Summary
The synthesis of five 2-arylnaphtho[2,3-d]oxazole- 4,9-dione derivatives was accomplished by refluxing 2-amino-3-bromo-1,4-naphthoquinone with appropriate benzoyl chloride analogs at elevated temperatures. In vitro anticancer evaluation of these compounds was performed on androgen-dependent, LNCaP, and androgen-independent, PC3, human prostate cancer cell lines. In general, these compounds displayed slightly stronger cytotoxicity on the androgen-dependent LNCaP than on the androgen-independent PC3 prostate cancer cell lines. The meta-substituted 2-(3-Chloro-phenyl)-naphtho[2,3-d]oxazole-4,9- dione (10) appear to display the best cytotoxicity on both cell lines with an IC50 of 0.03 μM on LNCaP and 0.08 μM on PC3 after 5 days of exposure.
Keywords: 2-arylnaphtho[2,3-d]oxazole-4,9-dione; Oxazolo-1,4-naphthoquinone; Prostate cancer; Naphthoquinone; Anticancer activities
Introduction
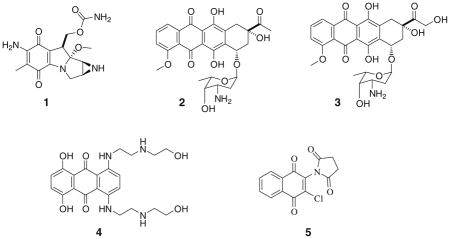
The quinone moiety is an important part of many biologically active natural products and their synthetic analogs. Both natural and synthetic quinones have been shown to exhibit a variety of biological activities, including anticancer activities. For example, anti-cancer drugs containing the quinone moiety such as mitomycin C (1) daunorubicin (2), doxorubicin (3) and mitoxantrone (4) have been used in the treatment of various types of cancers, including solid tumors, for many years [1, 2]. Quinonoid compounds are ideal for cancer chemotherapies as selective toxicity is obtained through a variety of pathways including DNA intercalation, alkylation of bio-nucleophiles, apoptosis induction, enzymatic inhibition (especially Topoisomerase II inhibition), and generation of reactive oxygen species through redox cycling [1, 2]. More recently, several quinone-containing compounds have been developed as inhibitors of enzymes and/or pathways implicated in cancer development and survival. These include inhibitors of heat shock proteins (Hsp90) [3], protein phosphatases (CDC25A, CDC25B, CDC25C) [4–6], and indoleamine- 2,3-dioxygenase (IDO, an immunosuppression enzyme implicated in immune escape strategy of many tumors) [7]. In addition, some quinonoids have been reported to target the human telomerase [8, 9], microtubule polymerization [10, 11], and the two-electron reductase NQO1 (DT-diaphorase) [12, 13]. We have previously developed 2- chloro-3-(N-succinimidyl)-1,4-naphthoquinone (5) and some of its analogs as MEK1 specific inhibitors of the Ras-MAPK pathway [14]. We subsequently reported the anti-carcinogenic activities of some of these imido-substituted 2-chloro-1,4-naphthoquinone derivatives on androgen-dependent, LNCaP, and androgen-independent, PC3 and DU145, human prostate cancer cell lines [15]. Similarly, we have studied the anticancer activity of 2,3- dichloro-5,8-dimethoxy-1,4-naphthoquinone on some breast and prostate cancer cell lines [16, 17]. In continuation of our work on developing novel quinones for study in our prostate and breast cancer drug development program, we here report the synthesis of some novel aryl- substituted oxazolo-1,4-naphthoquinone (2-arylnaphtho[2,3-d]oxazole- 4,9-dione) derivatives and their anticancer effects on LNCaP (androgen dependant) and PC3 (androgen-independent) human prostate cancer cell lines.
