Summary
Background
Prenatal maternal smoking and prematurity independently affect wheezing and asthma in childhood.
Objective
We sought to evaluate the interactive effects of maternal smoking and prematurity upon the development of early childhood wheezing.
Methods
We evaluated 1448 children with smoke exposure data from a prospective urban birth cohort in Boston. Maternal antenatal and postnatal exposure was determined from standardized questionnaires. Gestational age was assessed by the first day of the last menstrual period and early prenatal ultrasound (preterm<37 weeks gestation). Wheezing episodes were determined from medical record extraction of well and ill/unscheduled visits. The primary outcome was recurrent wheezing, defined as ≥ 4 episodes of physician documented wheezing. Logistic regression models and zero inflated negative binomial regression (for number of episodes of wheeze) assessed the independent and joint association of prematurity and maternal antenatal smoking on recurrent wheeze, controlling for relevant covariates.
Results
In the cohort, 90 (6%) children had recurrent wheezing, 147 (10%) were exposed to in utero maternal smoke and 419 (29%) were premature. Prematurity (odds ratio [OR] 2.0; 95% CI, 1.3-3.1) was associated with an increased risk of recurrent wheezing, but in utero maternal smoking was not (OR 1.1, 95% CI 0.5-2.4). Jointly, maternal smoke exposure and prematurity caused an increased risk of recurrent wheezing (OR 3.8, 95% CI 1.8-8.0). There was an interaction between prematurity and maternal smoking upon episodes of wheezing (p=0.049).
Conclusions
We demonstrated an interaction between maternal smoking during pregnancy and prematurity on childhood wheezing in this urban, multiethnic birth cohort.
Keywords: Smoking, Prematurity, Wheeze
Introduction
A growing body of literature has highlighted the importance of prenatal factors on the subsequent development of recurrent wheezing and asthma. In particular, in utero tobacco smoke exposure has been well established to promote respiratory morbidity. In utero exposure to tobacco smoke remains a public health concern as 13.2% of mothers in the United States were noted to smoke during pregnancy in 2006.1 Antenatal maternal smoking has been associated with the subsequent development of wheezing and asthma in childhood.2-7 Prematurity is also a well described risk factor in the development of respiratory morbidity and asthma 8-11 and continues to account for 12.7% of all births in the United States.1
Both in utero smoke exposure and prematurity are known to have effects on both airway caliber and lung function.12-18 We are aware of only one study directly evaluating the association between prematurity and antenatal maternal smoking on the development of asthma. This study noted that the effect of maternal smoking on asthma was minimally reduced when fetal growth and preterm delivery were accounted for, suggesting that only a small fraction of the effect of smoking on asthma is through these variables.19 In utero smoke exposure is associated with early immune dysregulation20,21 as well as changes in lung branching morphogenesis.22 The pulmonary effects of preterm delivery may be more related to effects on existing small airways15 as opposed to modulation of airway branching. Given the different effects of these factors and the limited literature published on the topic, evaluation of a possible synergistic effect of these prenatal factors deserves further attention.
The purpose of this study was to evaluate the independent and synergistic associations between both antenatal maternal smoking and prematurity on the outcome of wheezing in the Boston Birth Cohort, a large, urban, multiethnic cohort.
Methods
Patient Population
The Boston Birth Cohort (BBC) was initiated in 1998, and recruitment is ongoing at the Boston University Medical Center (BUMC). The Boston Birth Cohort was originally designed to study adverse birth outcomes, including preterm birth. It is a multiethnic cohort (56% black, 11% white, 20% Hispanic) with subjects from a range of socioeconomic strata, including primarily inner-city poor (50% with annual household income <30K) up to a smaller proportion of middle-class subjects. The inclusion criteria of the parent study are as follows: any woman admitted to the labor and delivery floor at BMC who delivered a singleton live infant was eligible. The Children's Memorial Hospital Institutional Review Board (IRB) and the BUMC IRB approved the parent study protocol. Under a separate BUMC IRB consent, all infants enrolled in the BBC are eligible for the postnatal follow-up study.
