INTRODUCTION
Sarcoidosis is a systemic disorder characterized by the aberrant development of granulomas within various organs in the body. The lungs are involved in 90% of patients, and the skin, eyes, and heart are affected in a significant fraction of patients. The disease remits within 3 years in most patients, whereas 10% to 30% of patients develop chronic disease requiring ongoing treatment.1 Significant variation in disease incidence and manifestations is well recognized. The incidence rate of sarcoidosis in Northern Europe is between 5 and 40 cases per 100,000 people, compared with a rate of 1 to 2 cases per 100,000 in Japan.2 Cardiac and ocular disease are more common in Japanese patients, whereas joint symptoms and erythema nodosum are more common in northern Europeans.3 In the United States, the age-adjusted annual incidence of sarcoidosis in black patients is 35.5 per 100,000, 3 times higher than that of white patients (10.9 per 100,000).4 Black patients are more likely to develop ocular and granulomatous skin involvement and more frequently suffer chronic, debilitating disease.4–6
Although there are no universal criteria for the diagnosis of sarcoidosis, a diagnosis is likely when a patient presents with signs or symptoms consistent with sarcoidosis and has granulomas shown on tissue biopsy. Most patients with sarcoidosis develop pulmonary involvement, which may be asymptomatic or may cause dyspnea, dry cough, or chest discomfort. Chest radiographs show abnormalities classified into 5 stages (Table 1). Laboratory testing may reveal an increased angiotensin-converting enzyme (ACE) level; however, this test lacks sufficient specificity to make a diagnosis of sarcoidosis. Examination of bronchoalveolar lavage fluid may help in the diagnosis, because a markedly increased ratio of CD4+ T cells to CD8+ T cells in the bronchoalveolar lavage fluid is relatively specific for sarcoidosis; however, diagnosis is generally confirmed by showing epithelioid granulomas on transbronchial biopsy.1,7 Diagnosis of sarcoidosis also requires the exclusion of other causes of granulomatous disease, including mycobacterial infections such as tuberculosis and leprosy, fungal infections such as coccidiomycosis and histoplasmosis, syphilis, exposures to particulates such as beryllium, and granulomatosis with polyangiitis.
Table 1.
Chest radiographic staging
| Stage | Radiographic Findings |
|---|---|
| Stage 0 | Normal radiograph |
| Stage I | Bilateral hilar lymphadenopathy |
| Stage II | Bilateral hilar adenopathy and parenchymal infiltrates |
| Stage III | Parenchymal infiltrates alone |
| Stage IV | Pulmonary fibrosis |
Extrapulmonary disease may manifest before, concurrent with, or after development of pulmonary disease, thus patients with sarcoidosis come to the attention of a range of providers depending on the location of symptoms. Awareness of the common and protean manifestations of sarcoidosis is required to recognize the disease and monitor for additional disease complications. In this article, first, the immunopathology of sarcoidosis is reviewed. The common extrapulmonary manifestations of sarcoidosis are then reviewed, and organ-specific considerations in treatment are discussed.
IMMUNOPATHOLOGY
The hallmark of sarcoidosis is the development of epithelioid granulomas. Autopsy and imaging studies suggest that granulomatous involvement can be more wide-spread in patients with sarcoidosis than is apparent clinically.8–10 Granulomas in different organs tend to conform to a similar histologic pattern, consisting of a dense collection of epithelioid macrophages and CD4+ T cells, with fewer CD8+ T cells restricted to the periphery. Part of the diagnostic challenge is that similar appearing granulomas may form in response to several different stimuli, some of which must be excluded to diagnose sarcoidosis. Exposure to beryllium causes a granulomatous disease similar in appearance to sarcoidosis; however, chronic beryllium disease is generally considered a distinct entity.1
Hints as to the cause of sarcoidosis have been derived from observations about the localization of lesions, spatial-temporal patterns of disease, immunophenotyping, and genetics; however, a cohesive understanding of the disease remains elusive. The pattern of tissue involvement, with a predominance of symptoms in the lungs, skin, and eyes, suggests that exposure to an external trigger plays a key role in initiating the disease. One potential set of triggers is environmental particulate matter. Just as particles of beryllium clearly cause a granulomatous reaction, other types of particulate matter have also been suspected in sarcoidosis. The most comprehensive evaluation for such a trigger was ACCESS (A Case Control Etiologic Study of Sarcoidosis),11 which evaluated exposure histories of more than 700 patients with recently diagnosed sarcoidosis. The study found no association of sarcoidosis with occupational exposure to wood dust, metal, silica, or talc but did report that occupational exposure to insecticides was associated with a modestly increased risk of sarcoidosis. The increased frequency of sarcoidosis in people exposed to dust and debris from the World Trade Center collapse further supports the association between particulates and sarcoidosis.12
An infectious cause of sarcoidosis has also been long suspected, in particular because certain well-characterized infections, such as tuberculosis and leprosy, also induce granulomas. Presentations of Löfgren syndrome were noted to cluster in the spring and early summer, suggesting a possible infectious agent.13 Person-to-person transmission was suggested by observations from the Isle of Man that patients diagnosed with sarcoidosis were more likely than healthy control patients to have been previously in contact with another person with sarcoidosis.14,15 The ACCESS study also noted a positive association between sarcoidosis and occupational exposure to areas with musty odors, which perhaps carry higher loads of bioaerosols containing molds and mycobacteria.11,12
The case for a mycobacterial infection underlying at least some cases of sarcoidosis is particularly strong.16 Several reports have described isolation of mycobacterial DNA from patients with sarcoidosis, and a meta-analysis of 31 studies17 showed that detection of mycobacterial DNA by polymerase chain reaction (PCR) has been reported in about one-quarter of patients with sarcoidosis tested, although evidence of publication bias was noted. Propionibacterium, Mycoplasma, viruses, and Borrelia have been implicated in some patients. Given the variety of possible exposures associated with this disease, it seems unlikely that a single trigger explains all of sarcoidosis. Rather, it is more likely that several triggers may be able to initiate a granulomatous response in a susceptible host, causing a clinical presentation of sarcoidosis.
EXTRAPULMONARY MANIFESTATIONS
Cardiac
Cardiac sarcoidosis is a leading cause of death in sarcoidosis, responsible for 13% to 25% of deaths caused by sarcoidosis in US patients with sarcoidosis, and strikingly, 58% to 85% of deaths in Japanese patients with sarcoidosis.4,9,18 Thus, evaluation for cardiac disease in all patients with sarcoidosis is particularly important. Cardiac involvement does not correlate with the severity of pulmonary involvement and can be difficult to diagnose in the context of active pulmonary disease.19 In the United States, about 5% of patients with sarcoidosis have clinical manifestations of cardiac sarcoidosis; however, autopsy analyses show that myocardial granulomas can be found in 20% to 30% of patients.1,9 More severe cardiac disease correlates with an increased risk of severe arrhythmias.9 Symptoms suggestive of conduction disease include significant palpitations, presyncope, and syncope. The presence of such symptoms increases the likelihood of cardiac sarcoidosis by 8-fold, with significant palpitations being the most informative symptom.19 Sarcoidosis has been found as a cause of previously unexplained atrioventricular block or early pacemaker dependence.20 Sarcoidosis may also cause a dilated cardiomyopathy, associated with typical symptoms of heart failure such as dyspnea, weight gain, and edema, and can rarely cause valvular involvement.
All patients with sarcoidosis should have an electrocardiogram as part of the initial evaluation, although electrocardiograms are an insensitive method of evaluating for cardiac sarcoidosis.19 Electrocardiographic abnormalities may include PR prolongation, atrioventricular nodal blockade, or atrial or ventricular premature beats. Patients suspected of having conduction disease from symptoms or an abnormal electrocardiogram should also undergo Holter monitoring. Rhythm abnormalities detected by Holter monitoring increase the likelihood of finding imaging abnormalities consistent with sarcoidosis by almost 20-fold.19 The presence of ventricular dysfunction can be evaluated by a transthoracic echocardiogram.
