Abstract
Purpose
Running and other strenuous sports activities are purported to increase osteoarthritis (OA) risk, more so than walking and less-strenuous activities. Analyses were therefore performed to test whether running, walking, and other exercise affect OA and hip replacement risk, and to assess BMI’s role in mediating these relationships.
Methods
Proportional hazards analyses of patients’ report of having physician-diagnosed OA and hip replacement vs. exercise energy expenditure (metabolic equivalents, METs).
Results
74,752 runners reported 2004 OA and 259 hip replacements during 7.1-year follow-up, while the 14,625 walkers reported 695 OA and 114 hip replacements over 5.7 years. Compared to running <1.8 METhr/d, the risks for OA and hip replacement decreased: 1) 18.1% (P=0.01) and 35.1% (P=0.03), respectively, for 1.8 to 3.6 METhr/d run; 2) 16.1% (P=0.03) and 50.4% (P=0.002), respectively, for 3.6 to 5.4 METhr/d run; and 3) 15.6% (P=0.02) and 38.5% (P=0.01), respectively, for ≥5.4 METhr/d run, suggesting that the risk reduction mostly occurred by 1.8 METhr/d. Baseline BMI was strongly associated with both OA (5.0% increase per kg/m2, P=2x10−8) and hip replacement risks (9.8% increase per kg/m2, P=4.8x10−5), and adjustment for BMI substantially diminished the risk reduction from running ≥1.8 METhr/d for OA (from 16.5%, P=0.01 to 8.6%, P=0.21) and hip replacement (from 40.4%, P=0.005 to 28.5%, P=0.07). The reductions in OA and hip replacement risk by exceeding 1.8 METhr/d did not differ significantly between runners and walkers. Other (non-running) exercise increased the risk of OA by 2.4% (P=0.009) and hip replacement by 5.0% per METhr/d (P=0.02), independent of BMI.
Conclusions
Whereas other exercise increased OA and hip replacement risk, running significantly reduced their risk due, in part, to running’s association with lower BMI.
Keywords: Prevention, exercise, epidemiology, cohort study
Osteoarthritis (OA) is the leading cause of disability in the aged, affecting between 7% and 25% of Caucasians >55 years old, and is projected to become the fourth most common medical condition in women [11]. There is a common perception that, over the long run, running is injurious to joints [11]. Running and vigorous sports activity do, in fact, increase the risk of knee trauma and injuries, which in turn are known risk factors for knee OA [11]. Alternatively, running reduces body weight [38,39], a known risk factor for OA [3], which may offset in part its pro OA-effects, resulting in diminished OA risk or even OA-protection. Exercise may also promote cartilage thickening [12,13] and prevent the loss cartilage proteoglycans [30,34], which provide cartilage’s viscoelastic properties [22]. These effects are important because cartilage thinning [11] and focal loss of proteoglycans [5] are prominent features of OA.
Case-control studies suggest a positive relationship between OA and physical sporting activities in general, and running in particular, with an odds ratio of approximately 2 for both [20]. There are prospective studies in humans that claim that exercise increases [2,3,6,16,23,31,32,37], has no significant effect [1,5,9,10,17,27], or decreases the risks for HIP replacement or OA [18,24]. There are also animal studies suggesting that exercise increases [14] or decreases OA risk [7,29].
With nearly 90,000 participants, the National Runners’ and Walkers’ Health Studies are the largest prospective cohorts specifically recruited for the study of health benefits and risk of physical activity. Their data provide a unique opportunity to test whether OA and hip replacement risk are: 1) increased by running, walking, and other exercise in relation to amount and intensity; 2) increased more for running than walking; and 3) reduced as a consequence of the leanness of higher mileage runners and walkers.