Data collection and measurements
Recruitment of the birth cohort
Mother-infant pairs were recruited 24 to 48 hours following delivery. After obtaining signed written informed consent, we interviewed subjects by using a standardized questionnaire. We reviewed maternal and infant medical records using a standardized abstraction form to obtain clinical data, including ultrasonagraphic findings, placental pathology reports, laboratory reports, pregnancy complications, labor and delivery course and birth outcomes.
Postnatal follow-up study
Subjects who sought primary care at the BUMC were invited to participate in the postnatal follow-up study beginning in 2004. After written informed consent was obtained from the biological mother, study visits were conducted at 6 to 12 months, 2 years, 4 years and 6 years in alignment with the child's pediatric primary care visit schedule. Mothers were interviewed with a standardized postnatal health questionnaire. We reviewed the study child's medical record using a standard medical record abstraction form to obtain clinical data, including type and site of visits, clinical diagnosis (including International Classification of Diseases, ninth revision, codes) and growth parameters.
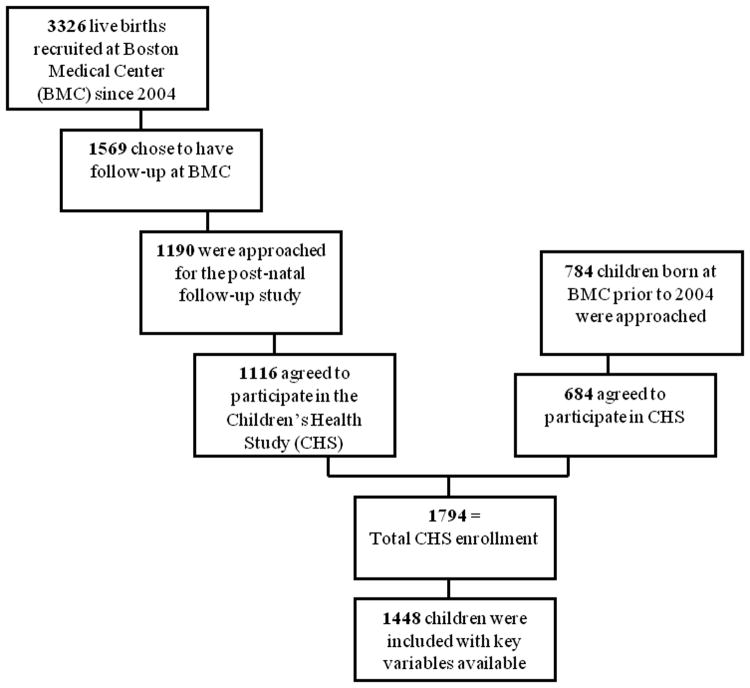
Since 2004, there were 3326 children newly enrolled into the BBC. Of those, 1569 identified the BMC as their preferred site for future pediatric primary care, and thus, were eligible for the follow-up study. 1190 were approached and 1116 agreed to participate in the Children's Health Study (CHS). Approximately 784 children who were born prior to 2004 also were approached and 678 agreed to participate, leading to the current CHS enrollment of 1794 subjects (enrollment of 91% of all those approached). Of those enrolled in the CHS, complete data for smoking in utero, prematurity and the outcome of recurrent wheezing was available for 1448 individuals as is shown in Figure 1.
Figure 1. Participant Flowchart.
Assessment of gestational age
Gestational age was assessed on the basis on the first day of the last menstrual period, as recorded in the medical record, and early (< 20 weeks) prenatal ultrasound. This approach has been used in large, hospital-based preterm studies and in our ongoing funded preterm studies.23,24
Assessment of maternal smoking
Maternal smoking during pregnancy was assessed based on answers to the maternal questionnaire at delivery. Mothers who answered yes to smoking during 6 months before pregnancy or smoking during the first trimester and those who answered yes to smoking during the second or third trimester in the maternal questionnaire were classified as having ongoing in utero smoke exposure through the pregnancy. In the follow-up CHS study, children whose mothers answered yes to smoking at the time of follow-up at any visit were classified as having postnatal exposure from the mother.