Imaging by cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET) have facilitated detection of cardiac sarcoidosis, and the combination of clinical assessment plus imaging has been reported to show cardiac involvement in almost 40% of patients with sarcoidosis.19 Cardiac MRI may show a pattern of late gadolinium enhancement in the basolateral area of the left ventricle, with lesions most frequently seen in the midcardial to epicardial regions, distinct from the subendocardial regions commonly affected by ischemia.21,22 The ability of cardiac MRI to differentiate active inflammation from previous injury is not fully defined; however, serial cardiac MRI evaluation has been suggested to have usefulness in following the response of cardiac sarcoidosis to corticosteroid treatment.23
Nuclear imaging by PET shows focally increased uptake of the radioactive tracer 18F-fluorodeoxyglucose (FDG), most often in the basal and midanteroseptal-lateral areas of the left ventricle.24,25 This method seems more sensitive than cardiac MRI in detecting cardiac sarcoidosis, with a reported sensitivity of 89% and specificity of 78%.26 Because FDG PET theoretically depends on the presence of inflammatory cells to take up the radiolabeled tracer, this modality may be particularly useful in monitoring disease activity.27 Recently, high-sensitivity cardiac troponin T has also been suggested as a means of assessing the presence and activity of cardiac sarcoidosis.28
The presence of granulomatous disease infiltrating the myocardium may be confirmed by endocardial biopsy. However, cardiac sarcoidosis is patchy and favors areas of the left ventricle, whereas endocardial biopsies are typically taken from the right side of the interventricular septum; thus, false-negative results are common because of limitations of sampling.29
Cutaneous
The skin is affected in 20% to 35% of patients with sarcoidosis, and skin lesions are often present at the time of diagnosis.30,31 Cutaneous manifestations of sarcoidosis that are caused by granulomas are referred to as specific for sarcoidosis, whereas other lesions are considered nonspecific. The most common nonspecific cutaneous manifestation is erythema nodosum, which typically manifests as painful nodules on the lower legs, usually in the setting of an acute presentation of sarcoidosis. Erythema nodosum is more common in women and northern Europeans, and is associated with a favorable overall prognosis.6,32 Histologically, the lesions show a septal panniculitis rather than granulomas.
Specific forms of cutaneous sarcoidosis occur in many patterns, with the most common being papular, maculopapular, and plaque lesions. Papular lesions occur commonly on the face, often around the eyes, whereas maculopapular lesions tend to favor the neck and trunk (Fig. 1).30 Both are associated with milder pulmonary disease and a good prognosis, whereas plaque lesions are more often associated with chronic disease requiring steroid treatment.33 Variants of papular and plaque sarcoidosis can take on many forms, including lesions that resemble psoriasis, lichen planus, verrucae, and lupus.30
Fig. 1.

Examples of cutaneous sarcoidosis. (A) Waxy papules over the eyelid of a patient with systemic sarcoidosis. (B) Granulomatous inflammation within the area of 1 color of a tattoo in a patient with systemic sarcoidosis. (Courtesy of Dr J. Merola, Brigham and Women’s Hospital, Boston, MA.)
A unique lesion of sarcoidosis, termed lupus pernio (unrelated to systemic lupus erythematosus) causes distinctive violaceous, indurated lesions on the face, often on the nasal alae. These lesions are often disfiguring and may damage underlying soft tissue and bony structures, causing nasal ulcerations, septal perforation, and deformity.34 Bony cysts may develop under affected areas.35 Lupus pernio occurs more frequently in female patients and is associated with more frequent pulmonary parenchymal involvement and more aggressive systemic disease.34,36
Cutaneous lesions in sarcoidosis may also be precipitated by skin trauma. So-called scar sarcoidosis can occur in response to abrasions, punctures, or tattoos. Reactions to tattoos may form in response to 1 or multiple colors within a tattoo, and may develop even years after placement of the tattoo.37 Such reactions can be the initial presentation of sarcoidosis and should prompt the investigation of systemic manifestations of sarcoidosis. Granulomas may also occur in the subcutaneous tissue below otherwise normal-appearing skin, causing painless or only mildly tender nodules.38,39 Such nodules, which may be the presenting sign of sarcoidosis, can be evaluated by ultrasonography or MRI, and biopsy reveals granulomas within the panniculus.38,40 Most patients with subcutaneous sarcoidosis have hilar lymphadenopathy, and many are subsequently found to have granulomas in other organs.39,41 Recognition of skin lesions is important in sarcoidosis, because identification of the disease by skin biopsy may obviate more invasive diagnostic procedures.
Ophthalmologic
The eye is the third most frequently involved organ, affected in between 10% and 60% of patients. Granulomatous disease may cause inflammation either within the eye or in adnexal structures. Ocular involvement occurs at higher rates in women and African Americans, and seems more common in Japanese cohorts.6,42
Uveitis is the most common ocular manifestation and can be vision-threatening; thus, all patients diagnosed with sarcoidosis should have an ophthalmologic evaluation (Fig. 2). Symptoms of uveitis may include tearing, photophobia, pain, and injection; however, about one-third of patients with uveitis caused by sarcoidosis have no ocular symptoms.43 There are 2 peaks of incidence: the first in the third decade (more often associated with an acute course) and the latter in the sixth to seventh decade (more often associated with a chronic course).43 Ocular inflammation is most often bilateral, and the anterior segment is involved in 70% to 85% of cases.44 Involvement of the posterior segment occurs less frequently but is seen more often in whites, particularly elderly women, and is associated with a higher risk of central nervous system (CNS) involvement.43,44 Symptoms of blurry vision, hyperopia, visual field deficits, or floaters may suggest the development of retinal vasculitis, which in sarcoidosis is usually a retinal periphlebitis, sparing the retinal arteries.45 Uveitis occurring concomitant with fever, parotitis, and facial nerve paralysis has been termed uveoparotid fever or Heerfordt syndrome.
Fig. 2.
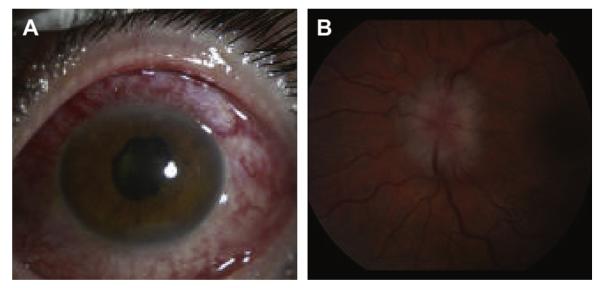
Examples of ocular sarcoidosis. (A) Scleritis in a patient with sarcoidosis. (B) Optic nerve swelling on fundoscopic exam. (Courtesy of Dr G. Papaliodis, Massachusetts Eye and Ear Infirmary, Boston, MA.)
Ocular inflammation may be the presenting symptom of sarcoidosis; however, showing granulomatous disease in the eye is often not feasible, because biopsy is generally not pursued in patients presenting with uveitis. Biopsy of another involved site, if available, is useful. Given the frequent lack of ocular histologic evidence, an international consensus conference delineated criteria for the diagnosis of ocular sarcoidosis, which include a description of 7 clinical signs on ophthalmologic examination suggestive of ocular sarcoidosis.42 The conference also outlined different levels of certainty regarding the diagnosis of ocular sarcoidosis based on ophthalmologic evaluation, laboratory investigation, and imaging (Table 2).42
Table 2.
Diagnosis of ocular sarcoidosis
| Certainty of Ocular Sarcoidosis Diagnosis42 | Findings |
|---|---|
| Definite | Uveitis compatible with sarcoidosis Biopsy of another organ supporting sarcoidosis |
| Presumed | Uveitis compatible with sarcoidosis Biopsy not performed Bilateral hilar adenopathy on chest imaging |
| Probable | 3 signs of uveitis compatible with sarcoidosis Biopsy not performed 2 other investigations supporting sarcoidosis: Chest computed tomography abnormalities Increased ACE or lysozyme level Abnormal liver enzyme tests Negative PPD in a BCG-vaccinated or previously PPD-positive patient |
| Possible | 4 signs of uveitis compatible with sarcoidosis Lung biopsy negative 2 other investigations supporting sarcoidosis: Chest computed tomography abnormalities Increased ACE or lysozyme level Abnormal liver enzyme tests Negative PPD in a BCG-vaccinated or previously PPD-positive patient |
In all cases, alternative causes of uveitis, in particular tuberculosis, must be excluded.
