Abstract
Objective
To elucidate the relationship between CpG-induced activation of innate immunity and pregnancy outcome.
Design
An animal model-based study.
Setting
Academic.
Animal(s)
Pregnant nonobese diabetic (NOD) mice were compared with nonimmunodeficient mice.
Intervention(s)
We mimic toll-like receptor 9 (TLR9) activation using CpG ODN administration in pregnant wild-type (WT) and natural killer (NK) cell–deficient NOD mice.
Main Outcome Measure(s)
Evaluation of fetal resorption and preterm birth in pregnant mice; flow-cytometric analysis and ELISA detection.
Result(s)
CpG-induced fetal resorption or preterm birth was observed steadily only in NOD mice but not in WT mice. Concurrently, CpG treatment triggered amplification of uterine macrophages and neutrophils. Moreover, CpG induced a substantial increase of serum mouse keratinocyte-derived cytokine (mKC) and tumor necrosis factor-α (TNF-α) that were produced by uterine CD11b+F4/80+ cells but not by NK or CD11b+Gr-1+ cells. In addition, depletion of F4/80+ cells abrogated a CpG-induced increase in TNF-α production and improved pregnancy outcomes in NOD mice treated with CpG.
Conclusion(s)
These results provide evidence that CpG-driven innate immune activation may lead to activation and amplification of macrophages followed by their migration to fetomaternal microenvironment, up-regulated TNF-α production, and consequent adverse pregnancy outcomes.
Keywords: Animal model, cell signaling, fetomaternal interface, immune modulation, immune tolerance
Miscarriage and preterm birth are the most common complications of pregnancy. In humans, it is estimated that 30% of embryos are lost before implantation and another 30% of embryos are lost before 6 weeks' gestation (preclinical/biochemical pregnancy loss) (1, 2). In addition, more than 10% of clinical pregnancies result in miscarriage that occurs mostly before 12 weeks' gestation. One to 2 percent of pregnant women experience recurrent pregnancy loss that is defined as the failure of three or more consecutive pregnancies (3). One of the most common causes for early and late pregnancy loss is intrauterine infection, in natural pregnancy and pregnancy after clinical assistance such as IVF and ET (4). It is proposed that uterine natural killer (NK) cells produce angiogenic factors and regulate local endovascular processes as well as trophoblast invasion (5–7). Our previous findings suggest that restoration of uterine NK cells by adoptive transfer of NK cells can successfully decrease the percentage of infertility and fetal resorption in NK cell–deficient nonobese diabetic (NOD) mice that exhibit low NK cell activity (8, 9). On the other hand, it is demonstrated that in some cases, uterine NK cells become harmful to pregnancy in response to bacterial toxins (10, 11). Thus, the mechanisms underlying cytotoxic activation of maternal immune cells by diverse pathogen-mediated inflammatory events require further exploration (12).
In some cases, systemic or intrauterine bacterial infection results in excessive production of hypomethylated CpG DNA motifs that are recognized by toll-like receptor 9 (TLR9) (13, 14). A number of observations imply that CpG motifs are capable of triggering strong and polarized immune responses that may be beneficial or harmful to pregnancy depending on the intrinsic microenvironment (12, 15). Thus, it is necessary to determine whether overreaction of the immune system in response to CpG can impair pregnancy outcomes.
In this study, we examined the role of the CpG-TLR9 axis in an NK cell–deficient mouse model during pregnancy. We sought to elucidate the relationship between CpG-induced activation of innate immunity, impaired function in NK cells, and other possible immune cells such as macrophages, neutrophils, and dendritic cells in the fetomaternal microenvironment with respect to pregnancy loss.
Materials and Methods
Mice and Ethics
Mice used in this study, wild-type (WT) female BALB/c, female NOD on a BALB/c background, and male C57BL/6 (8–12 weeks old), were obtained from the Experimental Animal Center of Zhongshan University (Guangzhou, China). All mice were housed in a pathogen-free facility. The immunodeficiency of NOD mice was confirmed using methods as described elsewhere (16, 17). All animal procedures followed the national animal care guidelines, and Shanghai Jiao Tong University's Institutional Animal Care and Use Committee specifically approved this study. All related data were approved for publication by Shanghai Jiao Tong University's Institutional Review Board and Ethics Committee. Each experimental group contained at least four mice. The day of vaginal plug appearance was designated as gestational day 0.5 (E0.5).
In Vivo Treatment of Pregnant Mice
WT and NOD mice was injected IP with CpG oligodeoxynucleotide (ODN 1826; InvivoGen) at doses of 15, 25, 100, 300, or 400 μg on E6.5 or E14.5 (12). NK cell depletion was performed on E4.5, E6.5, and E9.5 or E9.5, E11.5, and E14.5 using 100 mL of anti-asialo GM1 (Wako) or nonimmune rabbit serum. Two hundred fifty micrograms of anti-Gr-1 (RB6-8C5; BD Bioscience) or isotype Ab (rat IgG1; BD Bioscience) was administered at E5.5. Two hundred fifty micrograms of anti-F4/80 (BM8; eBioscience) or isotype Ab (rat IgG2a, κ; eBioscience) was given on E5.5 and E7.5 or E13.5 and E15.5. Competitive antagonist experiments were performed in NOD mice and IP injections of 100 μg of antagonist ODN (ODN 2088; InvivoGen) were performed with 25 μg of CpG ODN on E6.5. Control experiments were performed using 1006 μg of antagonist ODN alone or 100 μg of antagonist ODN plus 25 μg of CpG ODN on E6.5. Monoclonal antitumor necrosis factor-α(TNF-α) Ab (Gr81-2626; BD Pharmingen) was administered IP at 250 μg on E5.5 and E7.5 with CpG ODN injection on E6.5 or on E13.5 and E15.5 with CpG ODN injection on E14.5 (12).