Assessment of fetal growth
Fetal growth was based on estimate of standardized birth weight (SBWT). SBWT is defined as birth weight standardized by mean and variance in the stratum of corresponding ethnic group, sex, and gestational week in the reference population by using approximately 15,000 births at BMC during 1998-2003. Fetal growth restriction (FGR) is defined as an SBWT of less than the 10th percentile of the SBWT in the reference population at the same gestational week.
Outcome measures
The primary outcome was recurrent wheezing, defined as >3 episodes of physician-documented wheezing. Given the mean age of 3 years in this cohort, we felt that classifying individuals as having recurrent wheezing using a cutoff of greater than 3 episodes would reflect a similar level of morbidity to other studies using asthma predictive indices in this age range.25-28 We also examined the number of episodes of wheezing. Wheezing episodes were determined from prospective ongoing data extraction from the patient's electronic medical record. All instances of contact with the BMC healthcare system for each of the subjects, including well and ill/unscheduled visits (office visits, urgent care, ED and inpatient encounters) were evaluated. This data collection provided the most complete and accurate assessment of wheezing episodes requiring medical attention of subjects receiving their urgent and emergent medical care at BMC.
Statistical analyses
A total of 1,448 children from the BBC completed at least one postnatal follow-up visit and had key variables available. For comparison of baseline characteristics of the population, t-test and χ2 test were used to characterize all continuous variables and all categorical variables respectively. Annual household income between groups was compared by ANOVA. We examined the associations of maternal in-utero smoking and prematurity with recurrent wheezing by multivariate logistic regression analysis. This included initial evaluation of these factors' independent effects on recurrent wheezing in a combined multivariate model. We also evaluated for joint affect by categorization into controls (nonsmoking mothers and term births), smoking mothers with term deliveries, non-smoking mothers with preterm deliveries, and smoking mothers with preterm deliveries.
Aside from the key determinant variables, demographic variables, and measures of socioeconomic status (SES) were retained in the model. Other potential confounding variables were retained in the model based on significance (p<0.2 for association with the outcome). Following univariate analyses, infant factors were limited to the child's current age, sex, breastfeeding, exposure to tobacco smoke in-utero, exposure to tobacco smoke post-natally, and preterm delivery (<37 weeks gestation). Other traditional infant risk factors such as firstborn status, fetal growth variables and type of delivery (c-section) were not confounders and were excluded. Maternal factors included maternal ethnicity (categorized as black or other), SES as measured by annual household income (> 30 K, <30K, and unknown), and maternal history of atopic diseases.
To decrease the potential for residual confounding which may be due to any differences in duration of follow up between those with recurrent wheezing and those who did not meet our criteria for recurrent wheezing, we carried out two subgroup analyses. First, we repeated the analyses for recurrent wheezing limited to those children who were more than 2 years of age (n=897) at time of last follow up. Second, we restricted the analysis in both groups to only those children aged 2-5 years of age. Finally, we also performed a zero inflated negative binomial regression on the number of episodes of wheeze as a count outcome. This analysis was performed to evaluate if the relationship between maternal smoking and prematurity is present when wheezing is not categorized at a set number of episodes which is more likely to be achieved with longer duration of follow-up. Variables inflated in this model included smoking, prematurity, gender, maternal atopy and age of the child at follow up.
All analyses were performed using statistical software STATA for Windows 9.20 (STATA, College Station, TX).
Results
Baseline characteristics of the cohort
This analysis included 1358 children without recurrent wheezing and 90 children with recurrent wheezing from the BBC. Of these, 147 children were exposed to antenatal maternal smoking and 419 were premature. The majority of the mothers were African American (60%, n=874) and 50% of mothers in the cohort reported an annual household income of < $30,000 per year (n=720). Baseline characteristics of the study population, including demographic measures, SES measures and maternal factors, are shown in Table 1, stratified by wheezing status. The mean age of the children at the last point of follow-up were 3.1±2.4 years in the non-wheezing group and 4.7±2.3 years in the wheezing group. Compared with children who had no recurrent wheezing, children with recurrent wheezing were of an older mean age, were more likely to be male, and had higher frequencies of gestational age < 37 weeks, maternal atopy and annual household income of < $30,000. Children with recurrent wheezing also had a higher frequency of having been born to mothers who smoked during pregnancy (24.4% vs. 15.4% in children without recurrent wheezing).