Abbreviations: BCG, bacille Calmette-Guérin; PPD, purified protein derivative.
Involvement of the orbit and adnexal structures is less common than uveitis, occurring in 8% to 27% of cases, and occurs independently of uveitis.46 Adnexal involvement occurs in the form of lacrimal gland infiltration, formation of an orbital mass, or less commonly, involvement of the lacrimal sac. The lacrimal gland is the most commonly affected site, with an estimated incidence of 5% to 16% in sarcoidosis.46 Patients may present with edema or erythema of the eyelid or symptoms of dry eye, which may mimic Sjögren syndrome. Progressive lacrimal gland disease may cause insufficient tear production; however, sicca symptoms do not necessarily correlate with lacrimal gland infiltration.47,48 Occasionally, patients may present with a palpable eyelid mass. A solid orbital mass may be caused by sarcoidosis; however, it is debated whether an isolated, solitary orbital granulomas should be considered sarcoidosis or a distinct entity.47
Neurologic
Neurologic symptoms affect an estimated 5% of patients with sarcoidosis and may also be the presenting manifestation of systemic sarcoidosis.31,49,50 Granulomatous inflammation can affect the cranial nerves, peripheral nerves, or brain parenchyma, and autopsy studies suggest that granulomas are frequently present in these areas in the absence of symptoms.8 Cranial nerve dysfunction is the most common neurologic manifestation. The facial nerve is the most frequently affected cranial nerve, followed by the optic and vestibulocochlear nerves, although any cranial nerve can be involved.51
Lesions occurring in the peripheral nervous system most often cause an axonal or sensory peripheral neuropathy, although sensory-motor and myopathic patterns are also seen.51 Suspected peripheral nerve lesions can be confirmed by evaluation with nerve conduction studies and electromyography. Histologically, granulomas form within the epineurium or perineurinum, frequently accompanied by some component of granulomatous angiitis.52 Involvement of the endoneurium may also occur, perhaps via inflammatory cell invasion along septae or via microvessels, which inflicts more severe injury to the nerve.52 Peripheral nervous system involvement tends to respond to corticosteroid treatment and carries a better prognosis than does CNS involvement.51
Granulomatous involvement of the brain parenchyma is one of the most serious complications of sarcoidosis. However, attributing neurologic dysfunction to sarcoidosis is challenging, particularly in the absence of identifiable granulomatous disease in other organs. Symptoms of headache, nausea, and ataxia raise suspicion for cerebellar or brainstem involvement. Visual impairment, diplopia, and seizures may also occur. The base of the brain is frequently affected, often with granulomatous infiltration of the hypothalamus and pituitary, leading to dysfunction of the hypothalamic-pituitary axis.53 Leptomeningeal involvement may yield an appearance of aseptic meningitis, and involvement of the spinal cord may result in myelopathy. Spinal cord involvement tends to occur in older patients with sarcoidosis and can be difficult to distinguish from cervical spondylosis.54 Neuropsychiatric symptoms are uncommon.53
Evaluation for neurosarcoidosis typically includes a lumbar puncture and brain MRI (Fig. 3). Cerebral spinal fluid analysis may reveal an increased cell count with a lymphocytic pleiocytosis, increased protein levels, and oligoclonal bands. A variety of lesions can be seen on brain MRI, including enhancing parenchymal lesions, leptomeningeal thickening or enhancement, and dural involvement.50 Periventricular white matter lesions may be easily mistaken for lesions from multiple sclerosis.55 A set of diagnostic criteria has been proposed to help classify patients as having definite, probable, or possible neurosarcoidosis (Table 3).49,56
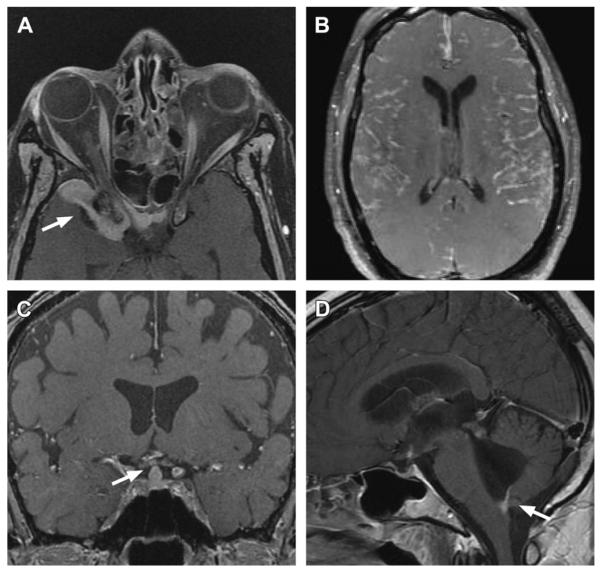
Fig. 3.
Examples of neurosarcoidosis. (A) Orbital and dural involvement (arrow) on gadolinium-enhanced T1-weighted fat-saturated MRI. (B) Diffuse nodular meningeal lesions on gadolinium-enhanced T1-weighted fat-saturated MRI. (C) Involvement of the pituitary infundibulum (arrow) on gadolinium-enhanced T1-weighted fat-saturated MRI. (D) Involvement of the foramen of Magendie (arrow) causing hydrocephalus. (Courtesy of Dr K. Talekar, Thomas Jefferson University Hospital, Philadelphia, PA.)
Table 3.
Diagnosis of neurosarcoidosis
| Certainty of Neurosarcoidosis Diagnosis49 | Findings |
|---|---|
| Definite | Presentation suggestive of neurosarcoidosis Nervous system histology with granulomatous disease |
| Probable | Presentation suggestive of neurosarcoidosis One abnormality indicating CNS inflammation: Increased CSF protein or cells CSF oligoclonal bands MRI findings consistent with sarcoidosis Either biopsy of another organ supporting sarcoidosis or 2 of the followinga: Increased ACE level Abnormal chest imaging Abnormal gallium scan |
| Possible | Presentation suggestive of neurosarcoidosis Criteria for probable neurosarcoidosis not met |
In all cases, alternative diagnoses must be excluded.
Abbreviation: CSF, cerebrospinal fluid.