Cell Preparation
Uterine mononuclear and granular cells (UMGCs) were isolated by mincing and mechanical dispersion of whole E9.5 or E15.5 uteroplacental tissue in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and L-glutamine. Single-cell suspensions from uterine horns were sifted through a 100-μm cell strainer followed by density gradient separation using Fico-6Lite LM (Atlanta Biologicals). These experiments were performed on the three layers obtained from Ficoll gradient separation to determine in which layer granulocytes accumulated. It was found that granulocytes collect directly below the monocyte layer, and both layers were harvested together for further experiments (12).
Flow Cytometry
Abs specific for CD45 (30-F11), CD3 (145-2C11), F4/80 (BM8), Gr-1 (RB6-8C5), and CD11b (M1/79) were purchased from BD Bioscience. FITC-conjugated Dolichos biflorus agglutinin (DBA)-lectin was purchased from Sigma-Aldrich. Ab specific for mouse keratinocyte-derived cytokine (mKC; cat. no. CL9179B; rabbit IgG) was purchased from Cedarlane, used together with FITC- or PE-conjugated secondary Ab. Isolated UMGCs were washed in phosphate-buffered saline (PBS) and resuspended in PBS containing 2% FBS (staining buffer). Combinations of Abs were added for extracellular staining for 30 minutes on ice, rinsed with staining buffer, and assayed on a FACS Calibur flow cytometer using CellQuest software (BD Biosciences). Isotype controls were established by staining of isotype control Abs to exclude false-positive cells (18, 19). Abs specific for TNF-α (MP6-XT22) and interferon-γ (IFN-γ; XMG1.2) were purchased for intracellular staining (BD Biosciences). UMGCs were washed with staining buffer and incubated in 96-well plates for 4–6 hours with Brefeldin A (BD Biosciences), PMA (Calbiochem), and ionomycin (Calbiochem). Cells were washed twice with staining buffer and stained for cell surface antigens as described above. For staining of intracellular antigens, UMGCs were washed with Perm Wash (BD Biosciences) and fixed with Cytofix/Cytoperm (BD Biosciences) for 25 minutes on ice and incubated with Abs for 30 minutes at room temperature. Cells were washed and analyzed with flow cytometry. Experiments were performed independently 4 times, and data were shown as mean ± SD (12, 20, 21).
ELISA
Blood samples were collected from the orbital vein and allowed to clot at room temperature. After centrifugation at 8,000 rpm for 20 minutes, the supernatant of each sample was collected and frozen for further analysis. Serum TNF-α, interleukin-12 (IL-12), IFN-γ, mKC, MIP-1α, and MIP-2 were measured using Quantikine ELISA kits (R&D Systems) according to the manufacturer's instructions. These experiments were repeated independently 10 times in each setting (12).
Statistical Analysis
Statistical significance of differential pregnancy outcomes between the compared groups was examined using the one-way analysis of variance method. All experiments where flow cytometry was plotted were analyzed independently using four animals per condition, and significance was assessed using the t test. Data were shown as means ± SD. P< .05 was considered statistically significant (12, 20, 21).
Results
CpG ODN Significantly Increased Pregnancy Loss in NOD Mice
The effects of CpG ODN on pregnancy were evaluated in allogeneic mating BALB/c × C57BL/6 and NOD × C57BL/6 mouse models. Both fetal resorption and preterm birth were assessed as described below. To assess the ability of CpG ODN to induce fetal resorption, the CpG ODN motif, CpG 1826, was injected IP at the indicated doses in WT mice and NOD mice at E6.5. A dose range from 15 to 400 μg/dam was used initially, and we found that the 25 μg/dam dose was sufficient to induce complete fetal resorption of uterine horns in NOD mice. Maternal wasting in NOD mice was observed when CpG ODN was given at higher doses (100 μg/dam or higher). As shown in Supplemental Figure 1A, fetal resorption was significantly higher in NOD mice than in WT mice even under the conditions where only control ODN was administered (22.6% [24 of 106] vs. 5.5% [6 of 110]; P< .05). CpG ODN caused a further increase in fetal resorption in NOD mice at the 25 μg/dam dose (88.7% [86 of 97]; P< .05 vs. NOD mice treated with control ODN). In contrast, CpG ODN at 25 μg/dam did not impair pregnancy outcome in WT mice (fetal resorption rate 6.3%; 7 of 112; P<.05 vs. NOD mice treated with CpG ODN at the same dose; Supplemental Fig. 1A).
To find a dose of CpG ODN that caused a negative pregnancy outcome in WT mice, a range of doses from 15 to 400 μg/dam was administered at E6.5. None of the tested doses impaired pregnancy outcome with respect to fetal resorption and cranial and distal limb malformation (n = 10/group; Supplemental Fig. 1A) (12, 14).
To assess the effects of CpG administration on preterm birth, CpG ODN 1826 was injected IP at E14.5, and mice were examined 4 times daily for signs of preterm birth of live pups. As shown in Supplemental Figure 1B, NOD mice consistently delivered pups at the dose of 25 μg/dam within 48 hours of injection (100% vs. 10% in control ODN-treated NOD mice; P<.05). Lower doses failed to induce preterm birth. Similar to fetal resorption data, no negative pregnancy outcome was observed after CpG ODN treatment in WT mice at the same dose or higher doses up to 400 μg/dam (n = 10/group). In addition, no cranial or distal limb malformation was found in the delivered pups.