Table 1. Baseline demographics of the Boston birth cohort by wheezing status.
| Variable | No Recurrent Wheezing (n=1358) | Recurrent Wheezing (n=90) | P value |
|---|---|---|---|
| Male Sex (%) | 49.2 | 62.2 | 0.02 |
| Age (y) (mean ± SD) | 3.1±2.4 | 4.7±2.3 | <0.001 |
| Gestational Age (wk) (mean ± SD) | 37.7±3.5 | 35.6±5.0 | <0.001 |
| Maternal Age (y), (mean ± SD) | 28.5±6.6 | 28.0±6.3 | 0.2 |
| Maternal Atopy (%) | 31.5 | 43.3 | 0.02 |
| Maternal smoking in pregnancy (%) | 9.8 | 15.6 | 0.10 |
| Maternal smoking at follow-up (%) | 15.4 | 24.4 | 0.03 |
| Cesarean section (%) | 33.2 | 35.6 | 0.65 |
| First born (%) | 41.2 | 42.2 | 0.93 |
| Breastfeeding (%) | 6.3 | 4.4 | 0.48 |
| Race (%) | |||
| Black | 59.3 | 76.7 | <0.001 |
| Non-black | 40.7 | 23.3 | |
| Annual Household Income (%) | |||
| <$30,000 | 49.2 | 57.8 | <0.001 |
| >$30,000 | 29.2 | 16.7 | |
| Unknown | 21.7 | 25.6 |
All continuous variables were characterized by t-test and all categorical variables were characterized by chi-square. Income was characterized by ANOVA.
Finally, while there were statistical differences in terms of race, maternal age, gestational age, and birth weight between those in the CHS cohort and those not followed due to the sample size, these differences are minimal in magnitude (see supplemental table 1). For example, the gestational age at delivery (mean±SD) was 37.6±3.6 weeks in CHS compared to 38.1±3.1 weeks in those not followed. Similarly, birth weight (mean±SD) in CHS was 2888.9.2±821.8 grams compared to those not followed whose mean birth weight was 2981.4±755.3 grams.
Associations of maternal smoking during pregnancy and prematurity with recurrent wheezing
We evaluated the independent associations of both maternal smoking during pregnancy and prematurity on the primary outcome of recurrent wheezing as shown in Table 2 with a multivariate logistic regression analysis. We found that prematurity was associated with increased risk of recurrent wheezing (odds ratio [OR], 2.0 95% Confidence Interval [CI] 1.3-3.1) even after adjusting for the child's gender, age, maternal history of atopy, race and income. By itself, maternal smoking during pregnancy did not have an effect on recurrent wheezing (OR 1.1, 95% CI 0.5-2.4). There was no difference in the risk of recurrent wheeze in mothers who smoked only in the six months prior to pregnancy (OR1.7, 95% CI 0.6-4.6) from those mothers who smoked during pregnancy (OR 1.7, 95% CI 1.0-3.0) (supplemental table 2).
Table 2. Association of maternal smoking and prematurity on recurrent wheezing*.
| Risk Factor | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Maternal Smoking in Pregnancy | 1.1 | 0.5-2.4 | 0.82 |
| Maternal Smoking at Follow-up | 1.4 | 0.7-2.7 | 0.34 |
| Prematurity | 2.0 | 1.3-3.1 | 0.002 |
| Gender | 1.5 | 1.0-2.4 | 0.052 |
| Maternal Atopy | 1.4 | 0.9-2.2 | 0.15 |
| Breastfeeding | 0.9 | 0.3-2.6 | 0.87 |
| Age | 1.2 | 1.1-1.3 | <0.001 |
| Black Race | 2.0 | 1.2-3.3 | 0.007 |
| Income < $30,000 | 1.4 | 0.8-2.5 | 0.30 |
| Income Unknown | 1.4 | 0.7-2.8 | 0.31 |
Recurrent wheezing: episodes of wheezing > 3.