Modifications have more recently been proposed to exclude the ACE level criterion and to include an increased CD4/CD8 T-cell ratio >3.5 in bronchoalveolar fluid or >5 in CSF.56
Musculoskeletal
Sarcoidosis may involve joints, muscles, and bones, causing a variety of musculoskeletal complaints through different mechanisms. Arthritic syndromes in sarcoidosis can be categorized as acute or chronic. Acute sarcoid arthritis often occurs concomitant with bilateral hilar lymphadenopathy and erythema nodosum, a constellation termed Löfgren syndrome. In Löfgren syndrome, the ankles are most commonly affected, followed by the knees, wrists, elbows, wrists, and metacarpophalangeal joints.57 Ultrasonographic evaluations have shown that swelling usually occurs in the soft tissue around joints, causing a periarthritis rather than a true arthritis.58 Joint symptoms tend to precede or occur concomitantly with development of erythema nodosum, and even in the absence of erythema nodosum, the combination of hilar lymphadenopathy and ankle periarthritis can be considered a variant of Löfgren syndrome.57,59
In a cohort of patients in the Netherlands, the presence of at least 3 of 4 criteria (symmetric ankle symptoms, age younger than 40 years, erythema nodosum, and symptoms of less than 2 months) had a 93% sensitivity and 99% specificity for the diagnosis of acute sarcoid arthritis.60 Enthesitis also occurred in about one-third of patients with sarcoidosis in this cohort, mainly at the Achilles tendon and heels.60 Soft tissue swelling at the ankles can be prominent, and biopsy of this soft tissue reveals a panniculitis similar to erythema nodosum, rather than granulomas.61,62 Löfgren syndrome carries an excellent prognosis. Symptoms typically resolve without therapy, and joint destruction does not occur; however, a small subset of patients continue to experience arthralgias after the acute inflammatory state has resolved.57
In contrast to acute arthritis, chronic arthritis in sarcoidosis usually occurs in the setting of more diffuse organ involvement.63 Ankles, knees, wrists, elbows, and hands may all be affected, often in a polyarticular pattern.63,64 Synovial fluid is usually noninflammatory or only mildly inflammatory, with a predominantly mononuclear infiltrate.65 When obtained, synovial biopsies may show synovial granulomas or nonspecific mononuclear infiltrates and synovial hypertrophy.65,66 Dactylitis similar to that seen in psoriatic arthritis, associated with pain, swelling, overlying skin erythema, and underlying bony changes may also occur.67 Rarely, Jaccoud arthropathy, a nonerosive deformity, develops.68,69
Bony lesions of osseous sarcoidosis occur in 3% to 13% of patients with sarcoidosis.31,70 The hands and feet are the most frequent sites of involvement, whereas the spine is less commonly affected (Fig. 4).71,72 Only about half of patients with bony lesions have pain and stiffness, whereas the other half remain asymptomatic.71 Radio-graphically, bone manifestations follow 3 distinct patterns, namely, lytic, permeative, and destructive. A lytic pattern results from focal areas of imbalanced bone destruction and bone formation, resulting in net bone resorption and cyst formation. Bone cysts are often associated with overlying skin disease, either on the hands and feet, for example associated with dactylitis, or on the face underlying skin lesions of lupus pernio.34,67,73 The second pattern, termed permeative, results in a tunneled, reticular appearance of the cortex. The third pattern, termed destructive, is uncommon and causes severe bone damage and fractures. In some cases, MRI may be more sensitive in detecting bony lesions; however, sarcoidosis lesions on MRI can be difficult to distinguish from bony metastasis.74
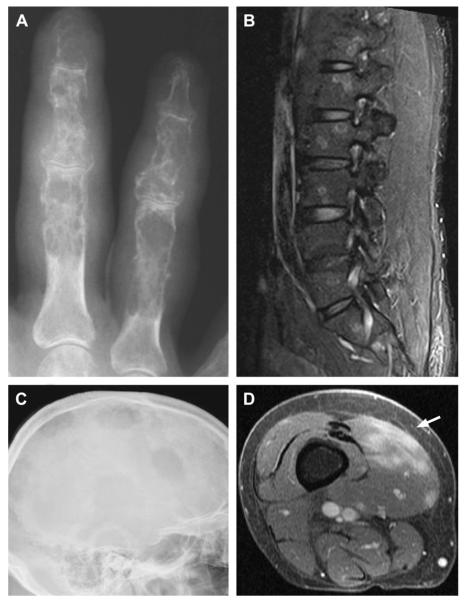
Fig. 4.
Examples of musculoskeletal involvement in sarcoidosis. (A) Punched-out cortical lesions and coarsened trabeculae yield a characteristic lacelike pattern in the proximal, middle, and distal phalanges. (B) Focal targetlike lesions in the vertebral bodies seen on sagittal short-tau inversion recovery sequence MRI. (C) Multiple large, lytic lesions within the skull of a patient with sarcoidosis. (D) Lobular lesions (arrow) within the vastus medialis muscle on gadolinium-enhanced MRI. (Courtesy of [A, C, D] Dr S. Smith, Brigham and Women’s Hospital, Boston, MA; and [B] Dr K. Talekar, Thomas Jefferson University Hospital, Philadelphia, PA.)
Sarcoid myopathy occurs clinically in less than 5% of patients; however, granulomatous involvement of muscle can be found histologically in most patients with sarcoidosis.75 Muscle involvement occurs in 3 general forms: acute myopathy, chronic myopathy, and nodular myopathy. Chronic myopathy, the most common form of sarcoid myopathy, typically causes symmetric proximal weakness. Creatine kinase levels are often normal but can be markedly increased.76 Electromyography shows a myopathic pattern with abnormal spontaneous activity with fibrillation potentials.76 MRI of affected muscles shows nonspecific muscle edema or fatty atrophy and is unlikely to indicate a specific diagnosis; however, such imaging may guide muscle biopsy site selection to avoid sampling atrophic areas.77
Additional imaging with radionucleotide scanning can be useful in sarcoidosis.67 Gallium scanning has been reported to identify extensive areas of granulomatous muscle infiltration in the absence of significant musculoskeletal symptoms.10 PET scanning has also been suggested to have usefulness in identifying muscle inflammation and in monitoring response to therapy.78,79 Histologically, granulomas in sarcoid myopathies show a pattern similar to that seen in other organs, with collections of macrophages and activated CD4+ T cells throughout the granulomas.80 In contrast to patterns seen in polymyositis, CD4+ cells outnumber CD8+ cells in sarcoid myopathy, and muscle fibers do not express increased levels of major histocompatibility complex class I.80
Nodular myopathy is an uncommon form of sarcoid myopathy characterized by the accumulation of large granulomas and dense connective tissue into nodules. These lesions, which can resemble tumors, may be painful but rarely cause weakness. On MRI, nodules are long and display a distinctive pattern on T2-weighted axial images of a dark, star-shaped lesion surrounded by a bright rim.81
Acute myopathy is the least common form of sarcoid myopathy. It presents with a rapid onset of proximal weakness and myalgias over several weeks, often associated with fever. The presentation may appear similar to polymyositis, with serum creatine kinase levels usually, although variably, increased.82 Biopsy of acute sarcoid myopathy shows pronounced lymphocytic infiltration associated with granulomas.82
Gastrointestinal
Sarcoidosis frequently involves the liver; however, a variety of insults may induce liver granulomas; thus, it is important to distinguish granulomas caused by sarcoidosis from those with other causes. Generally, granulomatous disease in a second organ, plus exclusion of alternate causes, is needed to consider a patient with liver granulomas to have sarcoidosis.83 Based on observations from autopsies and liver biopsies, granulomas within the liver can be found in 50% to 80% of patients with sarcoidosis, although the fraction of patients with clinically evident liver disease is smaller.83 As with the eye, skin, and bone marrow, liver involvement is more common in African American patients than whites.6 Symptoms from liver involvement include abdominal pain, pruritus, and less commonly, jaundice and ascites.83 Increase in liver enzyme tests occur in about one-third of patients with sarcoidosis.84
Sarcoidosis may cause biliary disease and cholestasis via several mechanisms. Pathologic patterns resembling either sclerosing cholangitis or biliary cirrhosis have been described. Chronic damage may result in ductopenia, or loss of small biliary ducts, and once this stage is reached, treatment is unlikely to improve function. Granulomatous disease rarely progresses to cirrhosis; however, portal hypertension may still occur caused by granulomatous obstruction of blood flow through the portal circulation, or through development of portal vein thrombosis or Budd-Chiari syndrome.83,85
It is uncommon for sarcoidosis to affect the luminal gastrointestinal tract. When it occurs, the gastric antrum is the area most frequently affected.83 Symptoms may include abdominal pain, nausea, early satiety, and progressive weight loss. Even without involvement of the gut wall, symptoms of obstruction may be caused by external compression of the gut lumen by enlarged lymph nodes or adjacent large granulomas.83 Granulomas of sarcoidosis can be found within the pancreas in approximately 5% of patients with sarcoidosis.86 Nodular lesions may infiltrate the pancreas diffusely or cause a mass in the head of the pancreas. The lesions are rarely symptomatic, but when significant, may cause pain, jaundice, anorexia, and increase in serum lipase levels.