Methods
The current analyses involve data from follow-up of three cohorts: the first and second National Runners’ Health Study cohorts (NRHS-I and NRHS-II) and the National Walkers’ Health Study (NWHS) [38,39,40]. NRHS-I was recruited between 1991 and 1994 (primarily 1993), and follow-up questionnaires were obtained between 1999 and 2002 [38,39]. Eighty percent of NRHS-I provided follow-up information or were known deceased. The NRHS-II and NWHS were recruited primarily between 1998 and 2001 [40]. The 2006 partial re-survey of the National Runners’ Health Study II and the National Walkers’ Health Study [40] were obtained to identify and qualify approximately 50,000 runners and walkers for a proposed clinical trial, rather than a prospective follow-up study per se. These represented approximately a third of the original walker (33.2%), and one-half of the original runner surveyed (51.7%). Questions for ascertaining distance run and walked, body weight and height, and waist circumference, and their reproducibility, have been previously described [38,39]. The study protocols were approved by the University of California Berkeley committee for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Participants reported whether a physician had told them they had osteoarthritis and if they had a hip replacement since their baseline questionnaire and reported the year of diagnosis or replacement. Diagnoses and replacements dated the same year as the baseline survey or earlier were excluded in order that the end points represented only newly diagnosed incidence of disease. Usual pace (minutes per mile) was reported for walking in walkers, and for running in NRHS-II runners. Non-running energy expenditures in the runners, and non-walking energy expenditures in the walkers, were calculated using published tables of metabolic equivalents (MET) [40], where one MET is the energy expended sitting at rest (3.5 ml O2•kg−1•min−1). Physical activities that expended 3 to 6 METs were classified as moderate-intensity exercise, >6 METs as vigorous, <3 METs as light [40]. In walkers, METhr/d walked was calculated by converting reported distance into duration (i.e., distance/mph) which was then multiplied by the MET value for the reported pace. In runners, METhr/d run was calculated as km run*1.02 METhr/km [40]. Statistical analyses (Cox proportional hazard analyses) were performed using JMP (SAS institute, Cary NC, version 5.1). Hazard ratios (HR) and 95% confidence intervals (95% CI) were reported for the risk of incident OA and hip replacement per METhr/d of energy spent running, walking, and other exercise, running performance, race participation, and kg/m2 of body mass index (BMI) using covariates identified as being associated with OA or hip replacement risk.
Results
Of the 91,928 subjects (15,945 walkers, 42,923 NRHS-I runners, 33,060 NRHS-II runners) who were eligible for analyses, we eliminated 1789 for pre-existing OA or hip replacement, and 762 for unknown dates for their diagnosis, leaving 89,377 subjects (14,625 walkers, runners, 42,443 NRHS-I, 32,309 NRHS-II runners) for analyses. There were 2004 runners (1175 NRHS-I, 829 NRHS-II) who reported being diagnosed by a physician for OA, and 259 runners (159 NRHS-I, 100 NRHS-II) who reported receiving a hip replacement during their (mean±SD) 7.1±1.8 year follow-up. Walkers reported 696 and 114 diagnoses for OA and hip replacement, respectively, during their 5.7±1.2 year follow-up. The runners’ and walkers’ baseline characteristics are displayed in Table 1.
Table 1.
Characteristics (mean±SD) of runners and walkers by self-reported physician diagnosed osteoarthritis.
| Runners | Walkers | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| N | 46,819 | 27,933 | 3,122 | 11,503 |
| Incident OA (cases) | 1141 | 863 | 74 | 621 |
| Incident hip replacement (cases) | 208 | 51 | 36 | 78 |
| Age (years) | 46.09±10.73 | 39.89±10.38 | 61.48±11.15 | 52.45±11.95 |
| Education (years) | 16.59±2.46 | 16.15±2.34 | 16.33±2.69 | 15.28±2.53 |
| Smokers (%) | 1.33 | 1.65 | 3.40 | 3.59 |
| Meat (serving/d) | 0.42±0.38 | 0.26±0.30 | 0.46±0.41 | 0.37±0.34 |
| Fruit (pieces/d) | 1.56±1.19 | 1.58±1.07 | 1.62±1.23 | 1.69±1.13 |
| Alcohol (g/d) | 10.03±13.93 | 5.80±8.37 | 9.15±13.39 | 4.89±8.98 |
| Body mass index (kg/m2) | 23.91±2.54 | 21.43±2.42 | 26.58±4.01 | 25.30±5.01 |
| Exercise energy expenditure (METhr/d) | ||||
| Running | 5.44±3.24 | 4.98±3.09 | ||
| Walking | 2.20±1.65 | 2.17±1.64 | ||
| Other exercise | 2.45±3.64 | 2.90±3.82 | 2.14±3.69 | 1.84±3.25 |
| Years run or walked | 13.20±8.13 | 9.81±6.76 | 11.30±10.42 | 8.30±7.61 |
| Marathons (# in 5 years) | 1.95±4.26 | 1.15±2.82 | ||
| 10-km performance (m/s) | 3.89±0.55 | 3.42±0.53 | ||
Older age was the strongest risk factor, increasing OA risk by 3.9% per year in men (95% CI: 3.3 to 4.4%, P<10−15) and 6.1% in women (95% CI: 5.5 to 6.8%, P<10−15), and hip replacement risk by 7.4% per year in men (95% CI: 6.1 to 8.7%, P<10−15) and 10.6% in women (95% CI: 8.1 to 13.0%, P<10−15). The age-adjusted OA risk was significantly greater for women than men (HR: 1.86; 95% CI: 1.70 to 2.06, P<10−15), whereas the risk for hip replacement was not (P=0.06). The best age-adjustment model for OA included separate intercepts, and separate linear and quadratic regression terms for men and women. When adjusted for age, neither menstrual status (OA risk: P=0.57, hip replacement: P=0.95) nor estrogen use (OA risk: P=0.76, hip replacement: P=0.44) affected risk, whereas estrogen/progesterone hormone use did for OA (HR: 1.34; 95% CI: 1.08 to 1.65, P=0.008) but not hip replacement (P=0.72). The risk for OA also increased with years of education (HR: 1.03; 95% CI: 1.01 to 1.04 per year, P=0.007) and intake of red meat (HR: 1.17; 95% CI: 1.04 to 1.33 per serving/d, P=0.01), but not fruit (P=0.41) or alcohol intake (P=0.09) or smoking (P=0.62). Based on these results, the analyses that follow were all adjusted for running cohort (NRHS-I vs. NRHS-II), sex, sex-specific age effects (age, age2), education, estrogen/progesterone hormone use, and intake of red meat.