Joint association of maternal smoking and prematurity with recurrent wheezing
We evaluated the joint association of maternal smoking during pregnancy and prematurity on the primary outcome of recurrent wheezing as displayed in Table 3. We did not find a statistically significant joint association or interaction between prematurity and smoking on the outcome of recurrent wheeze. Prematurity alone increased the risk of recurrent wheezing (OR 1.8; 95% CI 1.1-2.8). Exposure to maternal smoking in utero without prematurity did not increase the risk of recurrent wheezing. The combination of both prematurity and a history of maternal smoking during pregnancy increased the risk of wheezing above prematurity alone (OR 3.8, 95% CI 1.8-8.0). Two secondary analyses were performed restricting the age of subjects analyzed to either children >2 years or even more conservatively to children between the ages of 2 and 5 years at age of last follow up. These secondary analyses were carried out to account for variable length of follow-up in the study sample. The same patterns of findings were seen in children older than 2 years of age as were seen in the total sample (Table 3). In children between the age of 2 and 5 years, we found the same patterns in this analysis (supplemental table 3) again with an increased risk of wheezing in children with both prematurity and maternal smoke exposure (OR 4.3, 95% CI 1.2-15.2, p value 0.024). In a separate model, evaluating an interaction term, we did not find a significant interaction between maternal smoke exposure in utero and prematurity on the outcome of recurrent wheezing. (p value = 0.41)
Table 3. The joint association of maternal smoking and prematurity with recurrent wheezing*.
| N | Maternal Smoking | Prematurity | Odds Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|---|---|
| All Children (n=1448) | ||||||
| Control | 941 | No | No | - | - | - |
| Prematurity Only | 360 | No | Yes | 1.8 | 1.1-2.9 | 0.018 |
| Maternal Smoking Only | 88 | Yes | No | 0.7 | 0.2-2.3 | 0.57 |
| Maternal Smoking and Prematurity | 59 | Yes | Yes | 4.0 | 1.9-8.6 | <0.001 |
| Only Children > 2 yrs (n=851) | ||||||
| Control | 555 | No | No | - | - | - |
| Prematurity Only | 215 | No | Yes | 1.7 | 1.0-2.8 | 0.06 |
| Maternal Smoking Only | 49 | Yes | No | 0.8 | 0.2-2.8 | 0.75 |
| Maternal Smoking and Prematurity | 32 | Yes | Yes | 5.8 | 2.5-13.8 | <0.001 |
Covariates adjusted in the model: infant factors: child's age, sex and exclusive breastfeeding; maternal factors: maternal ethnicity, maternal atopy and household income. Other covariates include the associations of maternal smoking and prematurity on recurrent wheezing.
Recurrent wheezing: episodes of wheezing >3.
Association of maternal smoking and prematurity with number of episodes of wheezing
To more fully characterize the interaction between the variables of maternal smoking during pregnancy and prematurity on childhood wheezing, further evaluation was undertaken looking at the outcome of number of wheezing episodes. In full term children with maternal smoke exposure, there was no effect on the number of episodes of wheezing. However, in premature children with exposure to antenatal maternal smoking, there was an increase by 1.4 fold in the number of episodes of wheeze. Additionally, in a model mutually adjusted for prematurity and in utero smoke exposure, a clearly significant interaction was found between the two factors on the number of episodes of wheezing (p value 0.029).
Discussion
In this prospective birth cohort study, we found that the combination of prematurity and maternal smoking during pregnancy increases both recurrent wheezing and the number of episodes of wheezing in early childhood. Prematurity alone was associated with an increased likelihood of recurrent early childhood wheezing. We found a significant interaction between prematurity and maternal smoking in utero on the outcome of number of episodes of wheezing, but not on the outcome of recurrent wheezing (≥4 episodes of wheeze) which has been suggested to be an important predictor of asthma in some studies.29
Our finding that prematurity was associated with an increased risk of recurrent wheezing is in keeping with the literature. A number of studies including a meta-analysis of 19 papers 10 suggest that children born prematurely have an increased risk of asthma and wheezing when compared to term babies.8,9,11 Prematurity also results in alterations in childhood lung function noted both at 1 year of age 15 and later in childhood. 16,17 Premature infants with wheezing have greater evidence of air trapping implicating abnormalities of the small airways in particular which may contribute to wheezing.15-17,30
We did not find a statistically significant effect of antenatal maternal smoking on recurrent wheezing, possibly due to sample size. This is not in keeping with previous literature. Past studies have consistently shown that maternal smoking during pregnancy correlates with an increased risk of wheezing and/or development of asthma.2-7 Further evidence for the in-utero effect of smoke exposure comes from genetic studies which find that maternal genetic polymorphisms in nicotine metabolism modify the effect of smoke exposure in utero on subsequent development of asthma.31,32 It is likely that with a larger sample, we would have found significant associations of in utero smoke exposure and childhood wheeze.