Requirement of TLR9 for CpG-Induced Pregnancy Loss
CpG 1826 is known to be a molecule that may signal not only through the TLR9 pathway, but also through intracellular receptors (12, 22, 23). For instance, DNA-dependent protein kinase, but not TLR9, is required for CpG-mediated Akt activation under certain conditions (16). Thus, we questioned whether increased fetal resorption and preterm birth in NOD mice required TLR9 signaling. To address this issue, CpG ODN 2088 (antagonist ODN to CpG ODN 1826) was used. CpG 2088 is known as an ODN sequence that binds to intracellular TLR9 with high affinity but does not induce downstream stimulation of the TLR9 pathway (12, 24). In the present study, no adverse pregnancy outcome was observed after CpG 2088 injection (Supplemental Fig. 2A and B). When CpG 2088 was administered with CpG 1826 at a dose ratio of 4:1, CpG 1826-induced fetal resorption and preterm birth were blocked by CpG 2088 (Supplemental Fig. 2A and B). These results suggest that the TLR9 antagonist ODN 2088 is able to block the TLR9-mediated signaling by competing with CpG 1826 in binding to TLR9 and to prevent CpG 1826-induced fetal resorption and preterm birth.
CpG ODN Treatment in NOD Mice Induces Placental Accumulation of Macrophages but Not NK Cells
Under physiological conditions, uNK cells, macrophages, and a small number of T cells are present in the murine uterus at the early stages of pregnancy (9, 25). In the present study, flow cytometry was used to assess the percentage of uNK cells (DBA-lectin+CD3−), macrophages (CD11b+F4/80+), neutrophils (CD11b+Gr-1+), and dendritic cells (CD11c+) in WT and NOD mice treated with or without CpG ODN.
In the experiments where conditions for fetal resorption were used, uterine tissue and spleen were harvested at E9.5, whereas in the case of preterm birth, uterine tissue and spleen were harvested at E15.5 (24 hours after CpG injection). The percentage of uNK cells (DBA-lectin+) in CD45+ mononuclear and granular cells from NOD mice was lower than that from WT mice (47.9% ± 4.6% vs. 55.2% ± 5.8% at E9.5 and 13.2% ± 1.5% vs. 17.5% ± 2.1% at E15.5; P<.05 for both; Supplemental Fig. 3A and B). In addition, no significant change of the uNK cell population was observed in WT or NOD mice in E9.5 (52.6% ± 5.3% in NOD and 58.2% ± 6.9% in WT) and E15.5 tissues (17.5% ± 2.2% in NOD and 19.5% ± 3.0% in WT) after CpG administration (Supplemental Fig. 3A and B). Depletion of uNK cells using anti-asialo GM1 antiserum did not alter CpG-induced fetal resorption and preterm birth in NOD mice but slightly increased fetal resorption and preterm birth rates in WT mice (Supplemental Fig. 3C and D). A significant increase was found in the macrophage population (CD11b+F4/80+). The population increased from 12.2% ± 1.1% to 26.7% ± 2.1% when analyzed at E9.5 in the fetal resorption model (P<.05; Supplemental Fig. 4A and B) and from 7.3% ± 0.9% to 24.4% ± 2.1% when analyzed at E15.5 in the preterm birth model (P< .05). At the same dose used to induce fetal resorption or preterm birth in NOD mice, no significant change was observed in either of the cell populations in WT mice (from 14.1% ± 1.5% to 16.0% ± 2.5% when analyzed at E9.5 and from 6.9% ± 1.6% to 7.4% ± 1.3% when analyzed at E15.5; Supplemental Fig. 4A and B).
A significant increase in the neutrophil (CD11b+Gr-1+) population was found in CpG ODN-treated NOD mice as compared with control ODN-treated NOD mice (26.3% ± 2.7% vs. 7.0% ± 1.5%; P< .05; Supplemental Fig. 4C and D). A similar increase was observed in the neutrophil population in CpG ODN-treated NOD mice in the preterm birth experiments (22.9% ± 1.4% vs. 2.1% ± 0.5%; P<.05; Supplemental Fig. 4C and D). No significant change was observed in this cell population in WT mice treated with CpG ODN and control ODN (7.5% ± 0.9% vs. 7.9% ± 1.0% in the fetal resorption model, and 4.5% ± 0.5% vs. 3.8% ± 0.8% in the preterm birth model; Supplemental Fig. 4C and D). In addition, no significant change was observed in the dendritic cell population in WT and NOD mice (data not shown).
Chemokine mKC Is Significantly Increased in CpG-Treated NOD Mice
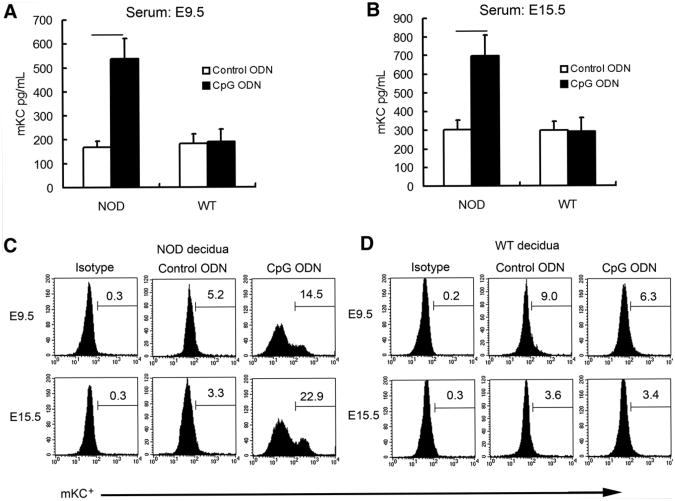
We speculated that certain chemokines were induced in response to CpG ODN treatment in NOD mice and that the induced chemokines recruited monocytes and granulocytes into the uterus during pregnancy (12). Initial screening was performed to measure serum levels of MIP-1α, MIP-2, and mKC using chemokine-specific ELISA. No significant difference was observed in serum levels of MIP-1α or MIP-2 between control ODN- and CpG-treated mice (data not shown). However, mKC, the mouse homologue of human IL-8 and a known chemoattractant of macrophages and neutrophils (12, 26), was significantly increased in serum samples from CpG-treated NOD mice but not WT mice (n = 10 for each group). Serum mKC at E9.5 in NOD mice was 168.0 ± 22.4 pg/mL in control ODN-treated mice and 535.2 ± 86.9 pg/mL in CpG ODN-treated mice (P<.05; Fig. 1A). In comparison, no significant difference was observed in WT mice under the same conditions (183.3 ± 37.5 pg/mL in control ODN-treated mice and 188.8 ± 52.0 pg/mL in CpG-treated mice, respectively; Fig. 1A).