Renal
Granulomatous involvement of the kidney can be found in 7% to 23% of patients with sarcoidosis; however, significant renal impairment is less common.86,87 The most common lesion is a granulomatous interstitial nephritis, which usually occurs in the setting of more diffuse disease and is associated with hypercalcemia and hypergammaglobulinemia.88,89 Sterile pyuria, proteinuria, and microscopic hematuria may occur.87 Rarely, a granulomatous pseudotumor may develop, mimicking malignancy in 1 or both kidneys.90
Hypercalcemia is common in sarcoidosis, occurring in 10% to 20% of patients, as a result of excess conversion of 25(OH) vitamin D to 1,25(OH) vitamin D by macrophage 1-α-hydroxylase within granulomas.91 Hypercalciuria occurs in half of patients with sarcoidosis and predisposes to nephrolithiasis, which develops in about 10% to 15% of patients.87,89 Symptomatic nephrolithiasis may be the initial indication of systemic sarcoidosis in approximately 1% of sarcoidosis cases.92
TREATMENT
General Principles
It is important to recognize that not all patients with sarcoidosis require immunosuppressive treatment. Patients with acute presentations of Löfgren syndrome usually remit spontaneously and require only symptomatic therapy with nonsteroidal antiinflammatory drugs (NSAIDs). Patients with stable pulmonary function without symptoms and limited disease elsewhere, including an absence of cardiac, neurologic, and vision-threatening ocular involvement, may be monitored closely without therapy and often maintain a stable course.93 On the other hand, patients with progressive pulmonary disease or cardiac, neurologic, or ocular involvement require prompt treatment. The mainstay of treatment of sarcoidosis is glucocorticoid therapy. Steroids can be used to treat virtually all manifestations of sarcoidosis; however, it is not clear that corticosteroid treatment has any beneficial effect on long-term disease outcomes.94 Higher doses are used for neurologic and cardiac involvement, whereas lower doses usually suffice for other affected organs. Efforts should be made to minimize the potential complications of glucocorticoids by using the lowest effective dose, using local therapy when possible, and promptly initiating adjunctive therapies to prevent glucocorticoid-induced bone loss and opportunistic infections.
In cases in which the steroid dose cannot be tapered without disease recurrence, or when steroid therapy is not tolerated, steroid-sparing agents should be considered. Methotrexate has been frequently used in these situations and can be effective in both pulmonary and extrapulmonary disease.95 Antimalarial agents such as chloroquine and hydroxychloroquine are also effective, particularly for cutaneous disease and hypercalcemia.96 Use of immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclophosphamide has also been described, although controlled trials are lacking. Given the importance of tumor necrosis factor (TNF) in granuloma formation, anti-TNF agents are increasingly being used to treat sarcoidosis.97,98 The anti-TNF antibodies infliximab and adalimumab seem to be more effective than the soluble receptor etanercept.99 However, reports of sarcoidosislike granulomatous reactions in patients treated with anti-TNF therapies for other indications have prompted heightened caution with this approach.97,100 Recently, attention has also focused on the role of rituximab in treating sarcoidosis, with reports of efficacy in both ocular sarcoidosis and neurosarcoidosis.101,102 In the following sections, some of the organ-specific considerations of sarcoidosis treatment are discussed.
Pulmonary
Patients with little or no change in respiratory symptoms and pulmonary function tests over the preceding 3 months can be monitored closely without corticosteroid treatment.93 Patients with progressive disease based on symptoms, lung function tests, or radiography should be treated with systemic cortico steroids, usually in the range of 0.5 to 1 mg/kg of prednisone, tapered slowly over the course of a year. Inhaled corticosteroids have not been shown to reduce pulmonary disease but may help reduce cough and symptoms of airway hyperresponsiveness.94,103
Cardiac
Higher doses of corticosteroids, often in the range of 1 to 1.5 mg/kg of prednisone, are used to suppress cardiac inflammation and fibrosis, with the goal of limiting damage to the conduction system, reducing arrhythmias, and preventing the development of systolic dysfunction. Steroids are tapered slowly, and relapse once off steroids is not uncommon.104 Optimal strategies for monitoring disease activity while tapering steroids have not been established, although serial evaluation by PET and monitoring of circulating cardiac troponin T have been proposed.27,28 High-grade conduction system disease or complete heart block necessitates permanent pacemaker implantation. Placement of an implantable cardioverter-defibrillator (ICD) should be pursued in patients with sustained ventricular tachycardia or ventricular fibrillation and those who meet other standard criteria.105 ICD implantation can also be considered in patients with cardiac sarcoidosis and evidence of active disease on cardiac imaging, although there is no consensus on this issue.104 Symptoms of heart failure are managed with ACE inhibitors, β-blockers, and diuretics as with other causes of dilated cardiomyopathies. Cardiac transplantation is rarely necessary; however, when performed, transplants performed for cardiac sarcoidosis fare as well if not better than those performed for most other indications.106
Neurologic
Parenchymal brain disease also requires higher-dose corticosteroids, usually in the range of 1 to 1.5 mg/kg. Severe or rapidly progressive symptoms are treated initially with pulse-dose intravenous solumedrol in the range of 1 g per day for 3 days, followed by oral prednisone. Some have recommended continued weekly pulse-dose solumedrol for several weeks until symptoms are controlled.49 Close monitoring of symptoms and serial evaluation of MRI abnormalities are monitored during steroid tapering. Multiple steroid-sparing agents have been used, with methotrexate and hydroxychloroquine being the most common.49 Experiences with azathioprine, cyclophosphamide, cyclosporine, and mycophenolate mofetil have also been described, although controlled data are lacking. Anti-TNF therapy with infliximab has been reported to have efficacy in multiple case reports and small case series.97 Patients with neurosarcoidosis should be monitored frequently for signs of hypothalamicpituitary dysfunction and treated with hormone replacement as needed. Development of hydrocephalus may require placement of a ventriculoperitoneal shunt, although this carries an increased risk of CNS infection, in particular in immunosuppressed patients.53 Cranial nerve disease and aseptic meningitis can be treated with less aggressive corticosteroid doses, often in the range of 0.5 to 1 mg/kg.51
Cutaneous
Treatment of cutaneous lesions is aimed at limiting disfigurement. Initial management is usually attempted with topical corticosteroids. Lesions on the trunk and extremities can be treated with high-potency topical corticosteroids with occlusion, whereas medium-potency corticosteroids are preferred on the face to reduce the risk of atrophy. However, facial lupus pernio lesions are particularly resistant to treatment and often require high-potency treatments. Lesions that do not respond to topical therapy can be treated with intralesional corticosteroids. For diffuse disease or lesions not responding to local therapies, systemic therapies are initiated. Recently, an algorithm has been described that suggests trying the steroid-sparing agents hydroxychloroquine, methotrexate, or tetracycline antibiotics first for mild to moderate disease, then moving to systemic corticosteroids, followed by combination therapy.30 On the other hand, for severe disease, corticosteroids plus a steroid-sparing agent, such as methotrexate, hydroxychloroquine, or mycophenolate mofetil, are tried first, followed by an anti-TNF for treatment failures. For lupus pernio, retrospective analyses suggest that anti-TNF antibodies are more effective than corticosteroids; therefore, accelerated use of anti-TNF antibodies has been suggested if lupus pernio does not respond to corticosteroids.30,107 Other agents that have been described include thalidomide, isotretinoin, allopurinol, and photodynamic therapy.
Ophthalmologic
Anterior uveitis is often treated first with topical corticosteroid eye drops. Cycloplegic agents are used in parallel to prevent formation of synechiae. Patients who do not respond to topical steroids may be treated with systemic steroids, usually at doses of 1 to 1.5 mg/kg of prednisone. Uveitis in the posterior compartment generally requires systemic therapy, given the increased risk of CNS involvement.108 Optic neuritis also merits treatment with systemic corticosteroids. For chronic or refractory uveitis, several steroid-sparing agents can be considered, although there are few published data to support their efficacy.99 Methotrexate has been reported to be an effective steroid-sparing agent in panuveitis.109 Mycophenolate mofetil, azathioprine, cyclosporine, and anti-TNF therapies are also used.99,110 Recently, rituximab was reported to improve granulomatous eye disease in 3 of 4 patients with ocular sarcoidosis.102 Granulomatous disease in the lacrimal gland or orbit usually responds to systemic corticosteroids.