Despite the fact that the literature would suggest that prematurity and maternal smoking appear to have similar influences on wheezing, few studies have evaluated a synergistic or interactive effect of both variables on the development of recurrent wheezing. Jaakkola and Gissler published data on over 60,000 children from the Finnish Medical Birth Registry. They found a 25% higher risk of development of asthma by age 7 if mothers smoked < 10 cigarettes per day while pregnant and a 36% higher risk if mothers smoked >10 cigarettes per day.19 Preterm delivery as well as low birthweight was also found to increase the risk of asthma in this cohort. The authors noted that the effect of maternal smoking was minimally reduced when fetal growth and preterm delivery were accounted for, suggesting that only a small fraction of the effect of smoking on asthma is through these variables.19 In contrast, we found a joint effect of maternal smoking during pregnancy and prematurity on recurrent wheezing as well as an interaction of borderline significance between smoking and prematurity on the number of episodes of early childhood wheezing. This suggests that while the majority of the effect of smoking is not accounted for by promoting preterm labor, these co-morbid factors may have combined effects on early life respiratory morbidity.
Both maternal smoking during pregnancy12-14,33 and prematurity appear to have significant effects upon lung function. Even in pre-term children with no neonatal respiratory disease, there are decreases in lung function noted at 1 year of age34, yet the extent to which lung function remains diminished in later childhood is less clear. 18,35 However, these early life defects in lung function may have relevance to respiratory morbidity in early life. Similarly, both prematurity 36-38 and in utero smoke exposure6,39 are associated with increased morbidity associated with viral respiratory illnesses. With both prematurity and in utero smoke exposure playing a role in lung development and early lung function as well as in susceptibility to viral pathogens in early life, children exposed to both of these factors may have an increased number of wheezing episodes at a young age and increased susceptibility to recurrent wheezing.
The results of this study should be interpreted with several potential limitations in mind. First, the data used to evaluate the variable of maternal smoking were derived from self-reported questionnaire. There is no objective measure of maternal or child cotinine levels to validate the maternal report and no measure or report of other household smoke exposure. Yet, even cotinine levels do not provide measures of cumulative tobacco exposure or even necessarily representative levels of exposure due to the fact that they are point estimates40. It is also possible that the retrospective collection of smoking data by questionnaire may result in some degree of recall bias. Second, our findings are limited by the duration of patient follow-up. As such, we are not able to comment on the outcomes of asthma and do not have lung function measures available for these children. The mean age of children at the time of follow-up was 3.1 to 4.7 years respectively in non-wheezers and wheezers. This difference in length of follow-up may have influenced the number of children able to meet our recurrent wheezing definition of ≥4 episodes. However, we accounted for this by controlling for the age of the child as the age of last follow-up and we also completed two sensitivity analyses restricting age to account for this variable length of follow-up. We did not have a large enough sample to stratify by pre-pregnancy smoking, antenatal maternal smoking, postnatal maternal smoking, and the various combinations of timing of this exposure. Finally, we carried out a zero-inflated negative binomial model to look at the outcome of number of wheezing episodes when taking age into account as a covariate. These additional analyses all provided similar results showing the same trend for increased episodes of wheezing with maternal smoke exposure and prematurity.