Figure 1.
CpG ODN treatment increased serum and decidual mKC production in NOD mice. Serum mKC was measured using ELISA at E9.5 (A) and E15.5 (B) in NOD and WT mice. Data represent means ± SD from 10 different serum samples in each group. Decidual mKC was assayed by flow cytometry at E9.5 (C) and E15.5 (D) in NOD and WT mice using single-cell suspensions of UMGCs. Data shown are representative of experiments that were performed independently 4 times per condition. A significant increase in the proportion of mKC+ cell subpopulation was observed in CpG ODN-treated NOD mice above control ODN-treated NOD mice (P<.05) (C).
In the preterm birth experiments, serum mKC at E15.5 in NOD mice displayed similar trends upon CpG ODN treatment, whereas no significant change was observed in WT mice (n = 10 for each group). CpG induced a substantial increase in serum mKC at E15.5 in NOD mice (697.3 ± 110.0pg/mLin CpG-treated mice and 302.9 ± 51.2 pg/mL in control ODN-treated mice; P< .05). In contrast, CpG did not alter serum mKC at E15.5 in WT mice (296.8 ± 50.0 pg/mL in control ODN-treated mice vs. 291.7 ± 76.1 pg/mL in CpG-treated mice; Fig. 1B).
Then we determined the proportion of the mKC+ cell population in decidual cells by flow cytometry using single-cell suspensions of UMGCs. We found that the percentage of mKC+ cells was significantly higher in CpG ODN-treated NOD mice both at E9.5 and E15.5 than in control ODN-treated NOD mice (Fig. 1C). In contrast, no significant change was observed in WT mice with the same treatment (Fig. 1D).
TNF-α Functions as the Regulator of CpG ODN-Mediated Pregnancy Loss
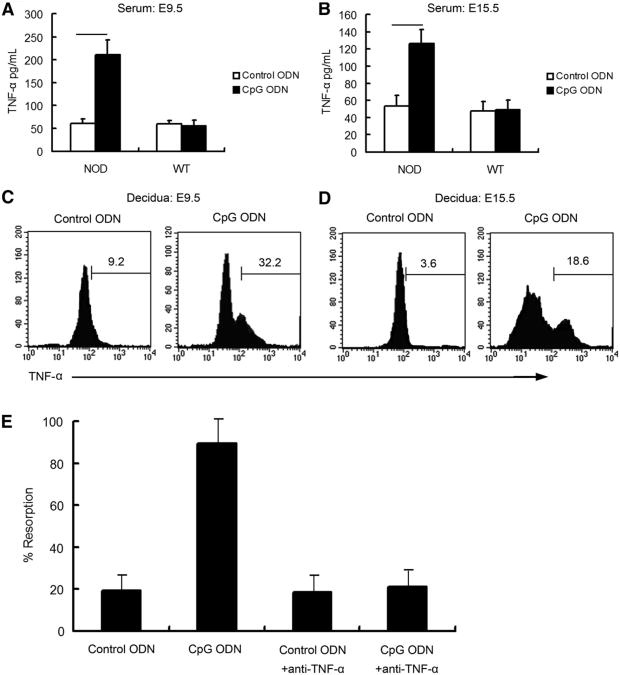
We hypothesized that a macrophage- and/or neutrophilderived cytotoxic factor mediates adverse effects of CpG ODN on pregnancy outcomes in NOD mice. CpG ODN is known to induce the production of a variety of cytotoxic factors, including IFN-γ, TNF-α, and IL-12 (27). In our present study, serum IFN-γ, TNF-α, and IL-12 were measured by ELISA in mice treated with CpG ODN or control ODN. In NOD mice, CpG ODN treatment resulted in a substantial increase in the serum TNF-α level in the fetal resorption models (210.3 ± 32.4pg/mL vs. 60.9 ± 9.6 pg/mL in the control ODN group;P< .05; Fig. 2A) and in the preterm birth models (126.0 ± 16.1 pg/mL vs. 53.3 ± 12.3 pg/mL in the control ODN group; P< .05; Fig. 2B). On the other hand, TNF-α did not exhibit any change in WT mice exposed to the same treatment. No significant difference was observed in IFN-γ and IL-12 between CpG ODN-treated and control ODN-treated NOD or WT mice (data not shown).
Figure 2.
TNF-α is the key cytotoxic factor leading to fetal resorption or preterm birth upon CpG ODN stimulation in NOD mice. Serum TNF-α was measured using ELISA in NOD and WT mice at E9.5 (A) or E15.5 (B). Data represent means ± SD from 10 different serum samples. UMGCs were collected at E9.5 (C) or E15.5(D) from NOD mice treated with control ODN or CpG ODN to determine the proportion of TNF-α+ cell population. Histograms are gated on CD45+ cells and are representative of data from four independently conducted experiments. (E) Neutralizing anti-TNF-α mAb was given IP to inhibit TNF-α activity induced by CpG ODN. The percentage of fetal resorption was analyzed at E9.5 (n = 10 in each group).
