tissue depots. We have shown that these proteomic approaches have not only confirmed several known physiological actions of GH, but also resulted in novel protein biomarkers and targets that may be indicative of the aging process and/or new functions of GH. These results may generate new directions for GH and/or aging research.
Introduction
Growth hormone (GH) is a hormone secreted by the anterior pituitary and circulates throughout the body to exert important actions on growth and metabolism. In most organs, GH stimulates the secretion of another hormone called insulin-like growth factor-I (IGF-I), which mediates several, but not all, of the actions of GH. The GH/IGF-I axis has recently been recognized as an important regulator of aging, as attenuation of the GH/IGF-I axis results in increased lifespan [1].
We have generated two mouse lines in which GH signaling is affected. The first is a transgenic mouse that expresses the bovine (b) GH gene, whose transcription is directed by the mouse metallothionein regulatory sequences [2]. This mouse is giant due to elevated levels of GH/ IGF-I and is hyperinsulinemic in spite of a lean phenotype. The bGH mouse has a lifespan of approximately 12–18 months and dies prematurely due to heart, liver, and kidney complications. The second mouse strain is one in which the GH receptor (R) gene has been disrupted [3]. This GHR−/− mouse is dwarf with low levels of IGF-I. Since there is no expression of the GHR, the mouse is GH insensitive or resistant, similar to human Laron Syndrome (LS) patients, who have mutations in the GHR gene [4]. The GHR−/− mouse is also obese with fat accumulating primarily in the subcutaneous depot. Surprisingly, this mouse has an extended lifespan of approximately 30–36 months. As such, the GHR−/− mouse is used by many for longevity studies.
In this review, we describe proteomic parameters of plasma and white adipose tissue in bGH and GHR−/− mice. In plasma we focused on putative biomarkers of GH action and aging. In individual adipose depots we searched for proteins affected by GH action and/or aging.
Endocrine parameters and aging issues in bGH and GHR−/− mice
Some of the primary actions of GH involve the regulation of glucose and lipid metabolism, as well as insulin sensitivity, which are relevant in view of GH’s role in aging. Below we review the major metabolic characteristics of GH transgenic and GHR−/− mice. For the GH transgenic mice, we have included references that use other lines (denoted as ‘GH transgenic’ instead of ‘bGH transgenic’), which show similar metabolic characteristics to our bGH mouse line.
GH transgenic mice
Insulin, glucose and insulin sensitivity
GH is a diabetogenic hormone, i.e., it inhibits the action of insulin [5, 6]. Thus, GH transgenic mice are giant with high insulin levels and normal or low glucose [7–15] (Table 1), suggesting these animals are insulin-resistant. Interestingly, they show normal or better glucose tolerance than WT littermates [12, 13, 16], at least when young, perhaps due to a robust pancreas [17] that secretes increased insulin in response to a glucose challenge [13]. Peripheral tissues such as liver, muscle and fat all have been shown to be insulin resistant [8, 9, 18], consistent with the whole-body insulin-resistant state due to the elevated levels of GH. Plasma adiponectin, an insulin sensitizer, is decreased, whereas resistin, tumor necrosis factor (TNF)-α and interleukin (IL)-6 are increased in these mice [15], which may contribute to their insulin resistance.
Table 1.
Characteristics of GH transgenic mice and GHR−/− mice
| Phenotype | GH transgenic mouse vs. control | GHR−/− mouse vs. control |
|---|---|---|
| Body weight and length (nose to anus) | Higher | Lower |
| IGF-I level | Higher | Lower |
| Lifespan | Shorter | Longer |
| Age-related diseases | Increased incidence of liver inflammation, liver tumors, glomerulosclerosis and oxidative stress [25, 29–34] | Lower incidence of cancer [51] |
| Glucose level | Normal or lower [7–15] | Lower (normal in older animals) [12, 14, 20, 36–41] |
| Insulin level | Higher [7–11, 13–15] | Lower [14, 20, 36–43] |
| Insulin sensitivity | Lower [8, 9, 18] | Higher in muscle and heart [43, 45– 47], mixed results for liver [36, 41, 43, 44, 89] |
| Glucose tolerance | Higher [12, 16] | Lower [12, 40] |
| Body composition | Less fat mass [2, 19], especially in the subcutaneous fat depot [14] | More fat mass, primarily in the subcutaneous depot [2, 14, 20] |
| Plasma free fatty acids | Lower or no change [10, 11, 15] | No change [48] |
| Plasma triglycerides | Mixed results [10, 12, 13, 15] | Normal or lower [12, 39, 42, 48] |
| Plasma cholesterol | Higher [10, 12, 13, 15] | Lower [12, 39, 42, 48] |
Body composition and lipid profile
Adult bGH mice have increased lean mass and reduced fat mass, consistent with GH’s lipolytic effect on fat and anabolic action on muscle [2, 19]. They are also resistant to diet-induced obesity [13, 14]. Interestingly, bGH mice younger than 4 months have a greater normalized fat mass than WT mice [19] suggesting a role of GH in adipocyte differentiation and proliferation early in development. However, adult bGH mice have proportionally less subcutaneous fat than other adipose depots when compared to WT mice [14], suggesting a more pronounced lipolytic action of GH in subcutaneous fat [20].
GH transgenic mice have altered lipid profiles compared to controls (Table 1). Remarkably they have increased cholesterol including high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol [10, 12, 13, 15].
Premature aging and reduced lifespan
bGH mice live on average for ~ 14 months, significantly shorter than WT littermates, which live for ~ 2 years [2, 21, 22]. The pathophysiology of these mice shares several similarities with acromegalic humans, who have excess GH levels due to pituitary adenomas. Acromegalic patients often have kidney problems [23] and cardiovascular diseases [24]. Likewise, GH transgenic mice develop progressive glomerulosclerosis [25] and cardiovascular diseases [26–28]. In addition, GH transgenic mice show liver inflammation and liver tumors at old ages [29–31]. One of the causes of premature aging may be increased oxidative stress in GH transgenic mice in the kidney, liver and carotid artery at later ages [32–34].
In summary, chronic GH excess promotes insulin resistance, abnormal pathology of multiple organs with increased oxidative stress. Interestingly, many of these adverse phenotypes are observed as the mice age, indicating the importance of studying GH action in the context of lifespan rather than at a single age.
GHR−/− mice
GHR−/− mice, which were generated in our laboratory in the mid-1990s [3], show many phenotypes and endocrine parameters analogous to Laron Syndrome (LS) patients, who have disrupted GH signaling due to mutations in the GHR gene. These characteristics in both mice and men have been recently reviewed [4, 35]. Below, we will summarize several phenotypes related to aging.