There is the potential for some selection bias as only a proportion of the cohort who had planned to receive care at BMC were eligible for the post-natal follow-up study. As noted, there were statistical differences in terms of race, maternal age, gestational age, and birth weight between those in the CHS cohort and those not followed. However, these differences were minimal in magnitude. Finally, the Boston Birth Cohort is an urban, multiethnic cohort with a high proportion of African American children and has a high incidence of prematurity which limits the generalizability of our findings. The high proportion of African Americans and lower proportion of Whites in our sample may account in some part for the lower prevalence of in utero smoke exposure compared to the US population.41 Finally, given the low numbers of whites, we were unable to stratify the sample to separately evaluate the associations with childhood wheezing within each race.
In summary, we found that maternal smoking in utero and prematurity appear to have a joint effect on recurrent wheezing and an interactive effect on the number of episodes of early childhood wheezing. Further investigation in larger cohorts is justified to evaluate whether the interaction on childhood wheezing persists in other patient populations. Similarly, studies of early life lung function to evaluate the interaction of these risk factors on lung growth and development would be important. If these data are confirmed, our findings have significant implications to public health concerns as smoking is a modifiable behavior which not only is associated with preterm birth42, but also appears to act in conjunction with prematurity in this high risk cohort.
Supplementary Material
Acknowledgments
Funding: The parent study is in part supported by the March of Dimes PERI grants (PI: Wang, 20-FY02-56), NIEHS (PI: Wang, R21 ES011666), and NICHD (PI: Wang, R01 HD041702). The follow-up study is in part supported by Food Allergy Project, NIAID (PI Wang, R21AI079872), and U.S. Department of Defense (PI Wang, W81XWH-10-1-0123). Dr. Kumar is also supported by the NHLBI (PI: Kumar, K23HL093023). None of the authors have a conflict of interest pertaining to this work.
Abbreviations used
- BBC
Boston birth cohort
- BUMC
Boston University Medical Center
- BM
Boston Medical Center
- CHS
Children's Health Study
- SES
socio-economic status
References
- 1.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117(1):168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 2.Alati R, Al Mamun A, O'Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006;17(2):138–144. doi: 10.1097/01.ede.0000198148.02347.33. [DOI] [PubMed] [Google Scholar]
- 3.Braback L, Bjor O, Nordahl G. Early determinants of first hospital admissions for asthma and acute bronchitis among Swedish children. Acta Paediatr. 2003;92(1):27–33. doi: 10.1111/j.1651-2227.2003.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163(2):429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola JJ, Kosheleva AA, Katsnelson BA, Kuzmin SV, Privalova LI, Spengler JD. Prenatal and postnatal tobacco smoke exposure and respiratory health in Russian children. Respir Res. 2006;7:48. doi: 10.1186/1465-9921-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lannero E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE) Respir Res. 2006;7:3. doi: 10.1186/1465-9921-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pattenden S, Antova T, Neuberger M, Nikiforov B, De Sario M, Grize L, Heinrich J, Hruba F, Janssen N, Luttmann-Gibson H, Privalova L, Rudnai P, Splichalova A, Zlotkowska R, Fletcher T. Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006;15(4):294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dik N, Tate RB, Manfreda J, Anthonisen NR. Risk of physician-diagnosed asthma in the first 6 years of life. Chest. 2004;126(4):1147–1153. doi: 10.1378/chest.126.4.1147. [DOI] [PubMed] [Google Scholar]
- 9.Elder DE, Hagan R, Evans SF, Benninger HR, French NP. Recurrent wheezing in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74(3):F165–171. doi: 10.1136/fn.74.3.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118(4):823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Yuan W, Fonager K, Olsen J, Sorensen HT. Prenatal factors and use of anti-asthma medications in early childhood: a population-based Danish birth cohort study. Eur J Epidemiol. 2003;18(8):763–768. doi: 10.1023/a:1025390420122. [DOI] [PubMed] [Google Scholar]