Then we aimed to determine whether TNF-α was produced within the uterine tissue of CpG ODN-treated NOD mice. Single-cell suspensions of UMGCs were isolated, and intracellular staining for TNF-α was performed and analyzed by flow cytometry. Uterine CD45+ cells from CpG ODN-treated mice showed a significant increase in intracellular TNF-α production compared with control ODN-treated mice from E9.5 tissue (9.3% ± 1.4% vs. 32.3% ± 2.8%; P<.05; Fig. 2C). Similar results were obtained in the preterm birth model from E15.5 tissue (3.6% ± 2.8% vs. 17.9% ± 2.1%; P<.05; Fig. 2D).
A neutralizing Ab was used for in vivo inactivation of TNF-α. In the fetal resorption experiments, anti-TNF-α was injected at E5.5 and E7.5 in combination with a CpG injection on E6.5. Fetal resorption was assayed at E9.5. The fetal resorption rate was 19.0% in control ODN-treated NOD mice and 89.6% in CpG ODN-treated NOD mice, respectively (Fig. 2E). Anti-TNF-α Ab did not alter the resorption rate in NOD mice treated with control ODN (18.4%) but reduced the fetal resorption rate in CpG ODN-treated NOD mice to a level similar to those treated with control ODN (21.2%) (Fig. 2E).
Gr-1+ Cell Depletion Does Not Alter Pregnancy or TNF-α Production in NOD Mice
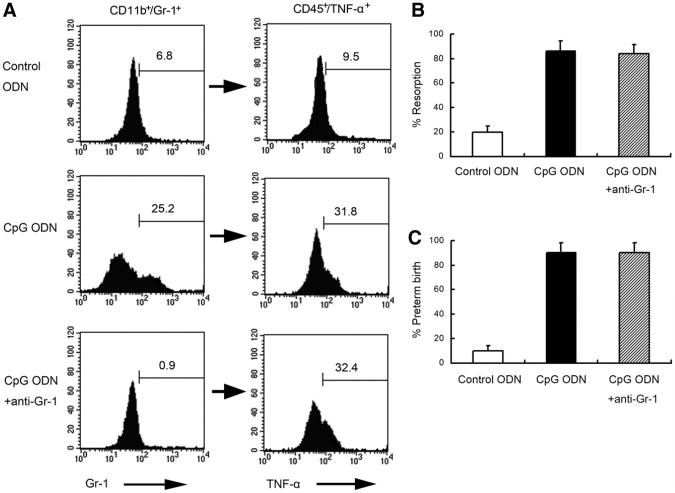
We set out to determine the cellular origin of TNF-α and the role that CD11b+Gr-1+ populations may play in CpG ODN-mediated fetal loss in NOD mice. Anti-Gr-1 Ab was administered IP once at E5.5 with a CpG ODN injection at E6.5. Fetal resorption rate was determined at E9.5. As shown in Figure 3A, anti-Gr-1 Ab treatment successfully depleted Gr-1+ cells in CpG ODN-treated NOD mice. However, this event was not associated with the depletion of a CD45+TNF-α+ cell population as assessed simultaneously by intracellular flow cytometry analysis (Fig. 3A). In addition, depletion of Gr-1+ cells did not rescue pregnancy. The fetal resorption rate was 19.8% in control ODN-treated NOD mice and 86.1% in CpG ODN-treated NOD mice (P<.05). No significant difference in the fetal resorption rate was observed in NOD mice treated with CpG ODN combined with anti-Gr-1 Ab (83.9%) when compared with those treated with CpG ODN alone (Fig. 3B). Similarly, no significant difference in the preterm birth rate was observed in NOD mice treated with CpG ODN plus anti-Gr-1 Ab (90.0%) when compared with those treated alone with CpG ODN (90.0%; Fig. 3C).
Figure 3.
Anti-Gr-1 mAb successfully depleted Gr-1, but Gr-1 depletion does not rescue CpG ODN-induced fetal resorption increase in NOD mice. (A) Anti-Gr-1 was used to deplete Gr-1+ uterine cells. The proportion of Gr-1+ cells in the CD11b+ subpopulation (left panel) and the corresponding proportion of TNF-α+ cells in the CD45+ subpopulation (right panel) were analyzed by flow cytometry. Histograms are representative of independently conducted experiments, 4 times per condition. The percentage of fetal resorption (B) and preterm birth (C) was shown. Ten animals were used under each condition in both fetal resorption and preterm birth models.
F4/80+ Cell Depletion Abrogates TNF-α Production and Rescues Pregnancy in NOD Mice
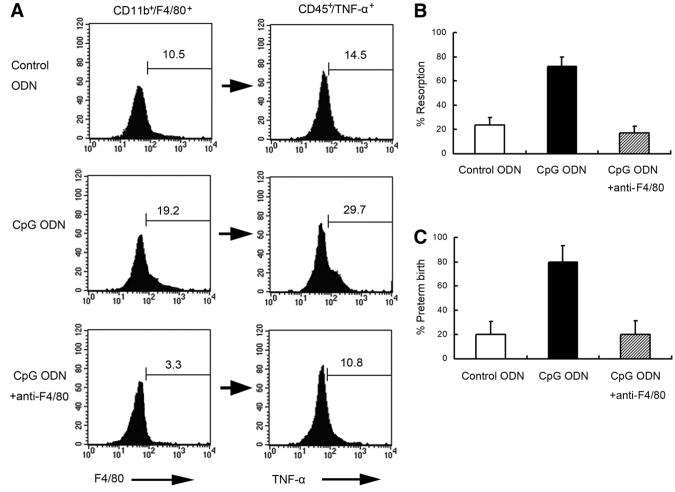
To confirm that F4/80+ cells were depleted, CD45+ UMGCs collected at E9.5 were analyzed by flow cytometry for the presence of the F4/80+ population. F4/80+ cells were successfully depleted below those of control levels (3.3% ± 0.9% in anti-F4/80 Ab-treated NOD mice vs. 10.2% ± 1.0% in control ODN-treated and 19.4% ± 2.0% in CpG ODN-treated NOD mice; P<.05 for both; Fig. 4A). Additionally, intracellular TNF-α production was assessed at E9.5 by FACS in CpG ODN-treated NOD mice depleted or not depleted for F4/80+ cells. In the F4/80+ cell-depleted mice, there was a decreased staining for intracellular TNF-α (10.6% ± 1.0%) compared with in those treated alone with CpG ODN (29.2% ± 1.6%; P<.05), and this level was comparable to that in control ODN-treated NOD mice (14.3% ± 1.9%; Fig. 4A).