Insulin, glucose and insulin sensitivity
GHR−/− mice have low-normal levels of glucose [12, 14, 20, 36–41] with very low levels of insulin throughout their lifespan compared to WT mice [14, 20, 36–43]. Glucose tolerance, however, is impaired perhaps due to a decreased size of the pancreas [39, 44], which results in reduced insulin secretion relative to WT mice upon a glucose stimulus [40]. Regarding tissue specific insulin-induced signaling, the heart and muscle of these mice are more insulin sensitive compared to control mice [43, 45–47].
Body composition and lipid profile
GHR−/− mice are obese and accumulate excess subcutaneous fat independent of sex or age [2, 14, 20, 39]. Interestingly, this ‘obese’ mouse actually shows unchanged or lower fat mass in the perigonadal depot [20], suggesting the importance of depot-specific adipose tissue in health and longevity. The fact that bGH mice have less subcutaneous fat implies that subcutaneous fat is the most GH sensitive (and perhaps insulin sensitive) among all fat pads. Also, in contrast to bGH mice, GHR−/− mice have decreased plasma triglyceride and cholesterol levels [12, 39, 42, 48], as summarized in Table 1.
Increased lifespan and reduced incidence of age-related diseases
GHR−/− mice are long-lived, in fact they are the longest lived laboratory mouse strain. The molecular mechanism(s) responsible for this extended lifespan is the object of intense research. Calorie restricted (CR) mice share many similar phenotypes with GHR−/− mice [49]. Although CR extends the lifespan in organisms as diverse as C. elegans and mice [50], CR does not further improve the lifespan of GHR−/− mice [43], indicating a possible overlap in the mechanisms for the increased longevity.
Consistent with their longer lifespan, GHR−/− mice suffer rarely from certain age-related diseases such as cancer [51]. GHR−/− mice also show increased insulin sensitivity compared to WT controls throughout lifespan, and are protected from nephropathy caused by experimentally-induced diabetes [52]. Importantly, these coincide with the reduced cancer and diabetes incidences found in LS individuals [53, 54]. Thus, from mouse to man, disruption of the GH receptor gene results in reduced age-related diseases such as diabetes and cancer, which may be the reason why GHR−/− mice are long-lived.
In summary, GHR−/− mice are obese, have altered lipid profiles, yet have increased insulin sensitivity and longevity. Together with GH transgenic mice, they make excellent animal models to study GH action(s) as it relates to aging. However, many issues remain unresolved such as the molecular mechanism(s) of longevity associated with GH signaling. Genomic and Proteomic results will certainly add to the knowledge base concerning GH’s physiological effects as they relate to aging.
Plasma proteomic profiles in bGH and GHR−/− mice
Although the results of many physiological studies have been reported in these GH-related mouse models, little has been done at the proteomic level. We have analyzed the proteomic profiles of these animals as a function of age at both the plasma and the adipose tissue level. In this section, we will summarize the findings on the plasma proteome.
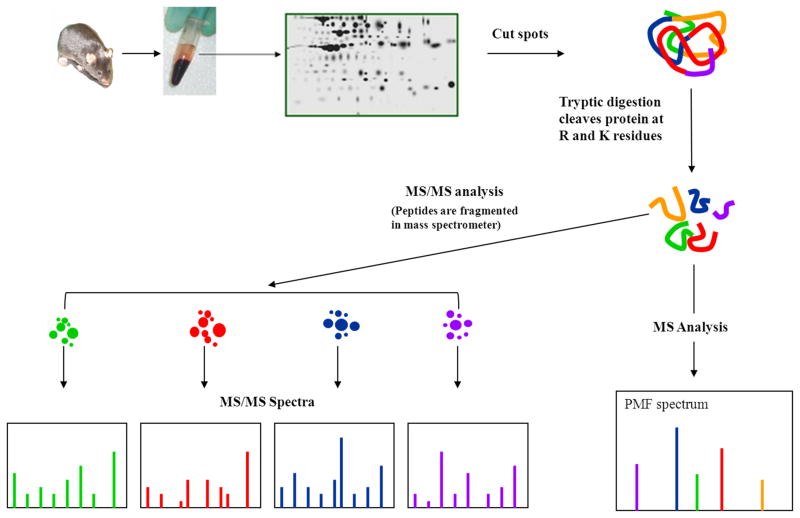
Plasma proteins have long been used as biomarkers for diseases. Our objective was to identify biomarkers of GH action and/or aging using a proteomic approach. We have characterized the plasma proteomes in bGH and GHR−/− mice of various ages (2 months to 24 months) in two longitudinal cohorts using 2-DE and MS/MS [55, 56]. The general workflow is shown in Figure 1. We have developed a sample processing protocol where the plasma sample is diluted in a buffer containing urea, thiourea, non-ionic (e.g., Triton X-100) and zwitterionic (e.g., CHAPS) detergents, a reducing agent (tributylphosphine), carrier ampholytes (e.g., Biolytes 3–10) and protease inhibitors, as reviewed previously [57, 58]. We have discovered that the majority of plasma proteins migrate between isoelectric points (pIs) of 5 and 8, therefore we used first dimensional 17-cm immobilized pH gradient strips of pH 3–10 and subsequently sectioned the strip to obtain the portion between pH 5–8, which was used for the second dimension (SDS-PAGE). In the case of plasma samples, we found good resolution of proteins below ~50 kDa in 15% polyacrylamide gels, without the interference of albumin, which is a highly abundant plasma protein. In this system, we did not deplete albumin since we found albumin fragments to be affected by aging [59]. The final gels were stained with SYPRO Orange, a fluorescent dye that shows good linearity with protein quantity. The gel images were analyzed using PDQuest (Bio-Rad). Selected spots were excised from the gels and analyzed by MS and MS/MS using MALDI-TOF and MALDI-TOF-TOF, respectively. The MS and MS/MS data were subjected to online searches using MASCOT at Matrixsciences.com. For a detailed protocol, refer to [59]. Approximately 160 mouse plasma protein spots were resolved in this system. The identification of selected proteins that exhibit significant differences in bGH and/or GHR−/− mice is shown in Figure 2.
Figure 1.
A schematic flow chart of protein isolation by 2-DE, then identification by MS and MS/MS. 2-DE: two-dimensional electrophoresis, MS: mass spectrometry, PMF: peptide mass fingerprint, R: arginine; K: lysine. This figure was adapted from that found on the Michigan Proteome Consortium web page.