- 12.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167(6):917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 13.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, Avol E, Peters JM. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55(4):271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348(9034):1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 15.Broughton S, Thomas MR, Marston L, Calvert SA, Marlow N, Peacock JL, Rafferty GF, Greenough A. Very prematurely born infants wheezing at follow-up: lung function and risk factors. Arch Dis Child. 2007;92(9):776–780. doi: 10.1136/adc.2006.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle LW. Respiratory function at age 8-9 years in extremely low birthweight/very preterm children born in Victoria in 1991-1992. Pediatr Pulmonol. 2006;41(6):570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 17.Siltanen M, Savilahti E, Pohjavuori M, Kajosaari M. Respiratory symptoms and lung function in relation to atopy in children born preterm. Pediatr Pulmonol. 2004;37(1):43–49. doi: 10.1002/ppul.10402. [DOI] [PubMed] [Google Scholar]
- 18.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306(6881):817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94(1):136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28(4):721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 21.Noakes PS, Holt PG, Prescott SL. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy. 2003;58(10):1053–1058. doi: 10.1034/j.1398-9995.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 22.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L611–618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, Pearson C, Ortiz K, Zuckerman B, Bauchner H, Cerda S, Stubblefield PG, Xu X, Wang X. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on GxE interactions and pathogenic pathways. Hum Genet. 2008;123(4):359–369. doi: 10.1007/s00439-008-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 25.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, Larsen G, Lemanske RF, Liu A, Mauger DT, Sorkness C, Szefler SJ, Strunk RC, Taussig LM, Martinez FD. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114(6):1282–1287. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi NA, Spycher BD, Strippoli MP, Frey U, Silverman M, Kuehni CE. Validation of the Asthma Predictive Index and comparison with simpler clinical prediction rules. J Allergy Clin Immunol. 127(6):1466–1472. e1466. doi: 10.1016/j.jaci.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Discriminative properties of two predictive indices for asthma diagnosis in a sample of preschoolers with recurrent wheezing. Pediatr Pulmonol. doi: 10.1002/ppul.21493. [DOI] [PubMed] [Google Scholar]
- 29.Caudri D, Wijga A, CM AS, Hoekstra M, Postma DS, Koppelman GH, Brunekreef B, Smit HA, de Jongste JC. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol. 2009;124(5):903–910. e901–907. doi: 10.1016/j.jaci.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 30.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165(1):83–87. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 31.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002;166(4):457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 32.Li YF, Gauderman WJ, Conti DV, Lin PC, Avol E, Gilliland FD. Glutathione S-transferase P1, maternal smoking, and asthma in children: a haplotype-based analysis. Environ Health Perspect. 2008;116(3):409–415. doi: 10.1289/ehp.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155(1):36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 34.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158(3):700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 35.Narang I, Baraldi E, Silverman M, Bush A. Airway function measurements and the long-term follow-up of survivors of preterm birth with and without chronic lung disease. Pediatr Pulmonol. 2006;41(6):497–508. doi: 10.1002/ppul.20385. [DOI] [PubMed] [Google Scholar]
- 36.Greenough A, Cox S, Alexander J, Lenney W, Turnbull F, Burgess S, Chetcuti PA, Shaw NJ, Woods A, Boorman J, Coles S, Turner J. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child. 2001;85(6):463–468. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon A, Ammann RA, Wilkesmann A, Eis-Hubinger AM, Schildgen O, Weimann E, Peltner HU, Seiffert P, Suss-Grafeo A, Groothuis JR, Liese J, Pallacks R, Muller A. Respiratory syncytial virus infection in 406 hospitalized premature infants: results from a prospective German multicentre database. Eur J Pediatr. 2007;166(12):1273–1283. doi: 10.1007/s00431-007-0426-y. [DOI] [PubMed] [Google Scholar]
- 39.Carroll KN, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Wu P, Enriquez R, Hartert TV. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119(6):1104–1112. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Curtis LM, Khiani S, Moy J, Shalowitz MU, Sharp L, Durazo-Arvizu RA, Shannon JJ, Weiss KB. A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. J Allergy Clin Immunol. 2008;122(4):754–759. e751. doi: 10.1016/j.jaci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Geronimus AT, Neidert LJ, Bound J. Age patterns of smoking in US black and white women of childbearing age. Am J Public Health. 1993;83(9):1258–1264. doi: 10.2105/ajph.83.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr Perinat Epidemiol. 2008;22(2):162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.