Figure 4.
Anti-F4/80 Ab depletes F4/80+ cells, diminishes TNF-α activity, and abrogates CpG-induced fetal resorption and preterm birth. (A) Anti-TNF-α Ab was used to deplete F4/80+ uterine cells. The proportion of F4/80+ cells in the CD11b+ subpopulation (left panel) and the corresponding proportion of TNF-α+ cells in the CD45+ subpopulation (right panel) was analyzed by flow cytometry. Histograms are representative of independently conducted experiments, 4 times per condition. The percentage of fetal resorption (B) and preterm birth (C) was shown. Ten animals were used under each condition in both fetal resorption and preterm birth models.
In the fetal resorption model, anti-F4/80 Ab was injected IP at E5.5 and E7.5 and CpG ODN was injected IP at E6.5. The fetal resorption rate was determined at E9.5. For the preterm birth model, anti-F4/80 Ab was injected at E13.5 and CpG ODN was injected at E14.5. The preterm birth rate was determined. Anti-F4/80 Ab treatment decreased fetal resorption (71.9% to 17.3%; P<.05; Fig. 4B) and preterm birth rate (90% to 20%; P<.05) in CpG ODN-treated NOD mice (Fig. 4C).
Discussion
It is not yet understood how the TLR system responds to external signals that originate from intrauterine infection or inflammation, particularly when combined with intrinsic deficiencies in immune cells (e.g., uNK cells) or molecules that are critical to pregnancy success. One interesting signaling pathway is the TLR9 pathway, which is triggered by interaction between TLR9 and unmethylated CpG OGN motifs.
In this study, we examined the gestational age–dependent consequences of CpG ODN administration in pregnant WT and NK cell–deficient NOD mice. We found that when injected IP at E6.5 or E14.5, CpG ODN caused increased fetal resorption or preterm birth in NOD mice, respectively. In contrast, a similar or a higher dose of CpG ODN does not cause any harm to pregnancy in WT mice. In addition, it appears that CpG ODN-mediated impairment of pregnancy in NOD mice is TLR9 specific because an antagonistic CpG ODN rescued pregnancy failure caused by pathogenic CpG ODN.
Upon CpG ODN stimulation, NOD mice displayed a series of interesting responses. In NOD mice, CpG ODN inducesa significant increase in uterine CD11b+F4/80+ macrophages and CD11b+Gr-1+ neutrophils. This is associated with a marked increase of mKC in serum and uterus. Overexpression of this chemokine in the fetomaternal interface in response to CpG ODN stimulation is highly detrimental via robust recruitment of cytotoxic macrophages. We propose that an increased level of mKC in response to CpG ODN treatment is responsible for the increased presence of CD11b+F4/80+ and CD11b+Gr-1+ cells in the uterine microenvironment.
Moreover, uterine F4/80+ macrophages, but not Gr-1+ neutrophils, display a significantly higher expression of TNF-α. Depletion of F4/80+ cells, but not Gr-1+ cells, significantly decreased the percentage of fetal resorption and preterm birth in CpG ODN-treated NOD mice. In vivo neutralization of TNF-α displays similar effects on these mice. No such result is observed in WT mice under similar conditions.
Notably, depletion of Gr-1+ cells does not rescue pregnancy or reduce TNF-α production. These results suggest that CpG ODN and TLR9 interaction may trigger cytotoxic activation of macrophages and TNF-α production and exert severe pathological effects on pregnancy in NOD mice. During this signaling course, macrophages may play a more critical role than neutrophils. These results are important in light of the numerous reports on the association of bacterial infections with early or late pregnancy loss in humans (4, 28).
TLR9 is expressed by uterine immune cells and localized in intracellular compartments (12, 29). TLR9 signaling has been found to be essential for recruitment and activation of NK cells and dendritic cells in a setting of Gram-negative bacterial infection (30). However, in our current study, no significant increase in fetal resorption or preterm birth was observed when uNK cells were depleted by IP injection of anti-asialo GM1 antiserum in NOD mice. In addition, the proportion of DBA-lectin+ uNK cells at E9.5 and E15.5 was slightly lower in NOD than in WT mice, but no significant change was observed after CpG ODN stimulation at the indicated dose. These results imply that uNK cells play a less important role than macrophages in TLR9-mediated fetal resorption and preterm birth. So what is the immune deficiency in NOD mice that triggered pregnancy complications after the CpG challenge? Our previous work found that the percentage of IL-10+ cells in the decidual CD45+ cell population derived from NOD mice was significantly lower than that in WT mice (31). NK cells have been shown to produce IL-10 in response to viral infection (32). It is then possible that NOD NK cells are deficient in IL-10 production and this contributes to an inflammatory milieu. Thus NOD mice may respond to CpG in a manner that is prevalent in IL-10−/− mice. The findings in this study are in strong agreement with those observed in IL-10−/− mice (12).