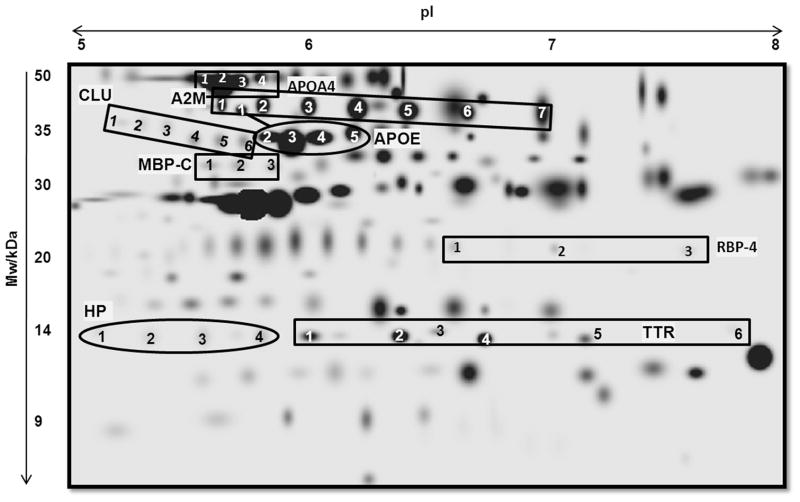
Figure 2.
2-DE gel map of mouse plasma proteome with annotations of proteins that are significantly different in bGH and/or GHR−/− mice. Mw = molecular weight; pI = isoelectric point; A2M = alpha-2 macroglobulin; APOA4 = apolipoprotein A-4; APOA1 = apolipoprotein A-1; APOE = apolipoprotein E; CLU= clusterin; HP = haptoglobin; MBP-C = mannose-binding protein-C; RBP-4 = retinol-binding protein-4; TTR = transthyretin.
We have found that apolipoprotein E (APOE), mannose-binding protein C (MBP-C) and haptoglobin (HP) increased in bGH mice and decreased in GHR−/− mice, whereas RBP-4 decreased in bGH mice and increased in GHR−/− mice [55, 56] (Table 2). Several other proteins changed significantly in one but not the other genotype. For example, transthyretin (TTR) was significantly down-regulated in bGH mice, but not significantly altered in GHR−/− mice. On the other hand, apolipoprotein A-4 (APOA4) was up-regulated in GHR−/− mice but not changed in bGH mice. This indicates that in these two mouse strains, while the levels of certain plasma proteins are primarily regulated by GH (e.g., APOE, MBP-C, HP and RBP-4), others may be regulated by GH as well as other factors. The significance of the proteomic changes were discussed previously [55, 56]. Below, we focus on these novel biomarkers of GH relevant to the phenotypes of bGH and GHR−/− mice including insulin sensitivity, lipid metabolism and aging.
Table 2.
Summary of protein changes in bGH transgenic mice and GHR−/− mice
| Plasma protein | Change in bGH vs. WT [55] | Change in GHR−/− vs. WT [56] | Main Function |
|---|---|---|---|
| A2M | Isoforms 1 and 2 ↑; 5 and 6 ↓ | ↔ | Protease inhibitor |
| APOA4 | ↔ | Isoforms 3, 4 and total ↑ | Transports HDL cholesterol |
| APOE | Isoforms 1–5 and total ↑ | Isoforms 1,3,4 and total ↓ | Transports HDL, LDL and VLDL |
| CLU | Isoforms 1,2 and total ↓ in bGH mice at young ages but ↑ in bGH at old ages | ↔ | Clearance of cellular debris and apoptosis |
| HP | Isoforms 1–4 and total ↑; 1–4 and total ↑ in bGH aging | Isoforms 1–3 ↓ (p<0.05) | Acute phase protein |
| MBP-C | Isoform 1 ↑ | Isoform 1 ↓ (p<0.05) | Acute phase protein |
| RBP-4 | Isoform 2 and total ↓ [90] | Isoforms 1–3 and total ↑ | Transports retinol; involved in insulin resistance |
| TTR | Isoforms 1–6 and total ↓ | ↔ | Transports RBP-4 and thyroxin T4 |
Numbers represent protein isoform# as shown in Figure 1. ‘Total’ is the sum of intensity of all protein isoforms. ↑ increase, ↓ decrease, ↔ unchanged. All changes are at a significance level of p<0.01 unless otherwise indicated (p<0.05 means 0.01<p<0.05).
Insulin sensitivity
RBP-4 is secreted by the liver and adipose tissue and is the major transporter of vitamin A in the circulation. RBP-4 has been found to be associated with obesity and insulin resistance [60]. Moreover, RBP-4 has a stronger association with visceral versus subcutaneous fat [61]. The mechanism of RBP-4-induced insulin resistance is not clear, but RBP-4 is reported to attenuate the insulin-induced phosphorylation of insulin receptor substrate-1 (IRS-1) and ERK1/2 in primary human adipocytes [62]. It has also been reported to induce proinflammatory cytokines in macrophages via c-jun N-terminal kinase and Toll-like receptor-4 [63]. In brown adipose tissue, peroxisome proliferator-activated receptors (PPAR) -α and -γ, and cAMP-mediated pathways regulate RBP-4 expression [64]. Most reports on RBP-4 are in the context of obesity and insulin resistance; very little is known about GH’s effect on RBP-4. It seems paradoxical that bGH mice which are insulin resistant have lower RBP-4 and, GHR−/− mice which are insulin sensitive have higher RBP-4. However, bGH mice are lean and GHR−/− mice are obese, therefore the RBP-4 levels may correlate to obesity rather than insulin resistance in these two mouse strains. In addition, RBP-4 may represent a link that uncouples insulin resistance from obesity in the bGH and GHR−/− mice, as these two mouse strains also show seemingly counter-intuitive phenotypes of adiposity and insulin sensitivity, i.e., the lean bGH mice being insulin resistant and the fat GHR−/− mice being insulin sensitive. A role of RBP-4 in these two mouse strains deserves further study.
Lipid metabolism
Since APOE is a major apolipoprotein that carries nearly all classes of cholesterol (VLDL, LDL and HDL), higher APOE in bGH mice and lower APOE in GHR−/− mice are consistent with the higher cholesterol levels in bGH mice and lower cholesterol levels in GHR−/− mice, respectively. On the other hand, GHR−/− mice have higher APOA4 levels, which are transporter proteins of HDL, the beneficial cholesterol. Consequently, in the long-lived GHR−/− mice, the ratio of levels of APOA4 versus APOE is higher than control mice. This ratio may represent a novel marker for fitness in terms of lipid metabolism. This adds to the existing knowledge that GHR−/− mice, although obese, preferably accumulate fat in the subcutaneous depot, and have low serum cholesterol and triglyceride levels. Together these characteristics may be indicators of beneficial lipid metabolism in terms of health and longevity..