Usual running distance
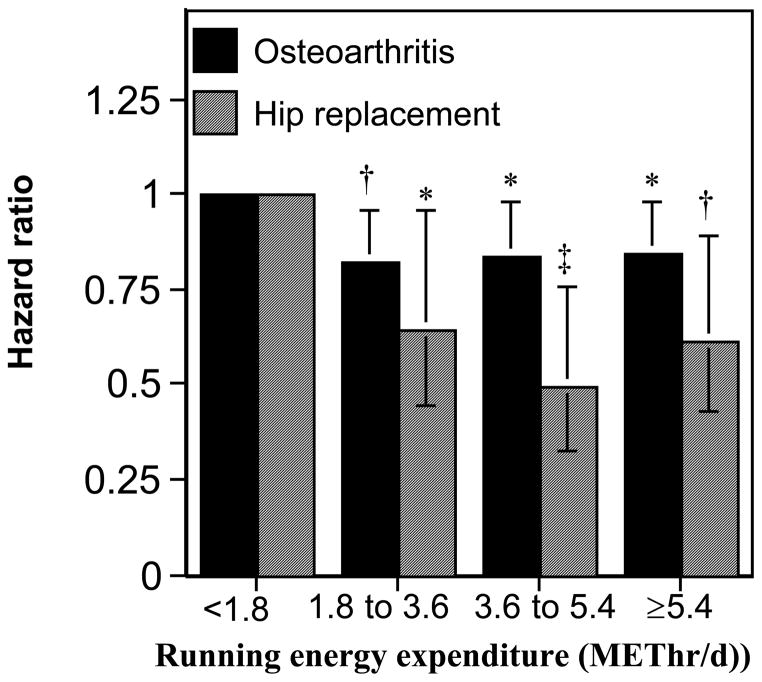
Figure 1 displays the reductions in the risks for OA and hip replacement by METhr/d run. Compared to running <1.8 METhr/d, OA risk decreased 18.1% for those between 1.8 and 3.6 METhr/d (P=0.01), 16.1% between 3.6 and 5.4 METhr/d (P=0.03), and 15.6% ≥5.4 METhr/d (P=0.02). The corresponding values for hip replacement risk were 35.1% (P=0.03), 50.4% (P=0.002), and 38.5% (P=0.01), respectively. Most of risk reduction appeared to have occurred by 1.8 METhr/d for both OA and hip replacement.
Figure 1.
Reduction in the risks for incident OA and hip replacement per METhr/d run, adjusted for age, sex, education, hormone use in women, and meat intake. Energy expenditure (X-axis) is categorized in terms of the upper limit of the minimum recommended physical activity levels (750 METmin/wk=1.8 METhr/d), e.g., 1 to 2-fold higher activity covers from 1.8 to 3.6 METhr/d, etc. Error bars represent 95% confidence intervals. Significant levels relative to the least active runners coded: * P<0.05; † P<0.01, and ‡ P=0.002.
Marathon and 10-km footrace
There were 43,782 subjects who never ran a marathon during the previous 5 years, 22,705 that ran at least one during the previous 5 years but less than one marathon annually, 5,753 who ran between 1 and less than 2 marathons annually, 2,170 who ran between 2 and less than 5 marathons annually, and 342 who ran 5 or more marathons annually. Marathon frequency at baseline was unrelated to both OA risk (HR 1.00, 95% CI: 0.99 to 1.01 per marathon, P=0.43) and hip replacement risk (HR 0.99, 95% CI: 0.96 to 1.02 per marathon, P=0.69). Best marathon performance during the previous five years was also unrelated to both OA risk (HR 1.09, 95% CI: 0.95 to 1.25 per m/s, P=0.22) and hip replacement risk (HR 1.15, 95% CI: 0.77 to 1.70 per m/s, P=0.57). Similarly, 10-km race performance times did not affect the risks for OA (HR 0.91, 95% CI: 0.82 to 1.01 per m/s, P=0.08) or hip replacement (HR 0.98, 95% CI: 0.73 to 1.31 per m/s, P=0.88).