One the basis of recent findings and the data in this study, we propose that TNF-α mediates impairment of pregnancy induced by systemic administration of CpG ODN as well as other TLR agonists (12). Because neutralization of TNF-α rescues pregnancy in the presence of CpG ODN, TNF-α can be an appealing therapeutic target in fetal resorption and preterm birth induced by TLR9 activation. Our findings warrant further investigation of the mechanisms that regulate TNF-α production in the fetomaternal microenvironment with respect to pregnancy-associated disorders.
In our current study, we have used systemic administration of CpG to study adverse pregnancy outcomes in NOD mice. We and others have also suggested that intrauterine infection may use a different mode of action that is TNF-α independent (see reference 33 and unpublished data by S. Sharma). However, uterine immune cells are still the contributing factor, suggesting that altered cellular immunity and/or cytokine storm play a key role in the programming of adverse pregnancy outcomes in response to infections.
Supplementary Material
Supplemental Figure 1: CpG-mediated induction of fetal resorption and preterm birth in WT and NOD mice. CpG or control ODN was injected IP as indicated. (A) Mice were injected with CpG or control ODN at E6.5, and fetal resorption was assessed at E9.5. (B) Injection was performed at E14.5, and the preterm birth rate was assessed. Pup delivery before E18.5 was defined as preterm birth. *Maximum preterm birth (100%) was observed at the dose of CpG used in the experiments. Ten female mice were used in each group.
Supplemental Figure 2: CpG ODN-mediated fetal resorption in NOD mice is TLR9 dependent. (A) Mice were injected with CpG ODN 1826 alone at 25 μg/dam, or CpG ODN 2088 aloneat100 μg/dam, orCpG 1826 (25 μg) plus CpG 2088 (100 μg) at E6.5. Fetal resorption was assessed at E9.5. (B) Injection was performed at E14.5, and preterm birth rate was assessed. Pup delivery before E18.5 was defined as preterm birth; n = 10 for each group.
Supplemental Figure 3: The proportion of uNK cell populations does not change upon CpG ODN stimulation in NOD mice. (A) Mice were injected with CpG 1826 at 25 μg/dam at E6.5, and the proportion of uNK cells (DBA-lectin+) in CD45+ mononuclear and granular cells was analyzed at E9.5 using flow cytometry. (B) Injection was performed at E14.5, and the proportion of uNK cells (DBA-lectin+) in CD45+ mononuclear and granular cells was analyzed at E15.5 using flow cytometry. (C) In CpG-treated mice, the effect of anti-asialo GM1 on fetal resorption was assessed at E9.5. (D) In CpG-treated mice, the effect of anti-asialo GM1 on preterm birth was assessed. Pup delivery before E18.5 was defined as preterm birth; n = 10 for each group.
Supplemental Figure 4: CD11b+F4/80+ and CD11b+Gr-1+ uterine cell populations amplify in response to CpG ODN in NOD but not in WT mice. Single-cell suspensions of UMGCs were obtained from NOD (A and C) or WT (B and D) mice at E9.5 or E15.5 after being treated with control or CpG ODN. Cells were gated for CD11b+F4/80+ (A and B) and CD11b+Gr-1+ in the CD45+ subpopulation (C and D). A representative dot plot of the isotype controls was shown (E). The data shown are representative of experiments performed independently 4 times per condition.
Acknowledgments
The authors thank Jingfang Di and Shan Zeng for their technical assistance.
This work was supported by the National Funds for Distinguished Young Scientists, China (no. 81125004), the National Basic Research Program of China (no. 2013CB967404), National Natural Science Foundation of China (no. 31171439), the Funds for Outstanding Academic Leaders in Shanghai, China (no. 12XD1406600), the Guide Project of the Science and Technology Commission of Shanghai Municipality,China (no. 114119a1900), the Funds for the Shanghai Key Laboratory for Assisted Reproduction andReproductive Genetics (no. 12DZ2260600), and the Superfund Program Research National Institute of Environmental Health Sciences (SPR NIEHS grant no. P42ES013660).
Footnotes
Y.S. has nothing to disclose. X.Q. has nothing to disclose. B.S. has nothing to disclose. W.W. has nothing to disclose. Q.Z. has nothing to disclose. S.S. has nothing to disclose. J.W. has nothing to disclose. Y.L. has nothing to disclose.