Aging and age-related diseases
Aging is associated with a low grade inflammation [65]. MBP-C and HP are both acute phase proteins indicative of inflammation; therefore their higher levels correspond to an increased inflammatory state in bGH mice, whereas their lower levels indicate that GHR−/− mice have reduced inflammation. In this regard, it has been shown that the proinflammatory cytokines TNF-α and IL-6 are increased in bGH mice [15], and monocyte chemotactic protein-1 and IL-1β are decreased in female GHR−/− mice [20]. Plasma MBP-C and HP are secreted by many tissues, but primarily by the liver. As discussed earlier, GH transgenic mice develop liver tumors at old ages, and have sustained liver inflammation and hepatocellular injury with reduced natural killer T cells [31]. Consistent with the increased inflammation, GH transgenic mice have enlarged spleen and show altered immunity, including deficiency in T helper 2 cytokine production [66] and self-antibody production leading to arthritic disorders [67]. The finding that MBP-C and HP are elevated in the plasma of GH transgenic mice further confirms their increased inflammatory phenotype. Specifically, MBP-C and HP are two plasma proteins that respond to increased GH signaling, i.e., up-regulated in bGH mouse plasma and down-regulated in GHR−/− mouse plasma. These two biomarkers of GH provide a link of GH with inflammation, an interesting observation deserving further research.
Several proteins including APOE, A2M, CLU and MBP-C are involved in Alzheimer’s disease (AD), which is an age-related disease. APOE has been well recognized as a player in AD. In humans, the APOE allele 4 is associated with a higher risk of AD [68]. One allele of A2M confers increased risk of AD [69], CLU variants have also been found to be associated with AD risks [70, 71], and higher total levels are found in AD patients [72]. MBP-C binds to amyloid β and may play a role in tissue homeostasis [73]. It has been found that the cerebral spinal fluid levels of MBP-C are lower in AD patients compared to the controls, although serum levels do not differ [74]. Even though there is no direct evidence linking plasma levels of APOE, A2M and MBP-C with AD, the fact that GH regulates these proteins involved in AD is intriguing.
In summary, plasma proteomic studies have revealed that GH alters several proteins involved in lipid metabolism, insulin sensitivity, and inflammation and may also play a role in AD
White adipose tissue proteomic profiles in GHR−/− mice
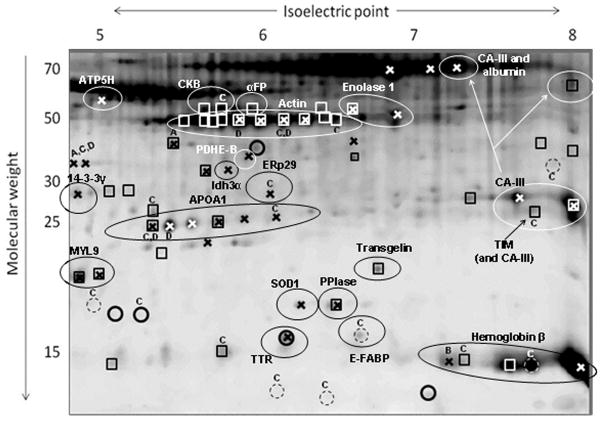
White adipose tissue (WAT) is classified into two main groups, subcutaneous (under the skin) and intra-abdominal or visceral (lining the internal organs, such as the stomach, kidneys, intestine and reproductive organs). Intra-abdominal WAT is more closely associated with classical obesity-related conditions (type-2 diabetes, insulin resistance, cardiovascular disease, etc.), whereas subcutaneous WAT is considered “less unhealthy” [75–78]. As described above, GHR−/− mice are dwarf, but exhibit a marked accumulation of WAT, localized mainly in the subcutaneous region. The fact that GHR−/− mice exhibit enlarged subcutaneous WAT depots while other depots remain unchanged highlights the depot-specific actions of GH on adipose tissue [20]. Also, given that GHR−/− mice are very insulin sensitive and show extended lifespan, understanding the physiology of WAT in different depots of these mice could be applicable to current human health issues. For these reasons, we studied the proteomic profiles of WAT from different depots from GHR−/− mice and WT littermates, including one subcutaneous depot (inguinal) and three intra-abdominal ones (retroperitoneal, mesenteric and epididymal). To better investigate the mechanisms of extended longevity in GHR−/− mice, we included two time points: one where mice are adult (12 months) and one representing old age (24 months). The main focus of our analysis was proteins that showed differences among WAT depots, between age groups, and genotypes. For 2DE, we used the procedures described above for plasma, following homogenization of the tissue and separation of lipids, which if present in a sample can generate problems in protein resolution. For a detailed protocol, refer to [79, 80]. A representative image of a 2D-gel of WAT is shown in Figure 2.
We observed that except for spot intensity differences, the resulting protein profiles of different WAT depots displayed similar spot patterns. Only a few protein spots (myosin regulatory polypeptide 9 and transgelin) were unique to mesenteric WAT and not detected in the other depots (Figure 2). These spots contained smooth muscle proteins which we believe to originate from large blood vessels more densely present in mesenteric fat [80].
Out of 166 well resolved spots, 70 showed significant differences among the groups evaluated. Some of the main observations resulting from the analysis of the differences among WAT depots and between age groups are summarized in Table 3. Regarding differences among the four WAT depots studied, protein profiles of inguinal (subcutaneous) WAT suggested a lower metabolic rate in this depot than in intra-abdominal (mesenteric and epididymal) fat pads. For example, subcutaneous WAT displayed lower levels of glycolytic enzymes and antioxidant proteins, among others [80]. A lower metabolic rate paralleled by a diminished production of ROS might result in decreased cellular stress in subcutaneous WAT which could contribute to its overall “healthiness” when compared to intra-abdominal fat pads (see above). When the effects of age were analyzed, similar changes were observed in all WAT depots, where enzymes involved in glycolysis, the Kreb’s cycle, and antioxidant proteins showed higher levels in aged mice than in adult animals [79]. Seemingly, advancing age leads to increased metabolic rate and ROS production in WAT, which are consistent with a decrease in insulin sensitivity in all depots. Considering the basal depot-differences just described, it was surprising to find similar changes in all WAT depots in terms of metabolic changes with aging. However, even at old age, the differences among WAT depots in metabolic enzyme levels seem to persist, suggesting that subcutaneous WAT remains the “less unhealthy” depot regardless of age. For instance, given that subcutaneous fat is progressively lost in humans with advancing age, the close link between aging and metabolic disease could partly depend on the abundance of the subcutaneous WAT depot [81].
Table 3.