Running intensity, longest run, and running history
Baseline usual running speed, longest usual run, and other vigorous and moderate intensity exercise were only collected for the second runners cohort (N=32,309), whose 6.4-year follow-up included 829 incident OA and 100 hip replacements. In this subset, OA risk was not increased by usual running speed (HR: 0.86, 95% CI: 0.72 to 1.01 per m/s, P=0.07), or longest usual run (HR: 1.00, 95% CI: 0.98 to 1.02 per km, P=0.98). Similarly, the risk for hip replacement was unrelated to usual running speed (HR: 0.67, 95% CI: 0.43 to 1.07 per m/s, P=0.09) or longest usual run (HR: 0.99, 95% CI: 0.93 to 1.04 per km, P=0.63). Running history (years run) appears to increase the risk for hip replacement (HR: 1.02, 95% CI: 1.00 to 1.03 per km, P=0.04) but not OA (HR: 1.00, 95% CI: 0.99 to 1.00 per year, P=0.50)
Other exercise
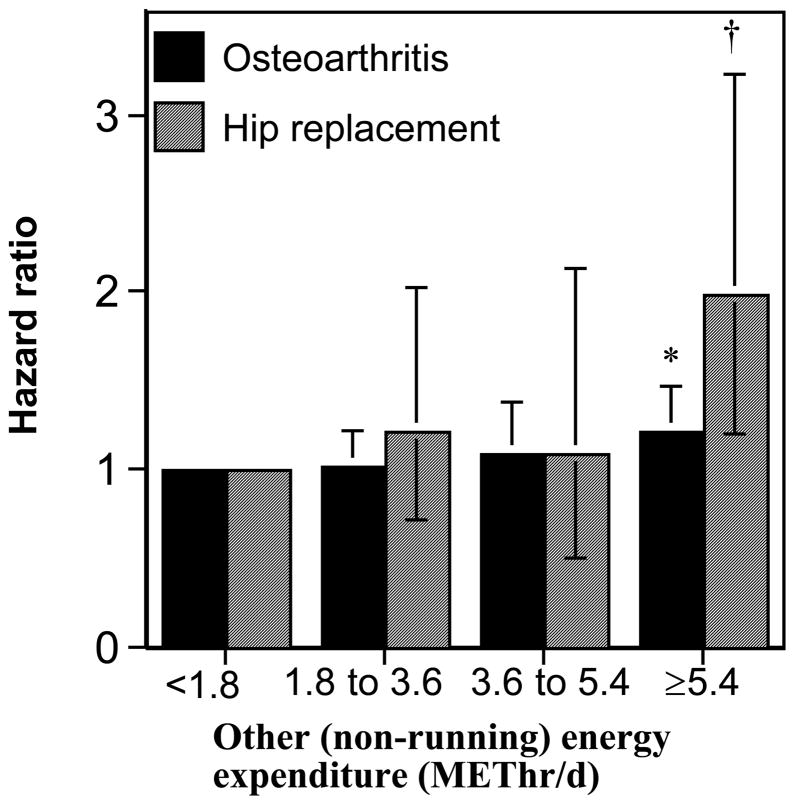
For both OA and hip replacement, baseline other (non running) exercise in the runners was associated with their increased risk (OA HR: 1.02, 95% CI: 1.01 to 1.04 per METhr/d, P=0.009; hip replacement HR: 1.05, 95% CI: 1.01 to 1.08 per METhr/d, P=0.02). Exercising ≥5.4 METhr/d other than running was associated with a 21% increased risk for incident OA (P=0.05) and 99% increased risk for hip replacement (P=0.009) as compared to <1.8 METh/d of other exercise (Figure 2). This association was specific to other vigorous exercise for hip replacement (other vigorous HR: 1.05, 95% CI: 1.01 to 1.11 per METhr/d, P=0.03; moderate intensity HR: 1.04, 95% CI: 0.93 to 1.12 per METhr/d, P=0.43), less so for OA (other vigorous HR:1.02, 95% CI: 1.00 to 1.04 per METhr/d, P=0.05; moderate intensity HR:1.04, 95% CI: 1.00 to 1.07 per METhr/d, P=0.03).
Figure 2.
Increase in the risks for incident OA and hip replacement per METhr/d of other (non-running) exercise adjusted for age, sex, education, hormone use in women, and meat intake. Error bars represent 95% confidence intervals. Significant levels relative to the runners who did the least other exercise: * P<0.05; † P<0.01.