Musculoskeletal
Acute sarcoid arthritis can be treated symptomatically with high-dose NSAIDs alone and does not require corticosteroids. If control is inadequate, low-dose corticosteroids in the range of 10 to 20 mg per day of prednisone are often effective.99 In a randomized clinical trial, methotrexate was shown to be effective in reducing musculoskeletal symptoms in acute sarcoidosis.111 Although controlled data are lacking, methotrexate seems helpful in chronic arthritis as well.112 Additional options include hydroxychloroquine, azathioprine, sulfasalazine, and biological therapies. Recently, an algorithmic approach to management of sarcoid arthritis incorporating these agents has been proposed.112
Gastrointestinal
Treatment of granulomatous liver disease in sarcoidosis is of uncertain usefulness, because corticosteroid treatment has not been clearly shown to improve abnormal liver enzyme tests.85 Corticosteroids may be tried for symptomatic liver disease with intermediate doses in the range of 0.5 to 1 mg/kg, tapered slowly over the course of a year. Treatment with ursodeoxycholic acid, a naturally occurring bile acid, has been suggested to reduce symptoms and biochemical abnormalities of cholestasis in patients with hepatic sarcoidosis.113–115
Renal
All patients with sarcoidosis with hypercalcemia should be cautioned to limit dietary calcium, avoid vitamin D supplementation and sun exposure, and limit oxalate intake to reduce the risk of kidney stone formation.87 Hypercalcemia usually requires corticosteroids, which can lower serum calcium within a few days.99,116 Hydroxychloroquine can also be particularly effective in treating hypercalcemia.117–119 Patients treated with hydroxychloroquine should undergo routine ophthalmologic evaluation monitoring for the accumulation of retinal deposits, although the risk is low with less than 5 years of therapy.120,121 Persistent hypercalcemia can be treated with ketoconazole, which inhibits macrophage 1 α-hydroxylase, the enzyme that converts 25(OH) vitamin D to calcitriol in sarcoidosis granulomas.87,122 Granulomatous interstitial nephritis tends to respond to corticosteroid therapy in the range of 1 mg/kg.87,123
SUMMARY
Granulomatous infiltration in systemic sarcoidosis may cause dysfunction in almost any organ; however, decades of observations have helped define the more common manifestations of sarcoidosis in various organs. An understanding of the typical patterns of involvement is critical for early detection and treatment of disease. Sarcoidosis is characteristically responsive to corticosteroids, which remain the mainstay of therapy. Disease-modifying antiinflammatory drugs, including anti-TNF agents, play an increasing role in managing refractory disease; however, further studies are required to clarify the roles of these agents in the treatment of systemic sarcoidosis.
KEY POINTS.
Sarcoidosis is a systemic granulomatous disease that most commonly affects the lungs, skin, and eyes.
All patients with sarcoidosis should be evaluated for cardiac involvement, which may lead to life-threatening arrhythmias.
Although many patients can be monitored without treatment, those with worsening pulmonary disease or cardiac, neurologic, or vision-threatening ocular disease require prompt therapy.
Most manifestations of sarcoidosis can be treated with corticosteroids, with the highest doses used for cardiac and neurologic involvement.
Disease-modifying antirheumatic drugs such as methotrexate and biological therapies such as antitumor necrosis factor agents are increasingly being used for refractory disease.
ACKNOWLEDGMENTS
We thank Dr Joseph Merola and Dr Rebecca Hunter for helpful discussions and critical review of parts of the article. We are grateful to Dr Joseph Merola, Dr George Papaliodis, Dr Stacy Smith, and Dr Kiran Talekar for generous image contributions.
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Pietinalho A, Hiraga Y, Hosoda Y, et al. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis. 1995;12:61–7. [PubMed] [Google Scholar]
- 4.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA. Genetics of sarcoidosis: candidate genes and genome scans. Proc Am Thorac Soc. 2007;4:108–16. doi: 10.1513/pats.200607-141JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 7.Costabel U. CD4/CD8 ratios in bronchoalveolar lavage fluid: of value for diagnosing sarcoidosis? Eur Respir J. 1997;10:2699–700. doi: 10.1183/09031936.97.10122699. [DOI] [PubMed] [Google Scholar]
- 8.Manz HJ. Pathobiology of neurosarcoidosis and clinicopathologic correlation. Can J Neurol Sci. 1983;10:50–5. doi: 10.1017/s0317167100044577. [DOI] [PubMed] [Google Scholar]
- 9.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 10.Suehiro S, Shiokawa S, Taniguchi S, et al. Gallium-67 scintigraphy in the diagnosis and management of chronic sarcoid myopathy. Clin Rheumatol. 2003;22:146–8. doi: 10.1007/s10067-002-0686-x. [DOI] [PubMed] [Google Scholar]
- 11.Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–30. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 12.Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145–50. doi: 10.1097/ACI.0b013e3283515173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badrinas F, Morera J, Fite E, et al. Seasonal clustering of sarcoidosis. Lancet. 1989;2:455–6. doi: 10.1016/s0140-6736(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 14.Hills SE, Parkes SA, Baker SB. Epidemiology of sarcoidosis in the Isle of Man–2: evidence for space-time clustering. Thorax. 1987;42:427–30. doi: 10.1136/thx.42.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkes SA, Baker SB, Bourdillon RE, et al. Epidemiology of sarcoidosis in the Isle of Man–1: a case controlled study. Thorax. 1987;42:420–6. doi: 10.1136/thx.42.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownell I, Ramirez-Valle F, Sanchez M, et al. Evidence for mycobacteria in sarcoidosis. Am J Respir Cell Mol Biol. 2011;45:899–905. doi: 10.1165/rcmb.2010-0433TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta D, Agarwal R, Aggarwal AN, et al. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30:508–16. doi: 10.1183/09031936.00002607. [DOI] [PubMed] [Google Scholar]
- 18.Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26–31. [PubMed] [Google Scholar]
- 19.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–35. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 20.Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–9. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 21.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–90. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Matoh F, Satoh H, Shiraki K, et al. The usefulness of delayed enhancement magnetic resonance imaging for diagnosis and evaluation of cardiac function in patients with cardiac sarcoidosis. J Cardiol. 2008;51:179–88. doi: 10.1016/j.jjcc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Bussinguer M, Danielian A, Sharma OP. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652–64. doi: 10.1007/s11936-012-0208-3. [DOI] [PubMed] [Google Scholar]
- 24.Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 25.Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6. [PubMed] [Google Scholar]
- 26.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 27.Keijsers RG, Heuvel DA, Grutters JC. Imaging the inflammatory activity of sarcoidosis. Eur Respir J. 2012 doi: 10.1183/09031936.00088612. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Baba Y, Kubo T, Kitaoka H, et al. Usefulness of high-sensitive cardiac troponin T for evaluating the activity of cardiac sarcoidosis. Int Heart J. 2012;53:287–92. doi: 10.1536/ihj.53.287. [DOI] [PubMed] [Google Scholar]
- 29.Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 30.Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. Cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1–e18. doi: 10.1016/j.jaad.2011.11.965. [quiz: 717–8] [DOI] [PubMed] [Google Scholar]
- 31.Siltzbach LE, James DG, Neville E, et al. Course and prognosis of sarcoidosis around the world. Am J Med. 1974;57:847–52. doi: 10.1016/0002-9343(74)90160-0. [DOI] [PubMed] [Google Scholar]
- 32.Honeybourne D. Ethnic differences in the clinical features of sarcoidosis in South-East London. Br J Dis Chest. 1980;74:63–9. [PubMed] [Google Scholar]