Summary of main WAT proteome differences among depots and age groups in GHR−/− and WT mice
| Protein function | Differences among WAT depots | Effects of Age on WAT |
|---|---|---|
| ATP generation | ↑ in mesenteric WAT (CKB) | ↑ in aged animals (ATP5H) |
| Glucose/lipid metabolism | ↑ in epididymal WAT; ↓ in inguinal WAT (TIM; CA-III) | ↑ in aged animals (ENO1; PDHE-B; Idh3 α; CA-III) |
| Lipid transport | ↑ in mesenteric WAT (APOA1) | ↑ in aged animals (APOA1) |
| Stress resistance | ↑ in epididymal and mesenteric WAT; ↓ in inguinal WAT (HSPβ1; PRDX2) | ↑ in aged animals (ERp29; SOD1; PPIase) |
| Smooth muscle markers | ↑ in mesenteric WAT (MYL9; transgelin) | No change detected |
| Other | ↑ hemoglobin β in mesenteric WAT | ↓ TTR in aged animals |
Values marked “↓” (low) or “↑” (high). Examples of proteins identified for a corresponding biological function are shown in parentheses.
Finally, differences between WAT depots in GHR−/− and wild-type mice involved several proteins that have been associated with aging processes or aging diseases [Apolipoprotein A-1 (APOA1) and TTR, among others]. This analysis is the subject of a recently submitted manuscript [Sackmann et al, submitted] and therefore will not be detailed here. Overall, the effects of GH on WAT depot proteomes were not as striking in terms of the number of proteins affected (12 out of 166 spots analyzed, Figure 2) as the effects observed in depot masses and adipocyte sizes, where differences between genotypes are impressive [20]. However, the extended lifespan of GHR−/− mice might result from a combination of effects of GH on the morphology, architecture and cellular biology of WAT depots (as well as other tissues).
Protein Isoforms in Mouse Plasma
In the 2-DE proteomic analysis, we routinely discover multiple isoforms identified to be the same protein, most likely due to post-translational modifications (PTMs). For example, in plasma, seven protein spots at approximately 35 kDa are identified as alpha-2 macroglobulin have been identified (A2M, Figure 1). In most situations, the isoforms of the same protein show similar expression changes between genotypes. For example, five plasma APOE isoforms are increased in bGH mice and decreased in GHR−/− mice, indicating that APOE is altered at the total protein level, but the proportions of different isoforms remain the same. However, for certain proteins, different isoforms behave differently from each other. For example, the isoforms 1 and 2 of A2M in plasma are up-regulated in bGH mice but isoforms 5 and 6 are down-regulated, with the total expression of A2M unaltered between bGH and control mice. In this case, GH does not change total A2M levels, but rather, regulates specific PTMs that result in selective enrichment of certain isoforms and loss of others. By conventional methods such as immunoblotting, A2M cannot be detected to be a target of GH action. PTMs can be easily detected by 2-DE, provided that the PTM results in changes in the charge of the protein, even if the molecular weight (MW) is only minimally altered.
We are interested in identifying the PTMs that cause these pI shifts of proteins in gels. For example, as a major target tissue of GH, liver is also a main source of plasma proteins, therefore it is natural to ask whether it is possible that GH induces a specific kinase that posttranlationally modifies certain liver proteins that are secreted into the blood, and whether these secreted proteins are affected by aging. One way of predicting or identifying PTMs is to theoretically calculate the pIs of the target protein for a given PTM (e.g., phosphorylation) and see whether they match the observed MWs and pIs on the 2-D gel. The mouse A2M is composed of two subunits- 165 kDa and 35 kDa, and the 35 kDa subunit is detected in our 2-DE system [55] shown in Figure 1. The 35kDa subunit of A2M has a theoretical pI of 7.09 and the seven observed isoforms had pI ranging from 5.65 to 7.08 (Table 4), which means isoform 7 (Figure 1) is most likely the un-modified form and isoforms 1–6 have been modified by PTM(s). A2M is known to be phosphorylated [82] and glycosylated on multiple Asn residues [83]. These PTMs are likely the reason for A2M to exist as multiple isoforms with similar sizes but different pIs on the 2D gel. Table 4 shows the theoretical and observed MWs and pIs of the seven A2M isoforms. Using an online tool to calculate the MW and pI of a protein with varying number of phosphorylated residues (http://scansite.mit.edu/calc_mw_pi.html), it was revealed that isoform 2 (observed pI 5.85) was consistent with the addition of three phosphate groups (predicted pI 5.81) and isoform 3 (observed pI 5.08) was consistent with the addition of two phosphate groups (predicted pI 5.08). The pIs of other isoforms cannot be explained by phosphorylation alone, but may be results of other PTM(s) (e.g., glycosylation) and/or a combination of phosphorylation and other PTM(s). It will be useful to develop tools to predict MW and pI with PTMs other than phosphorylation, as well as combinations of different PTMs.
Table 4.
The molecular weight and isoelectric point of A2M (35 kDa subunit) isoforms
| Isoform # | Theoretical MW (kDa) a | Observed MW (kDa) b | Theoretical pI a | Observed pI b | Theoretical pI with possible phosphorylation (P) c |
|---|---|---|---|---|---|
| 1 | 28.2 | 38.3 | 7.09 | 5.65 | 5.57 (4P) |
| 2 | 28.2 | 38.7 | 7.09 | 5.85 | 5.81 (3P) |
| 3 | 28.2 | 38.5 | 7.09 | 6.08 | 6.08 (2P) |
| 4 | 28.2 | 38 | 7.09 | 6.3 | 6.43 (1P) |
| 5 | 28.2 | 37.7 | 7.09 | 6.54 | |
| 6 | 28.2 | 37.5 | 7.09 | 6.80 | |
| 7 | 28.2 | 37.6 | 7.09 | 7.08 | 7.09 (0P) |
MW and pI were calculated at http://scansite.mit.edu/calc_mw_pi.html.
Observed MW and pI were estimated from the 2-D gel
Theoretical pIs assuming phosphorylation were calculated at http://scansite.mit.edu/calc_mw_pi.html. 1P means one phosphorylation and 2P means two phosphorylations, etc.
In addition to the theoretical calculation and prediction based on known PTMs, it is ultimately the experimental detection that demonstrates whether a PTM is present or not. Traditional MS and MS/MS can achieve this, but peptides with certain modifications such as phosphorylation are difficult to identify, resulting in under-representation of peptides with PTMs in the MS or MS/MS spectrum, leading to low detection rates of PTMs. Further, computer algorithms and programs that accurately and efficiently detect PTMs in the MS or MS/MS spectrum of a protein are needed given the large number of potential PTMs. Currently, the identification of PTMs, which are important elements to understand the regulation of novel biological processes by GH remains a bottle neck. For example, if A2M isoform 2 (which is up-regulated in bGH mouse plasma) proves to have two phosphorylation groups, then GH may regulate a kinase (either in liver cells or in plasma) that phosphorylates A2M, and it would be of interest to study that kinase for a physiological function regulated by GH.