Running vs. Walking
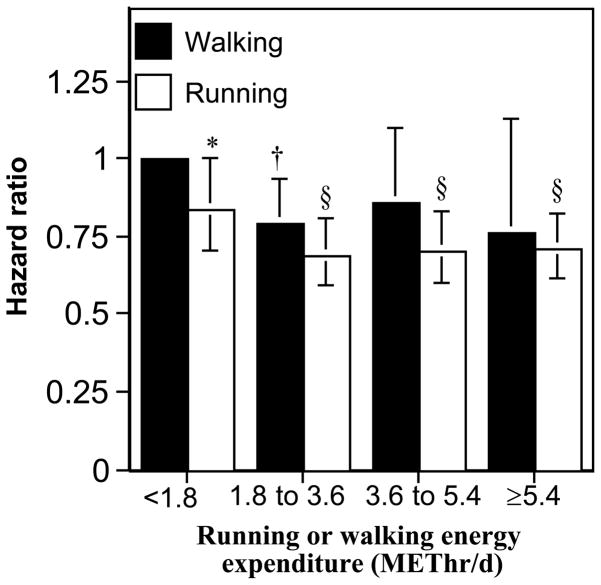
Figure 3 shows that most of the walkers’ reduction in OA risk also occurred by 1.8 METs, i.e., those that exceeded 1.8 METhr/d were at 18.3% lower risk than less active walkers (P=0.008). They were also at 23.2% lower risk for hip replacement, but for 114 replacements this did not achieve statistical significance (P=0.16). The figure compares the risks for incident OA of the runners to those of walkers. Runners who ran less than 1.8 METhr/d were at lower risk than walkers who walked less than 1.8 METhr/d, and this difference in risk appeared to be sustained at all activity levels. The decrease in risk from exceeding 1.8 METhr/d was not significantly different for running vs. walking for either OA (P=0.68 for difference) or hip replacement (P=0.31). Thus the risk for OA was reduced significantly by exceeding 1.8 METhr/d by walking or running, and their effects appeared comparable.
Figure 3.
Reduction in the risks for incident OA and hip replacement per METhr/d run or walked adjusted for age, sex, education, hormone use in women, and meat intake. Error bars represent 95% confidence intervals. Significant levels relative to the least active walkers (the referent group) coded: * P<0.05; † P<0.01, ‡ P<0.001 and § P<0.0001.
Effects of body weight
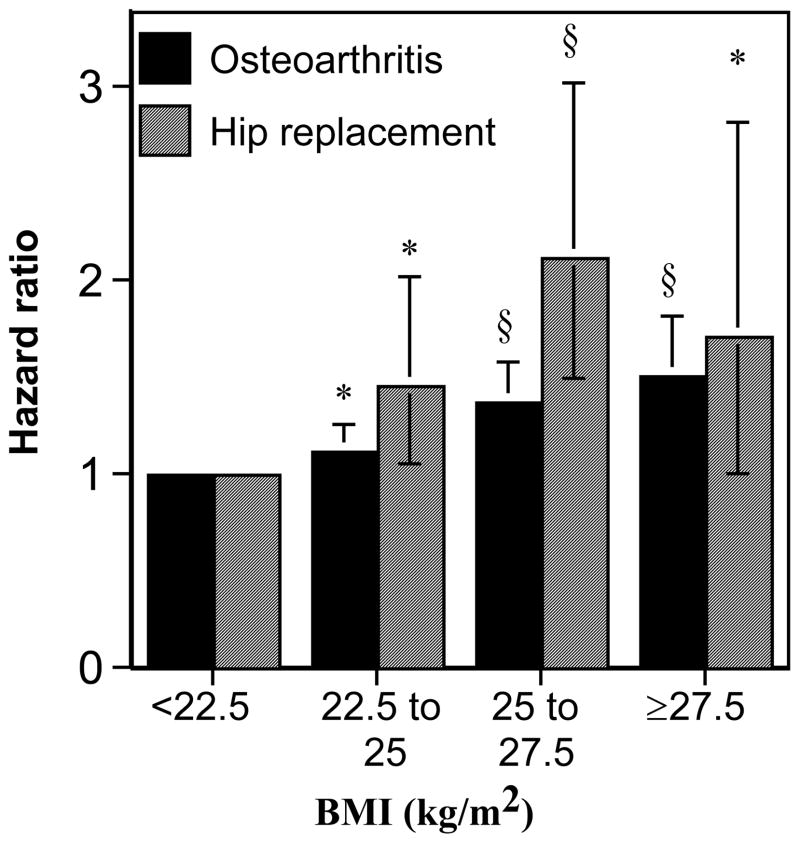
On average, the risk for OA increased 5.0% (P=2x10−8) and hip replacement increased 9.8% (P=4.8x10−5) per kg/m2 increase in BMI. Figure 4 displays the relationship of baseline BMI to incident OA and hip replacement during follow-up. Compared to subjects <22.5 kg/m2, OA risk increased 12.19% for those between 22.5 and 25.0 kg/m2 (P=0.04), 37.5% between 25 and 27.5 kg/m2 (P=5x10−6), and 50.4% ≥27.5 kg/m2 (P=4.5x10−5). The corresponding values for hip replacement risk were 44.8% (P=0.02), 111.8% (P=3.3x10−5), and 71% (P=0.05), respectively. Adjustment for BMI diminished the decrease in risk from running ≥1.8 METhr/d from 16.5% (P=0.01) to 8.6% (P=0.21) for OA, and from 40.4% (P=0.005) to 28.5% (P=0.07) for hip replacement, and diminished the decrease in risk from walking ≥1.8 METhr/d from 18.3% (P=0.008) to 10.3% (P=0.17) for OA. However, adjustment for BMI did not eliminate the effects of other (non-running) exercise on OA (adjusted HR: 1.02, 95% CI: 1.00 to 1.04 per METhr/d, P=0.01) or hip replacement (adjusted HR: 1.05, 95% CI: 1.01 to 1.08 per METhr/d, P=0.02).