- 33.Marcoval J, Mana J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol. 2011;36:739–44. doi: 10.1111/j.1365-2230.2011.04128.x. [DOI] [PubMed] [Google Scholar]
- 34.Spiteri MA, Matthey F, Gordon T, et al. Lupus pernio: a clinico-radiological study of thirty-five cases. Br J Dermatol. 1985;112:315–22. doi: 10.1111/j.1365-2133.1985.tb04859.x. [DOI] [PubMed] [Google Scholar]
- 35.Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295–302. doi: 10.1016/j.clindermatol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Yanardag H, Pamuk ON, Pamuk GE. Lupus pernio in sarcoidosis: clinical features and treatment outcomes of 14 patients. J Clin Rheumatol. 2003;9:72–6. doi: 10.1097/01.RHU.0000062509.01658.d1. [DOI] [PubMed] [Google Scholar]
- 37.Antonovich DD, Callen JP. Development of sarcoidosis in cosmetic tattoos. Arch Dermatol. 2005;141:869–72. doi: 10.1001/archderm.141.7.869. [DOI] [PubMed] [Google Scholar]
- 38.Vainsencher D, Winkelmann RK. Subcutaneous sarcoidosis. Arch Dermatol. 1984;120:1028–31. [PubMed] [Google Scholar]
- 39.Ahmed I, Harshad SR. Subcutaneous sarcoidosis: is it a specific subset of cutaneous sarcoidosis frequently associated with systemic disease? J Am Acad Dermatol. 2006;54:55–60. doi: 10.1016/j.jaad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Chen HH, Chen YM, Lan HH, et al. Sonographic appearance of subcutaneous sarcoidosis. J Ultrasound Med. 2009;28:813–6. doi: 10.7863/jum.2009.28.6.813. [DOI] [PubMed] [Google Scholar]
- 41.Dalle Vedove C, Colato C, Girolomoni G. Subcutaneous sarcoidosis: report of two cases and review of the literature. Clin Rheumatol. 2011;30:1123–8. doi: 10.1007/s10067-011-1731-4. [DOI] [PubMed] [Google Scholar]
- 42.Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop on Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–9. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 43.Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol. 2000;84:110–6. doi: 10.1136/bjo.84.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obenauf CD, Shaw HE, Sydnor CF, et al. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–55. doi: 10.1016/0002-9394(78)90184-8. [DOI] [PubMed] [Google Scholar]
- 45.Androudi S, Dastiridou A, Symeonidis C, et al. Retinal vasculitis in rheumatic diseases: an unseen burden. Clin Rheumatol. 2013;32(1):7–13. doi: 10.1007/s10067-012-2078-1. [DOI] [PubMed] [Google Scholar]
- 46.Demirci H, Christianson MD. Orbital and adnexal involvement in sarcoidosis: analysis of clinical features and systemic disease in 30 cases. Am J Ophthalmol. 2011;151:1074–1080.e1. doi: 10.1016/j.ajo.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Prabhakaran VC, Saeed P, Esmaeli B, et al. Orbital and adnexal sarcoidosis. Arch Ophthalmol. 2007;125:1657–62. doi: 10.1001/archopht.125.12.1657. [DOI] [PubMed] [Google Scholar]
- 48.Evans M, Sharma O, LaBree L, et al. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. 2007;114:325–33. doi: 10.1016/j.ophtha.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 49.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis–diagnosis and management. QJM. 1999;92:103–17. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 50.Shah R, Roberson GH, Cure JK. Correlation of MR imaging findings and clinical manifestations in neurosarcoidosis. AJNR Am J Neuroradiol. 2009;30:953–61. doi: 10.3174/ajnr.A1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gascon-Bayarri J, Mana J, Martinez-Yelamos S, et al. Neurosarcoidosis: report of 30 cases and a literature survey. Eur J Intern Med. 2011;22:e125–32. doi: 10.1016/j.ejim.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Said G, Lacroix C, Plante-Bordeneuve V, et al. Nerve granulomas and vasculitis in sarcoid peripheral neuropathy: a clinicopathological study of 11 patients. Brain. 2002;125:264–75. doi: 10.1093/brain/awf027. [DOI] [PubMed] [Google Scholar]
- 53.Joseph FG, Scolding NJ. Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009;80:297–304. doi: 10.1136/jnnp.2008.151977. [DOI] [PubMed] [Google Scholar]
- 54.Sakushima K, Yabe I, Nakano F, et al. Clinical features of spinal cord sarcoidosis: analysis of 17 neurosarcoidosis patients. J Neurol. 2011;258:2163–7. doi: 10.1007/s00415-011-6080-3. [DOI] [PubMed] [Google Scholar]
- 55.Scott TF, Yandora K, Kunschner LJ, et al. Neurosarcoidosis mimicry of multiple sclerosis: clinical, laboratory, and imaging characteristics. Neurologist. 2010;16:386–9. doi: 10.1097/NRL.0b013e3181b287df. [DOI] [PubMed] [Google Scholar]
- 56.Marangoni S, Argentiero V, Tavolato B. Neurosarcoidosis. Clinical description of 7 cases with a proposal for a new diagnostic strategy. J Neurol. 2006;253:488–95. doi: 10.1007/s00415-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 57.Gran JT, Bohmer E. Acute sarcoid arthritis: a favourable outcome? A retrospective survey of 49 patients with review of the literature. Scand J Rheumatol. 1996;25:70–3. doi: 10.3109/03009749609069210. [DOI] [PubMed] [Google Scholar]
- 58.Kellner H, Spathling S, Herzer P. Ultrasound findings in Lofgren’s syndrome: is ankle swelling caused by arthritis, tenosynovitis or periarthritis? J Rheumatol. 1992;19:38–41. [PubMed] [Google Scholar]
- 59.Mana J, Gomez-Vaquero C, Salazar A, et al. Periarticular ankle sarcoidosis: a variant of Lofgren’s syndrome. J Rheumatol. 1996;23:874–7. [PubMed] [Google Scholar]
- 60.Visser H, Vos K, Zanelli E, et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Ann Rheum Dis. 2002;61:499–504. doi: 10.1136/ard.61.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grunewald J, Eklund A. Sex-specific manifestations of Lofgren’s syndrome. Am J Respir Crit Care Med. 2007;175:40–4. doi: 10.1164/rccm.200608-1197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatham W. Rheumatic manifestations of systemic disease: sarcoidosis. Curr Opin Rheumatol. 2010;22:85–90. doi: 10.1097/BOR.0b013e328333ba74. [DOI] [PubMed] [Google Scholar]
- 63.Pettersson T. Rheumatic features of sarcoidosis. Curr Opin Rheumatol. 1997;9:62–7. doi: 10.1097/00002281-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Abril A, Cohen MD. Rheumatologic manifestations of sarcoidosis. Curr Opin Rheumatol. 2004;16:51–5. doi: 10.1097/00002281-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Palmer DG, Schumacher HR. Synovitis with non-specific histological changes in synovium in chronic sarcoidosis. Ann Rheum Dis. 1984;43:778–82. doi: 10.1136/ard.43.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokoloff L, Bunim JJ. Clinical and pathological studies of joint involvement in sarcoidosis. N Engl J Med. 1959;260:841–7. doi: 10.1056/NEJM195904232601701. [DOI] [PubMed] [Google Scholar]
- 67.Pitt P, Hamilton EB, Innes EH, et al. Sarcoid dactylitis. Ann Rheum Dis. 1983;42:634–9. doi: 10.1136/ard.42.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lima I, Ribeiro DS, Cesare A, et al. Typical Jaccoud’s arthropathy in a patient with sarcoidosis. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2318-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Sukenik S, Hendler N, Yerushalmi B, et al. Jaccoud’s-type arthropathy: an association with sarcoidosis. J Rheumatol. 1991;18:915–7. [PubMed] [Google Scholar]
- 70.Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321–30. doi: 10.1097/00002281-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 71.Neville E, Carstairs LS, James DG. Sarcoidosis of bone. Q J Med. 1977;46:215–27. [PubMed] [Google Scholar]
- 72.Boyaci B, Hornicek F, Rosenthal D, et al. Sarcoidosis of the spine: a report of five cases and a review of the literature. J Bone Joint Surg Am. 2012;94:e42. doi: 10.2106/JBJS.K.00062. [DOI] [PubMed] [Google Scholar]
- 73.Shorr AF, Murphy FT, Gilliland WR, et al. Osseous disease in patients with pulmonary sarcoidosis and musculoskeletal symptoms. Respir Med. 2000;94:228–32. doi: 10.1053/rmed.1999.0709. [DOI] [PubMed] [Google Scholar]
- 74.Moore SL, Kransdorf MJ, Schweitzer ME, et al. Can sarcoidosis and metastatic bone lesions be reliably differentiated on routine MRI? AJR Am J Roentgenol. 2012;198:1387–93. doi: 10.2214/AJR.11.7498. [DOI] [PubMed] [Google Scholar]
- 75.Silverstein A, Siltzbach LE. Muscle involvement in sarcoidosis. Asymptomatic, myositis, and myopathy. Arch Neurol. 1969;21:235–41. doi: 10.1001/archneur.1969.00480150025002. [DOI] [PubMed] [Google Scholar]