Protein isoforms in WAT
With respect to WAT, there are at least two interesting cases of PTMs in our proteomic analysis: APOA1 and carbonic anhydrase 3 (CA-III). The case of APOA1 is commonly observed in 2D-gels of many different tissues, appearing as a characteristic array of spots aligned at ~25 kDa between pIs of ~5.5 and ~6.5. In our analysis of WAT, six isoforms of APOA1 were found to vary significantly between age groups, and two showed differences among depots (Figure 3). When reviewing the literature, one can easily find associations of APOA1 with atherosclerosis, inflammation, and other age-related diseases [84, 85]. However, the PTMs giving origin to the detected isoforms of APOA1 remain uncharacterized. The theoretical MW and pI of secreted APOA1 are 27.9 and 5.42 respectively. The Uniprot database includes methionine sulfoxides at residues 110 and 136, a phosphoserine at 191 and a N-linked glycation at residue 263 as reported modifications of the human protein [86]. Among these, only serine-phosphorylation generates variations in pI and could therefore be responsible for the detection of a second isoform with similar MW but different pI. Nevertheless, the change in charge would result in a more negative pI, which is opposite to the increased pI detected in APOA1 spots. Given the important role played by APOA1 in lipid transport in the body and its consequences for human health, it is of great interest to unveil the nature of the PTMs present in its various isoforms and how they affect the protein’s activity.
Figure 3.
2-DE gel map of mouse white adipose tissue with annotations of proteins that are significantly different between GHR−/− mice and wild-type littermates (P<0.01). Main effects are labeled using (○) for genotype; (□) for depot; and (x) for age. Significant interactions are labeled with capital letters: genotype × depot (A); genotype × age (B); depot × age (C); and genotype × depot × age (D). Dashed circles mark spots that only show significant interaction effects. The identities of certain spots are annotated; αFP = α fetoprotein; APOA1 = apolipoprotein A-1; ATP5H = ATP synthase subunit β, mitochondrial; CA-III = carbonic anhydrase 3; CKB = creatine kinase B; E-FABP = Fatty acid-binding protein, epidermal; ERp29 = Endoplasmic reticulum resident protein 29; Idh3α = isocitrate dehydrogenase [NAD+] α; MYL9 = myosin regulatory light polypeptide 9; PDHE-B = Pyruvate dehydrogenase E1 β, mitochondrial; PPIase = Peptidyl-prolyl cis-trans isomerase A; SOD1 = superoxide dismutase [Cu–Zn]; TIM = triosephosphate isomerase; TTR = transthyretin.
CA-III is one of the most abundant proteins in white adipose tissue although its function is not well understood. This protein is thought to provide bicarbonate ions for reactions that involve carbon fixation (production of oxaloacetate from pyruvate for synthesis of triglycerides via glyceroneogenesis, and transformation of acetyl-CoA into malonyl-CoA for fatty acid synthesis). Apart from its putative role in anabolic reactions, CA-III might be an important regulator of intracellular pH when fatty acids are released during lipolysis [87, 88]. Therefore, the function of CA-III in WAT seems intrinsically related to the physiology of this tissue. In our proteomic analysis we found at least five spots containing CA-III that were different among WAT depots and/or age groups. These isoforms had MWs that varied from ~27 to ~70kDa and pIs from ~7.2 to ~8. The theoretical MW and pI for CA-III are 29.2 kDa and 6.97 respectively, indicating that various PTMs could be present in the spots detected. The Uniprot database reports the possible presence of two S-glutathionyl-cysteine residues at positions 182 and 187 [86]. However, more research is needed to find the PTMs that originate the shifts in MW and pI observed in our gels. Information on this matter could provide new mechanisms of regulation of CA-III activity, with possible influence in lipid metabolism in WAT.
Conclusion
The use of transgenic and knockout mouse strains to study GH activity has further established the actions of GH in terms of growth, metabolism and aging. However, systematic analysis of tissue proteomes in these mice has only recently emerged. The application of proteomics to study these mice will uncover novel aspects of GH action and represents one of the future research directions. Our recent proteomic studies have confirmed several phenotypes of GH-related mouse strains, e.g., the proteomic studies in mouse plasma indicate that chronic overexpression of GH or complete lack of GH regulate proteins that are involved in insulin sensitivity, lipid metabolism, inflammation and aging. Furthermore, novel biomarkers of GH have been discovered revealing new physiological targets (RBP-4, HP and MBP-C) in relation to aging and age-related diseases. Additionally, proteomic studies in mouse WAT provide interesting information on the physiology of subcutaneous and intra-abdominal depots, and their behavior during aging. The impairment of GH signaling in WAT leads to clear differences among depots and affects the levels of proteins associated to aging. However, the prolonged longevity observed in GHR−/− mice could be the result of GH’s combined action on several aspects of tissue morphology, architecture and cellular biology in WAT depots (and in other tissues). We anxiously await future results that will generate data on biomarkers of GH as it relates to normal physiology and the aging process.
Acknowledgments
This work was supported in part by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by NIH Grants DK075436, AG019899, and P01AG031736, by the Diabetes Research Initiative and the BioMolecular Innovation and Technology Partnership at Ohio University, and by AMVETS.