Figure 4.
Increase in the risks for incident OA and hip replacement by BMI adjusted for age, sex, education, hormone use in women, and meat intake. Error bars represent 95% confidence intervals. Significant levels relative to the leanest runners coded: * P<0.05; † P<0.01, ‡ P<0.001 and § P<0.0001.
Discussion
The number of runners studied here is larger than any prior study of physical activity and OA and hip replacement, and exceeds by over an order of magnitude the number of runners previously studied in all prior cross-sectional studies combined. Included among these were 863 runners who reported running ≥60 miles per week. Contrary to many previous reports [2,3,6,16,23,31,32,37], we find no evidence than running increases the risk of OA, including participation in marathon races, and, in fact, subjects that ran ≥1.8 METhr/d (≥12.4 km/wk) were at significantly lower risk for both OA and hip replacement. The reduction in risk for running ≥1.8METhr/d more than compensates for the 1.6% per year increase risk increase for hip replacement during the first 21 years. Moreover, there was no particular advantage to walking rather than running in reducing OA and hip replacement risk. In fact, runners were more likely to benefit from less OA and fewer hip replacements because a greater proportion exceeded 1.8 METhr/d (89.5% vs. 52.8%).
Previous studies suggesting a protective role for physical activity are much fewer than those showing a risk increase or no effect. In one, joint space loss was observed in nonrunners but not runners, suggesting that running preserved cartilage thickness [18]. In another, knee replacements decreased with increasing cumulative hours of recreational physical activity [24]. Our data even showed that neither marathon frequency, marathon intensity, nor 10-km intensity predicted any increase risk for OA or hip replacement, in contrast to Michaelsson et al report that skiers who repeatedly participated in a 90 km ski race increased OA risk in proportion to the number of races run and performance (speed) [26].
The OA-protective effects of running or walking appeared have already occurred by 1.8 METhr/d, suggesting the association may be due primarily to increased OA in the least active individuals. Articular cartilage thickness is reduced in animals subject to prolonged immobolization [36]. Cartilage is also thinned in the absence of normal joint loading in spinal cord injury patients [35]. In children, articular cartilage volume is increased in association with vigorous physical activity and muscle strength cross-sectionally [13], and those who engaged in more intense sport gained more cartilage over time [12]. Triathletes have thicker patellae cartilage than inactive subjects, albeit thinner medial femoral condyle cartilage [28]. Some [4], but not all [8], studies suggest that physical activity may enlarge the knee joint surface area in adults. Glycosaminoglycans are used in the synthesis of proteoglycans, which provide cartilage’s viscoelastic properties [22]. Early OA consists of a focal loss of proteoglycans [5]. Running increases the glycosaminoglycan content of human knee cartilage [34]. Roos and Dahlberg’s randomized trial showed that exercise produced a healthier distribution of proteoglycans in cartilage vis-à-vis non-exercising control [30]. Animal studies also suggest that the patellar cartilage of sedentary hamsters have a lower proteoglycan content than those that are active [29]. Moderate exercise has also been shown to inhibit the development of surgically induced OA in the rat [7]. In dogs, however, shifting from moderate to strenuous running eliminated increases in cartilage thickness and proteoglycan content produced with moderate running [14].
Our analyses showed that in contrast to running, other (non-running) exercise increased the risks for both OA and hip replacement. This result is consistent with >2-fold greater prevalence of tibiofemoral or patellofemoral OA in soccer players (29%) and weight lifters (31%) than runners (14%) reported by Kujala et al. [15]. Research on occupational activity show that OA is more common in jobs requiring knee-bends, kneeling, or squats [25], which may be more characteristic of exercise performed in gyms, circuit training, and aerobic classes than running or walking. Work-related knee bending exposure increases the odds for knee OA by up to 6-fold [21].