References
- 1.Salker M, Teklenbueg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the “black box” of early pregnancy loss. Hum Reprod Update. 2002;8:333–43. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 3.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 5.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fatal-maternal interface. Nat Med. 2006;12:1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 6.Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59:425–32. doi: 10.1111/j.1600-0897.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–92. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Lin Y, Zeng S, Li DJ. Improvement of fertility with adoptive CD25+ natural killer cell transfer in sub-fertile NOD mice. Reprod BioMed Online. 2009;18:95–103. doi: 10.1016/s1472-6483(10)60430-0. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Wang H, Wang W, Zeng S, Zhong Y, Li DJ. Prevention of embryo loss in non-obese diabetic mice using adoptive ITGA2+ISG20+ natural killer-cell transfer. Reproduction. 2009;137:943–55. doi: 10.1530/REP-08-0412. [DOI] [PubMed] [Google Scholar]
- 10.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–90. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 11.Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, et al. Evidence for participation of uterine NK cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308.e1–9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183:1144–54. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen G, Andresen L, Matthiessen MW, Rask-Madsen J, Brynskov J. Expression of toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141:298–306. doi: 10.1111/j.1365-2249.2005.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine. 2006;24:263–71. doi: 10.1016/j.vaccine.2005.07.105. [DOI] [PubMed] [Google Scholar]
- 15.Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116:992–7. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Chen Y, Zeng Y, Wang T, Zeng S. Lymphocyte phenotyping and NK cell activity analysis in pregnant NOD/SCID mice. J Reprod Immunol. 2005;68:39–51. doi: 10.1016/j.jri.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Rocha-Campos AC, Melki R, Zhu R, Deruytter N, Damotte D, Dy M, et al. Genetic and functional analysis of the Nkt1 locus using congenic NOD mice: improved Vα14-NKT cell performance but failure to protect against type 1 diabetes. Diabetes. 2006;55:1163–70. doi: 10.2337/diabetes.55.04.06.db05-0908. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Liang Z, Chen Y, Zeng Y. TLR3-involved modulation of pregnancy tolerance in double-stranded RNA-stimulated NOD/SCID mice. J Immunol. 2006;176:4147–54. doi: 10.4049/jimmunol.176.7.4147. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Xu L, Jin H, Zhong Y, Di J, Lin QD. CXCL12 enhances exogenous CD4+CD25+ T cell migration and prevents embryo loss in NOD mice. Fertil Steril. 2009;91:2687–96. doi: 10.1016/j.fertnstert.2008.01.109. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Wang W, Wang H, Zhong Y, Di J, Lin Y. Proteomic analysis of proteins differentially expressed in uterine lymphocytes obtained from wild-type and NOD mice. J Cell Biochem. 2009;108:447–57. doi: 10.1002/jcb.22271. [DOI] [PubMed] [Google Scholar]
- 21.Yin G, Li C, Shan B, Wang W, Chen H, Zhong Y, et al. Insufficient peroxiredoxin-2 expression in uterine NK cells obtained from a murine model of abortion. J Cell Biochem. 2011;112:773–81. doi: 10.1002/jcb.22893. [DOI] [PubMed] [Google Scholar]
- 22.Dragoi AM, Fu X, Ivanov S, Zhang P, Sheng L, Wu D, et al. DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. EMBO J. 2005;24:779–89. doi: 10.1038/sj.emboj.7600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verthelyi D, Zeuner AR. Differential signaling by CpG DNA in DCs and B cells: not just TLR9. Trends Immunol. 2003;24:519–22. doi: 10.1016/s1471-4906(03)00243-6. [DOI] [PubMed] [Google Scholar]
- 24.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 25.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal–fetal tolerance. Nat Immunol. 2006;7:241–6. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 26.Nardini E, Morelli D, Aiello P, Besusso D, Calcaterra C, Mariani L, et al. CpG-oligodeoxynucleotides induce mobilization of hematopoetic progenitor cells into peripheral blood in association with mouse KC (IL-8) production. J Cell Physiol. 2005;204:889–95. doi: 10.1002/jcp.20360. [DOI] [PubMed] [Google Scholar]
- 27.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:135–54. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- 28.Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis. 2006;25:562–9. doi: 10.1007/s10096-006-0190-3. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 30.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, ZengX, Moore TA, et al. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–46. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Ren L, Wang W, Di J, Zeng S, Saito S. Effect of TLR3 and TLR7 activation in uterine NK cells from non-obese diabetic (NOD) mice. J Reprod Immunol. 2009;82:12–23. doi: 10.1016/j.jri.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kim KS, FodilCornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206:2235–51. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200:93.e1–8. doi: 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: CpG-mediated induction of fetal resorption and preterm birth in WT and NOD mice. CpG or control ODN was injected IP as indicated. (A) Mice were injected with CpG or control ODN at E6.5, and fetal resorption was assessed at E9.5. (B) Injection was performed at E14.5, and the preterm birth rate was assessed. Pup delivery before E18.5 was defined as preterm birth. *Maximum preterm birth (100%) was observed at the dose of CpG used in the experiments. Ten female mice were used in each group.
Supplemental Figure 2: CpG ODN-mediated fetal resorption in NOD mice is TLR9 dependent. (A) Mice were injected with CpG ODN 1826 alone at 25 μg/dam, or CpG ODN 2088 aloneat100 μg/dam, orCpG 1826 (25 μg) plus CpG 2088 (100 μg) at E6.5. Fetal resorption was assessed at E9.5. (B) Injection was performed at E14.5, and preterm birth rate was assessed. Pup delivery before E18.5 was defined as preterm birth; n = 10 for each group.
Supplemental Figure 3: The proportion of uNK cell populations does not change upon CpG ODN stimulation in NOD mice. (A) Mice were injected with CpG 1826 at 25 μg/dam at E6.5, and the proportion of uNK cells (DBA-lectin+) in CD45+ mononuclear and granular cells was analyzed at E9.5 using flow cytometry. (B) Injection was performed at E14.5, and the proportion of uNK cells (DBA-lectin+) in CD45+ mononuclear and granular cells was analyzed at E15.5 using flow cytometry. (C) In CpG-treated mice, the effect of anti-asialo GM1 on fetal resorption was assessed at E9.5. (D) In CpG-treated mice, the effect of anti-asialo GM1 on preterm birth was assessed. Pup delivery before E18.5 was defined as preterm birth; n = 10 for each group.
Supplemental Figure 4: CD11b+F4/80+ and CD11b+Gr-1+ uterine cell populations amplify in response to CpG ODN in NOD but not in WT mice. Single-cell suspensions of UMGCs were obtained from NOD (A and C) or WT (B and D) mice at E9.5 or E15.5 after being treated with control or CpG ODN. Cells were gated for CD11b+F4/80+ (A and B) and CD11b+Gr-1+ in the CD45+ subpopulation (C and D). A representative dot plot of the isotype controls was shown (E). The data shown are representative of experiments performed independently 4 times per condition.