Footnotes
Conflict of interest
None
References
- 1.Holzenberger M. The GH/IGF-I axis and longevity 10.1530/eje.0.151S023. Eur J Endocrinol. 2004;151:S23. doi: 10.1530/eje.0.151s023. [DOI] [PubMed] [Google Scholar]
- 2.Berryman DE, List EO, Coschigano KT, Behar K, et al. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Xu BC, Maheshwari HG, He L, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laronâ ‰mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laron Z, Kopchick J. Laron Syndrome - From Man to Mouse. Springer; 2011. [Google Scholar]
- 5.HOUSSAY BA. The Hypophysis and Metabolism. N Engl J Med. 1936;214:961–971. [Google Scholar]
- 6.Rabinowitz D, Klassen GA, Zierler KL. Effect of Human Growth Hormone on Muscle and Adipose Tissue Metabolism in the Forearm of Man. J Clin Invest. 1965;44:51–61. doi: 10.1172/JCI105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valera A, Rodriguez-Gil JE, Yun JS, McGrane MM, et al. Glucose metabolism in transgenic mice containing a chimeric P- enolpyruvate carboxykinase/bovine growth hormone gene. FASEB J. 1993;7:791–800. doi: 10.1096/fasebj.7.9.8330686. [DOI] [PubMed] [Google Scholar]
- 8.Dominici FP, Cifone D, Bartke A, Turyn D. Alterations in the early steps of the insulin-signaling system in skeletal muscle of GH-transgenic mice. Am J Physiol Endocrinol Metab. 1999;277:E447–454. doi: 10.1152/ajpendo.1999.277.3.E447. [DOI] [PubMed] [Google Scholar]
- 9.Dominici FP, Cifone D, Bartke A, Turyn D. Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J Endocrinol. 1999;161:383–392. doi: 10.1677/joe.0.1610383. [DOI] [PubMed] [Google Scholar]
- 10.Frick F, Bohlooly-Y M, Linden D, Olsson B, et al. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. AJP - Endocrinology and Metabolism. 2001;281:E1230–E1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- 11.Olsson B, Bohlooly-Y M, Brusehed O, Isaksson OGP, et al. Bovine growth hormone-transgenic mice have major alterations in hepatic expression of metabolic genes. Am J Physiol Endocrinol Metab. 2003;285:E504–E511. doi: 10.1152/ajpendo.00444.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bartke A, Peluso MR, Moretz N, Wright C, et al. Effects of Soy-derived Diets on Plasma and Liver Lipids, Glucose Tolerance, and Longevity in Normal, Long-lived and Short-lived Mice. Horm Metab Res. 2004;36:550–558. doi: 10.1055/s-2004-825796. [DOI] [PubMed] [Google Scholar]
- 13.Olsson B, Bohlooly-Y M, Fitzgerald SM, Frick F, et al. Bovine Growth Hormone Transgenic Mice Are Resistant to Diet-Induced Obesity but Develop Hyperphagia, Dyslipidemia, and Diabetes on a High-Fat Diet. Endocrinology. 2005;146:920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- 14.Berryman DE, List EO, Kohn DT, Coschigano KT, et al. Effect of Growth Hormone on Susceptibility to Diet-Induced Obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the Regulation of Lipid Metabolism in Growth Hormone Transgenic and Calorie-Restricted Mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 16.Boparai RK, Arum O, Khardori R, Bartke A. Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol Chem. 2010;391:1149–1155. doi: 10.1515/BC.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 18.del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, et al. Growth Hormone Regulation of p85Îα Expression and Phosphoinositide 3-Kinase Activity in Adipose Tissue. Diabetes. 2007;56:1638–1646. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- 19.Palmer AJ, Chung MY, List EO, Walker J, et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353–1360. doi: 10.1210/en.2008-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berryman DE, List EO, Sackmann-Sala L, Lubbers E, et al. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrane MM, de Vente J, Yun J, Bloom J, et al. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988;263:11443–11451. [PubMed] [Google Scholar]
- 22.Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- 23.O’Shea MH, Layish DT. Growth hormone and the kidney: a case presentation and review of the literature. J Am Soc Nephrol. 1992;3:157–161. doi: 10.1681/ASN.V32157. [DOI] [PubMed] [Google Scholar]
- 24.Colao A, Marzullo P, Di Somma C, Lombardi G. Growth hormone and the heart. Clin Endocrinol (Oxf) 2001;54:137–154. doi: 10.1046/j.1365-2265.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 25.Doi T, Striker LJ, Quaife C, Conti FG, et al. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am J Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- 26.Bielohuby M, Roemmler J, Manolopoulou J, Johnsen I, et al. Chronic growth hormone excess is associated with increased aldosterone: A study in patients with acromegaly and in growth hormone transgenic mice. Proc Soc Exp Biol Med. 2009:0901-RM-0934. doi: 10.3181/0901-RM-34. [DOI] [PubMed] [Google Scholar]
- 27.Bogazzi F, Russo D, Raggi F, Ultimieri F, et al. Transgenic Mice Overexpressing Growth Hormone (GH) Have Reduced or Increased Cardiac Apoptosis through Activation of Multiple GH-Dependent or -Independent Cell Death Pathways. Endocrinology. 2008;149:5758–5769. doi: 10.1210/en.2008-0346. [DOI] [PubMed] [Google Scholar]
- 28.Bogazzi F, Raggi F, Ultimieri F, Russo D, et al. Regulation of cardiac fatty acids metabolism in transgenic mice overexpressing bovine GH. J Endocrinol. 2009;201:419–427. doi: 10.1677/JOE-08-0194. [DOI] [PubMed] [Google Scholar]
- 29.Snibson KJ, Bhathal PS, Hardy CL, Brandon MR, Adams TE. High, persistent hepatocellular proliferation and apoptosis precede hepatocarcinogenesis in growth hormone transgenic mice. Liver. 1999;19:242–252. doi: 10.1111/j.1478-3231.1999.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 30.Snibson KJ, Bhathal PS, Adams TE. Overexpressed growth hormone (GH) synergistically promotes carcinogen-initiated liver tumour growth by promoting cellular proliferation in emerging hepatocellular neoplasms in female and male GH-transgenic mice. Liver. 2001;21:149–158. doi: 10.1034/j.1600-0676.2001.021002149.x. [DOI] [PubMed] [Google Scholar]
- 31.Hardy CL, Bhathal PS, Snibson KJ, Adams TE. Comparison of intrahepatic lymphocytes from normal and growth hormone transgenic mice with chronic hepatitis and liver cancer. Immunology. 1997;90:412–420. doi: 10.1111/j.1365-2567.1997.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi SQ, Jacot TA, Sellitti DF, Hirszel P, et al. Growth Hormone Increases Inducible Nitric Oxide Synthase Expression in Mesangial Cells. J Am Soc Nephrol. 2000;11:1419–1425. doi: 10.1681/ASN.V1181419. [DOI] [PubMed] [Google Scholar]
- 33.Hauck SJ, Bartke A. Free Radical Defenses in the Liver and Kidney of Human Growth Hormone Transgenic Mice: Possible Mechanisms of Early Mortality. J Gerontol A Biol Sci Med Sci. 2001;56:B153–162. doi: 10.1093/gerona/56.4.b153. [DOI] [PubMed] [Google Scholar]
- 34.Andersson IJ, Johansson ME, Wickman A, Bohlooly YM, et al. Endothelial dysfunction in growth hormone transgenic mice. Clin Sci (Lond) 2006;110:217–225. doi: 10.1042/CS20050281. [DOI] [PubMed] [Google Scholar]
- 35.List EO, Sackmann-Sala L, Berryman DE, Funk K, et al. Endocrine Parameters and Phenotypes of the Growth Hormone Receptor Gene Disrupted (GHR−/−) Mouse. Endocr Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- 37.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 38.Coschigano KT, Holland AN, Riders ME, List EO, et al. Deletion, But Not Antagonism, of the Mouse Growth Hormone Receptor Results in Severely Decreased Body Weights, Insulin, and Insulin-Like Growth Factor I Levels and Increased Life Span 10.1210/en. 2003-0374. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 39.Liu JL, Coschigano KT, Robertson K, Lipsett M, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Lu Y, Houle D, Robertson K, et al. Pancreatic islet-specific expression of an insulin-like growth factor-I transgene compensates islet cell growth in growth hormone receptor gene-deficient mice. Endocrinology. 2005;146:2602–2609. doi: 10.1210/en.2004-1203. [DOI] [PubMed] [Google Scholar]