Our analyses confirmed the well-established association between BMI and incident risk of OA and hip replacement even within the purported healthy weight range, and attributed 45% and 28% of the running associated decrease in OA and hip replacement to BMI, respectively. In addition to promoting weight loss directly [39], running attenuates middle-age weight gain [38], such that higher mileage runners gain only half as much as low mileage runners. The prevention of weight gain is an additional mechanism for limiting risk OA and hip replacement risk. Body weight has a much weaker association with other exercise than with running [40], which may explain in part their different associations with OA and hip replacement, particularly given that adjustment for BMI did not affect the concordance between baseline other exercise and both OA and hip replacement.
There are important limitations to these analyses that warrant acknowledgement. The results are based on self-reported physician diagnosed OA and hip replacement rather than medical chart review or imaging. However, reviews suggest stronger associations have been reported for clinically assessed hip OA than its radiographic assessment [20]. Patient self-report of physician-diagnosed arthritis has been found by others to be the best predictor of radiologically ascertained OA, showing 64% specificity, a 57% positive predictive value, and 71% negative predictive value [33]. We do not believe that the declining incidence of OA and hip replacement with greater METhr/d walked or run was due to fewer opportunities for diagnosis in the more athletic men. The Health Professionals Follow-up Study reported that their more vigorously active participants had more routine medical check-ups than less active men [19]. It is possible that there is a higher pain threshold in longer distance runners, but it is unclear why this would not also be true for other exercise as well. It is unclear whether the exclusion of pre-existing injury would be warranted in assessing the OA risk in runners, if such injuries were the consequence of the exercise per se. Finally, we acknowledge that the analyses would have benefited from the complete follow-up of NRHS-II and NWHS. Heretofore, we have been unable to secure funding for their follow-up, and there is no evidence that the NRHS-I (80% follow-up) and NRHS-II (51.7% follow-up) show different relationships between METhr/d run and the risks for OA (P=0.45 for difference) or hip replacement (P=0.89 for difference). The lower follow-up of the walkers (33.2%) than NRHS-II (51.7) reflected our recruitment priorities rather than differences in the responsiveness of the walkers and runners, however we do not believe that this affected the comparison of walkers and runners given that comparable results were obtained when the analyses were restricted to the initial 33.2% of the NRHS-II runners recruited.
In conclusion, these results may not apply to truly elite athletes, but for recreational runners who even substantially exceed current guideline activity levels, and participate in multiple marathons annually, running does not appear to increase OA and hip replacement risk, and may, in fact, be preferable to other exercise.
Acknowledgments
This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have declared that no competing interests exist.
Disclosures
The authors have declared that no competing interests exist. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Ageberg E, Engström G, Gerhardsson de Verdier M, et al. Effect of leisure time physical activity on severe knee or hip osteoarthritis leading to total joint replacement: a population-based prospective cohort study. BMC Musculoskelet Disord. 2012;13:73. doi: 10.1186/1471-2474-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? J Clin Epidemiol. 2000;53:315–22. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Eckstein F, Faber S, Mühlbauer R, et al. Functional adaptation of human joints to mechanical stimuli. Osteoarthritis Cartilage. 2002;10:44–50. doi: 10.1053/joca.2001.0480. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum. 2007;57:6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 7.Galois L, Etienne S, Grossin L, et al. Moderate-impact exercise is associated with decreased severity of experimental osteoarthritis in rats. Rheumatology (Oxford) 2003;42:692–3. doi: 10.1093/rheumatology/keg094. [DOI] [PubMed] [Google Scholar]
- 8.Gratzke C, Hudelmaier M, Hitzl W, Glaser C, Eckstein F. Knee cartilage morphologic characteristics and muscle status of professional weight lifters and sprinters: a magnetic resonance imaging study. Am J Sports Med. 2007;35:1346–53. doi: 10.1177/0363546507299746. [DOI] [PubMed] [Google Scholar]
- 9.Hannan MT, Felson DT, Anderson JJ, Naimark A. Habitual physical activity is not associated with knee osteoarthritis: the Framingham Study. J Rheumatol. 1993;20:704–9. [PubMed] [Google Scholar]
- 10.Hootman JM, Macera CA, Helmick CG, Blair SN. Influence of physical activity-related joint stress on the risk of self-reported hip/knee osteoarthritis: a new method to quantify physical activity. Prev Med. 2003;36:636–44. doi: 10.1016/s0091-7435(03)00018-5. [DOI] [PubMed] [Google Scholar]