- 76.Le Roux K, Streichenberger N, Vial C, et al. Granulomatous myositis: a clinical study of thirteen cases. Muscle Nerve. 2007;35:171–7. doi: 10.1002/mus.20683. [DOI] [PubMed] [Google Scholar]
- 77.Moore SL, Teirstein A, Golimbu C. MRI of sarcoidosis patients with musculoskeletal symptoms. AJR Am J Roentgenol. 2005;185:154–9. doi: 10.2214/ajr.185.1.01850154. [DOI] [PubMed] [Google Scholar]
- 78.Marie I, Lahaxe L, Vera P, et al. Follow-up of muscular sarcoidosis using fluorodeoxyglucose positron emission tomography. QJM. 2010;103:1000–2. doi: 10.1093/qjmed/hcq055. [DOI] [PubMed] [Google Scholar]
- 79.Marie I, Levesque H, Manrique A, et al. Positron emission tomography in the diagnosis of muscular sarcoidosis. Am J Med. 2007;120:e1–2. doi: 10.1016/j.amjmed.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 80.Tews DS, Pongratz DE. Immunohistological analysis of sarcoid myopathy. J Neurol Neurosurg Psychiatry. 1995;59:322–5. doi: 10.1136/jnnp.59.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otake S. Sarcoidosis involving skeletal muscle: imaging findings and relative value of imaging procedures. AJR Am J Roentgenol. 1994;162:369–75. doi: 10.2214/ajr.162.2.8310929. [DOI] [PubMed] [Google Scholar]
- 82.Fujita H, Ishimatsu Y, Motomura M, et al. A case of acute sarcoid myositis treated with weekly low-dose methotrexate. Muscle Nerve. 2011;44:994–9. doi: 10.1002/mus.22222. [DOI] [PubMed] [Google Scholar]
- 83.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103:3184–92. doi: 10.1111/j.1572-0241.2008.02202.x. [quiz: 93] [DOI] [PubMed] [Google Scholar]
- 84.Vatti R, Sharma OP. Course of asymptomatic liver involvement in sarcoidosis: role of therapy in selected cases. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:73–6. [PubMed] [Google Scholar]
- 85.Valla D, Pessegueiro-Miranda H, Degott C, et al. Hepatic sarcoidosis with portal hypertension. A report of seven cases with a review of the literature. Q J Med. 1987;63:531–44. [PubMed] [Google Scholar]
- 86.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from the Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 87.Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006;48:856–70. doi: 10.1053/j.ajkd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 88.Bergner R, Hoffmann M, Waldherr R, et al. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:126–32. [PubMed] [Google Scholar]
- 89.Lebacq E, Desmet V, Verhaegen H. Renal involvement in sarcoidosis. Postgrad Med J. 1970;46:526–9. doi: 10.1136/pgmj.46.538.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.La Rochelle JC, Coogan CL. Urological manifestations of sarcoidosis. J Urol. 2012;187:18–24. doi: 10.1016/j.juro.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 91.Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med. 2000;6:442–7. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50:555–9. doi: 10.1136/thx.50.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hunninghake GW, Gilbert S, Pueringer R, et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med. 1994;149:893–8. doi: 10.1164/ajrccm.149.4.8143052. [DOI] [PubMed] [Google Scholar]
- 94.Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA. 2002;287:1301–7. doi: 10.1001/jama.287.10.1301. [DOI] [PubMed] [Google Scholar]
- 95.Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax. 1999;54:742–6. doi: 10.1136/thx.54.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baughman RP, Lower EE. Steroid-sparing alternative treatments for sarcoidosis. Clin Chest Med. 1997;18:853–64. doi: 10.1016/s0272-5231(05)70423-8. [DOI] [PubMed] [Google Scholar]
- 97.Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:76–89. [PubMed] [Google Scholar]
- 98.Egen JG, Rothfuchs AG, Feng CG, et al. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–84. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray PI, Bodaghi B, Sharma OP. Systemic treatment of sarcoidosis. Ocul Immunol Inflamm. 2011;19:145–50. doi: 10.3109/09273948.2010.542870. [DOI] [PubMed] [Google Scholar]
- 100.Massara A, Cavazzini L, La Corte R, et al. Sarcoidosis appearing during anti-tumor necrosis factor alpha therapy: a new “class effect” paradoxical phenomenon. Two case reports and literature review. Semin Arthritis Rheum. 2010;39:313–9. doi: 10.1016/j.semarthrit.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Bomprezzi R, Pati S, Chansakul C, et al. A case of neurosarcoidosis successfully treated with rituximab. Neurology. 2010;75:568–70. doi: 10.1212/WNL.0b013e3181ec7ff9. [DOI] [PubMed] [Google Scholar]
- 102.Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol. 2012;6:1613–8. doi: 10.2147/OPTH.S35521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198–204. [PubMed] [Google Scholar]
- 104.Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–46. doi: 10.1016/j.pcad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 105.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 106.Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant. 2007;26:714–7. doi: 10.1016/j.healun.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Stagaki E, Mountford WK, Lackland DT, et al. The treatment of lupus pernio: results of 116 treatment courses in 54 patients. Chest. 2009;135:468–76. doi: 10.1378/chest.08-1347. [DOI] [PubMed] [Google Scholar]
- 108.Rose AS, Tielker MA, Knox KS. Hepatic, ocular, and cutaneous sarcoidosis. Clin Chest Med. 2008;29:509–24. ix. doi: 10.1016/j.ccm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 109.Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology. 1999;106:111–8. doi: 10.1016/S0161-6420(99)90011-8. [DOI] [PubMed] [Google Scholar]
- 110.Wakefield D, Zierhut M. Controversy: ocular sarcoidosis. Ocul Immunol Inflamm. 2010;18:5–9. doi: 10.3109/09273941003597276. [DOI] [PubMed] [Google Scholar]
- 111.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60–6. [PubMed] [Google Scholar]
- 112.Sweiss NJ, Patterson K, Sawaqed R, et al. Rheumatologic manifestations of sarcoidosis. Semin Respir Crit Care Med. 2010;31:463–73. doi: 10.1055/s-0030-1262214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Becheur H, Dall’osto H, Chatellier G, et al. Effect of ursodeoxycholic acid on chronic intrahepatic cholestasis due to sarcoidosis. Dig Dis Sci. 1997;42:789–91. doi: 10.1023/a:1018816214640. [DOI] [PubMed] [Google Scholar]
- 114.Baratta L, Cascino A, Delfino M, et al. Ursodeoxycholic acid treatment in abdominal sarcoidosis. Dig Dis Sci. 2000;45:1559–62. doi: 10.1023/a:1005560927060. [DOI] [PubMed] [Google Scholar]
- 115.Alenezi B, Lamoureux E, Alpert L, et al. Effect of ursodeoxycholic acid on granulomatous liver disease due to sarcoidosis. Dig Dis Sci. 2005;50:196–200. doi: 10.1007/s10620-005-1300-2. [DOI] [PubMed] [Google Scholar]
- 116.Singer DR, Evans DJ. Renal impairment in sarcoidosis: granulomatous nephritis as an isolated cause (two case reports and review of the literature) Clin Nephrol. 1986;26:250–6. [PubMed] [Google Scholar]
- 117.Adams JS, Diz MM, Sharma OP. Effective reduction in the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Ann Intern Med. 1989;111:437–8. doi: 10.7326/0003-4819-111-5-437. [DOI] [PubMed] [Google Scholar]
- 118.Barre PE, Gascon-Barre M, Meakins JL, et al. Hydroxychloroquine treatment of hypercalcemia in a patient with sarcoidosis undergoing hemodialysis. Am J Med. 1987;82:1259–62. doi: 10.1016/0002-9343(87)90237-3. [DOI] [PubMed] [Google Scholar]
- 119.O’Leary TJ, Jones G, Yip A, et al. The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med. 1986;315:727–30. doi: 10.1056/NEJM198609183151203. [DOI] [PubMed] [Google Scholar]
- 120.Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003;110:1321–6. doi: 10.1016/S0161-6420(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 121.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:775–84. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 122.Adams JS, Sharma OP, Diz MM, et al. Ketoconazole decreases the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J Clin Endocrinol Metab. 1990;70:1090–5. doi: 10.1210/jcem-70-4-1090. [DOI] [PubMed] [Google Scholar]
- 123.Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009;88:98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]