- 41.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 42.Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, et al. Growth Hormone Receptor Deficiency Results in Blunted Ghrelin Feeding Response, Obesity and Hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2005;292:E1418–1425. doi: 10.1152/ajpendo.00181.2005. [DOI] [PubMed] [Google Scholar]
- 43.Bonkowski MS, Dominici FP, Arum O, Rocha JS, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Robertson K, Kopchick JJ, Liu JL. Growth hormone receptor gene deficiency causes delayed insulin responsiveness in skeletal muscles without affecting compensatory islet cell overgrowth in obese mice. Am J Physiol Endocrinol Metab. 2006;291:E491–498. doi: 10.1152/ajpendo.00378.2005. [DOI] [PubMed] [Google Scholar]
- 45.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Regaiey KA, Masternak MM, Bonkowski MS, Panici JA, et al. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62:18–26. doi: 10.1093/gerona/62.1.18. [DOI] [PubMed] [Google Scholar]
- 47.Giani JF, Bonkowski MS, Munoz MC, Masternak MM, et al. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol A Biol Sci Med Sci. 2008;63:788–797. doi: 10.1093/gerona/63.8.788. [DOI] [PubMed] [Google Scholar]
- 48.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Bonkowski MS, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- 49.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, et al. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol. 2002;16:2657–2666. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- 50.Shimokawa I, Chiba T, Yamaza H, Komatsu T. Longevity genes: insights from calorie restriction and genetic longevity models. Mol Cells. 2008;26:427–435. [PubMed] [Google Scholar]
- 51.Ikeno Y, Hubbard GB, Lee S, Cortez LA, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellush LL, Doublier S, Holland AN, Striker LJ, et al. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–168. doi: 10.1210/endo.141.1.7284. [DOI] [PubMed] [Google Scholar]
- 53.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science translational medicine. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 55.Ding J, Berryman DE, Kopchick JJ. Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res. 2011;20:1305–1320. doi: 10.1007/s11248-011-9499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding J, Berryman DE, Jara A, Kopchick JJ. Age- and Sex-Associated Plasma Proteomic Changes in Growth Hormone Receptor Gene-Disrupted Mice. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopchick JJ, List EO, Kohn DT, Keidan GM, et al. Perspective: proteomics--see “spots” run. Endocrinology. 2002;143:1990–1994. doi: 10.1210/endo.143.6.8882. [DOI] [PubMed] [Google Scholar]
- 58.Ding J, List EO, Okada S, Kopchick JJ. Perspective: Proteomic approach to detect biomarkers of human growth hormone. Growth Horm IGF Res. 2009;19:399–407. doi: 10.1016/j.ghir.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 2011;33:291–307. doi: 10.1007/s11357-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Q, Graham TE, Mody N, Preitner F, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 61.Kloting N, Graham TE, Berndt J, Kralisch S, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Ost A, Danielsson A, Liden M, Eriksson U, et al. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696–3704. doi: 10.1096/fj.07-8173com. [DOI] [PubMed] [Google Scholar]
- 63.Norseen J, Hosooka T, Hammarstedt A, Yore MM, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010–2019. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosell M, Hondares E, Iwamoto S, Gonzalez FJ, et al. Peroxisome proliferator-activated receptors-alpha and -gamma, and cAMP-mediated pathways, control retinol-binding protein-4 gene expression in brown adipose tissue. Endocrinology. 2012;153:1162–1173. doi: 10.1210/en.2011-1367. [DOI] [PubMed] [Google Scholar]
- 65.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalo JA, Mazuchelli R, Mellado M, Frade JM, et al. Enterotoxin septic shock protection and deficient T helper 2 cytokine production in growth hormone transgenic mice. J Immunol. 1996;157:3298–3304. [PubMed] [Google Scholar]
- 67.Ogueta S, Olazabal I, Santos I, Delgado-Baeza E, Garcia-Ruiz JP. Transgenic mice expressing bovine GH develop arthritic disorder and self-antibodies. J Endocrinol. 2000;165:321–328. doi: 10.1677/joe.0.1650321. [DOI] [PubMed] [Google Scholar]
- 68.Cosentino S, Scarmeas N, Helzner E, Glymour MM, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blacker D, Wilcox MA, Laird NM, Rodes L, et al. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 70.Harold D, Abraham R, Hollingworth P, Sims R, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambert JC, Heath S, Even G, Campion D, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 72.Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011;305:1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 73.Larvie M, Shoup T, Chang WC, Chigweshe L, et al. Mannose-binding lectin binds to amyloid beta protein and modulates inflammation. Journal of biomedicine & biotechnology. 2012;2012:929803. doi: 10.1155/2012/929803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lanzrein AS, Jobst KA, Thiel S, Jensenius JC, et al. Mannan-binding lectin in human serum, cerebrospinal fluid and brain tissue and its role in Alzheimer’s disease. Neuroreport. 1998;9:1491–1495. doi: 10.1097/00001756-199805110-00045. [DOI] [PubMed] [Google Scholar]
- 75.Kahn CR. Medicine. Can we nip obesity in its vascular bud? Science. 2008;322:542–543. doi: 10.1126/science.1165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 77.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 79.Sackmann-Sala L, Berryman DE, Lubbers ER, Vesel CB, et al. Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice. Age (Dordr) 2011 doi: 10.1007/s11357-011-9304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring) 2012;20:101–111. doi: 10.1038/oby.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghesquiere B, Van Damme J, Martens L, Vandekerckhove J, Gevaert K. Proteome-wide characterization of N-glycosylation events by diagonal chromatography. J Proteome Res. 2006;5:2438–2447. doi: 10.1021/pr060186m. [DOI] [PubMed] [Google Scholar]
- 84.Ramella NA, Rimoldi OJ, Prieto ED, Schinella GR, et al. Human apolipoprotein A-I-derived amyloid: its association with atherosclerosis. PLoS One. 2011;6:e22532. doi: 10.1371/journal.pone.0022532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teoh CL, Griffin MD, Howlett GJ. Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein & cell. 2011;2:116–127. doi: 10.1007/s13238-011-1013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2012;40:D71–75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coulson RA, Herbert JD. A role for carbonic anhydrase in intermediary metabolism. Ann N Y Acad Sci. 1984;429:505–515. doi: 10.1111/j.1749-6632.1984.tb12379.x. [DOI] [PubMed] [Google Scholar]
- 88.Herbert JD, Coulson RA. A role for carbonic anhydrase in de novo fatty acid synthesis in liver. Ann N Y Acad Sci. 1984;429:525–527. doi: 10.1111/j.1749-6632.1984.tb12381.x. [DOI] [PubMed] [Google Scholar]
- 89.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–684. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Ding J. PhD Dissertation. Department of Biological Sciences, Ohio University; 2009. A Proteomic Approach to Identify Biomarkers for Growth Hormone and Aging. [Google Scholar]