- 11.Hunter DJ, Eckstein F. Exercise and osteoarthritis. J Anat. 2009;214:197–207. doi: 10.1111/j.1469-7580.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G, Ding C, Glisson M, Hynes K, Ma D, Cicuttini F. Knee articular cartilage development in children: a longitudinal study of the effect of sex, growth, body composition, and physical activity. Pediatr Res. 2003;54:230–6. doi: 10.1203/01.PDR.0000072781.93856.E6. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000;43:2543–9. doi: 10.1002/1529-0131(200011)43:11<2543::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Kiviranta I, Tammi M, Jurvelin J, Arokoski J, Säämänen AM, Helminen HJ. Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin Orthop Relat Res. 1992;283:302–8. [PubMed] [Google Scholar]
- 15.Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38:539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 16.Lane NE, Hochberg MC, Pressman A, Scott JC, Nevitt MC. Recreational physical activity and the risk of osteoarthritis of the hip in elderly women. J Rheumatol. 1999;26:849–54. [PubMed] [Google Scholar]
- 17.Lane NE, Michel B, Bjorkengren A, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. J Rheumatol. 1993;20:461–8. [PubMed] [Google Scholar]
- 18.Lane NE, Oehlert JW, Bloch DA, Fries JF. The relationship of running to osteoarthritis of the knee and hip and bone mineral density of the lumbar spine: a 9 year longitudinal study. J Rheumatol. 1998;25:334–41. [PubMed] [Google Scholar]
- 19.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lievense AM, Bierma-Zeinstra SM, Verhagen AP, Bernsen RM, Verhaar JA, Koes BW. Influence of sporting activities on the development of osteoarthritis of the hip: a systematic review. Arthritis Rheum. 2003;49:228–36. doi: 10.1002/art.11012. [DOI] [PubMed] [Google Scholar]
- 21.Lievense AM, Bierma-Zeinstra SMA, Verhagen AP, Verhaar JAN, Koes B. Influence of work on the development of osteoarthritis of the hip: a systematic review. J Rheumatol. 2001;28:2520–8. [PubMed] [Google Scholar]
- 22.Lu XL, Sun DD, Guo XE, Chen FH, Lai WM, Mow VC. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Ann Biomed Eng. 2004;32:370–9. doi: 10.1023/b:abme.0000017534.06921.24. [DOI] [PubMed] [Google Scholar]
- 23.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 24.Manninen P, Riihimaki H, Heliovaara M, Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford) 2001;40:432–7. doi: 10.1093/rheumatology/40.4.432. [DOI] [PubMed] [Google Scholar]
- 25.McMillan G, Nichols L, McMillan G, Nichols L. Osteoarthritis and meniscus disorders of the knee as occupational diseases of miners. Occupat Environ Med. 2005;62:567–75. doi: 10.1136/oem.2004.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaëlsson K, Byberg L, Ahlbom A, Melhus H, Farahmand BY. Risk of severe knee and hip osteoarthritis in relation to level of physical exercise: a prospective cohort study of long-distance skiers in Sweden. PLoS One. 2011;6:e18339. doi: 10.1371/journal.pone.0018339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mork PJ, Holtermann A, Nilsen TI. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. Epidemiol Community Health. 2012;66:678–83. doi: 10.1136/jech-2011-200834. [DOI] [PubMed] [Google Scholar]
- 28.Muhlbauer R, Lukasz TS, Faber TS, Stammberger T, Eckstein F. Comparison of knee joint cartilage thickness in triathletes and physically inactive volunteers based on magnetic resonance imaging and three-dimensional analysis. Am J Sports Med. 2000;28:541–6. doi: 10.1177/03635465000280041601. [DOI] [PubMed] [Google Scholar]
- 29.Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletier JP, Milici AJ. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41:2068–76. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 31.Sandmark H, Vingård E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279–84. doi: 10.1111/j.1600-0838.1999.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 32.Szoeke CE, Cicuttini FM, Guthrie JR, Clark MS, Dennerstein L. Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: data from the longitudinal Melbourne Women’s Midlife Health Project. Bone. 2006;39:1149–55. doi: 10.1016/j.bone.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Szoeke CE, Dennerstein L, Wluka AE, et al. Physician diagnosed arthritis, reported arthritis and radiological non-axial osteoarthritis. Osteoarthritis Cartilage. 2008;16:846–50. doi: 10.1016/j.joca.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Tiderius CJ, Svensson J, Leander P, Ola T, Dahlberg L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn Reson Med. 2004;51:286–90. doi: 10.1002/mrm.10714. [DOI] [PubMed] [Google Scholar]
- 35.Vanwanseele B, Eckstein F, Knecht H, Spaepen A, Stüssi E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. 2003;48:3377–81. doi: 10.1002/art.11367. [DOI] [PubMed] [Google Scholar]
- 36.Vanwanseele B, Lucchinetti E, Stüssi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002;10:408–19. doi: 10.1053/joca.2002.0529. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Simpson JA, Wluka AE, et al. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. J Rheumatol. 2011;38:350–7. doi: 10.3899/jrheum.091138. [DOI] [PubMed] [Google Scholar]
- 38.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–809. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT. Asymmetric weight gain and loss from increasing and decreasing exercise. Med Sci Sports Exerc. 2008;40:296–302. doi: 10.1249/mss.0b013e31815b6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLoS One. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]