Experimental procedure
Materials
All reactions were carried out using laboratory grade materials and solvents. Purification was done by trituration in 100% diethyl ether or a mixture of diethyl ether/hexane followed by column chromatography on silica gel. Melting points were determined in open capillary tubes on a Mel-Temp melting point apparatus and are uncorrected. The IR spectra were recorded on a Perkin Elmer PE 100 spectrometer with an Atenuated Total Reflectance (ATR) window. The 1H- and 13C-NMR spectra were obtained on a Bruker Avance 400 MHz spectrometer in deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6). The 1H- and 13C-NMR spectra of 12 was obtained in trifluoroacetic acid-d: CDCl3 mixture. Chemical shifts are in δ units (ppm) with TMS (0.00 ppm), CHCl3 (7.26 ppm), or (CH3)2SO (2.52 ppm) as the internal standard for 1H-NMR, and CDCl3 (77.00 ppm) or (CD3)2SO (39.50 ppm) for 13C-NMR. High resolution electrospray ionization mass spectrometry was recorded on a Jeol AccuTOF-CS ESI-TOF. Dichloromethane was used as the solvent, and all the mass spectra showed signals corresponding to the molecular ion plus proton (H+). The known intermediates were prepared according to procedures that are reported in the literature. 2-Amino-3-bromo-1,4-naphthoquinone was prepared by refluxing commercially available 2,3-dibromo-1,4-naphthoquinone with ammonia/ammonium hydroxide mixture in ethanol.
In vitro cytotoxicity assay
Assessment of cytotoxicity
The MTT cell proliferation assay was employed in assessing the cytotoxicity profiles of the analogs. All the cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin-streptomycin, gentamycin, sodium pyruvate, glutamine and non essential amino acids and grown at 37°C in 5% CO2 humidified environment. PC-3 cells were seeded into 96-well plates (except for wells that served as blank control) at a density of 1.5×104 cells per well. On the other hand, the slow—growing LNCaP cells were seeded at a density of 2.0×104 cells per well. Cells were allowed to attach to the wells for 24 h. The RPMI 1640 medium used in seeding the cells were then aspirated and replaced with 200 μl of media containing different concentrations (10−3–102μM) of the test compound. 200 μl of the treatment medium containing 0.001% DMSO but without the test compound served as the control. Eight replicates of each concentration and the control were run in parallel. Viability of cells was determined quantitatively by treatment with the reducing agent 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and spectrophotometrically determining the optical densities of the solution of the resulting formazan product. The MTT assay was performed after 72 h and 120 h exposure of the individual cell lines to each concentration of each analog. 30 μl of MTT reagent (0.5 mg/ml) in PBS containing 10% HEPES was added into each well and incubated at 37°C for 4 h. The reaction medium was aspirated and the plates allowed to dry overnight at room temperature. Formazan crystals resulting from the reduction of MTT were solubilized with 100 μl of 0.04 N HCl in 2-propanol. Absorbance of the formazan solution in each was determined by placing the 96 well plate in μQuaint plate reader (KC Junior) and read at a wavelength of 570 nm. Cell viability was determined by comparing the OD of the treated vs. control (i.e. Cell viability = Corrected OD of treated/Corrected OD of Control ×100%). Correction of the OD was performed by subtraction of the background noise represented by the OD of the blank from the ODs of both the control and test.
Chemical synthesis
2-phenyl-naphtho[2,3-d]oxazole-4,9-dione (8)
A mixture of 2-amino-3-bromo-1,4-naphthoquinone (213 mg, 0.845 mmol) and benzoylchloride (2 mL) was refluxed for 5 h at high temperature. The reaction mixture was cooled to room temperature, filtered under suction, washed with diethyl ether and air dried to obtain a brown solid. The crude product was triturated in diethyl ether and then further purified by column chromatography on silica gel using 100% CH2Cl2 to obtain a yellow solid (104 mg, 44.7%). Mp 259–261°C; IR (cm−1) 3070.35, 1689.78, 1672.67, 1592.97, 1475.79, 1199.87. 1H NMR (CDCl3) δ 7.53–7.64 (m, 3 H), 7.79–7.83 (dt, J=9.0, 3.3 Hz, 2 H), 8.22–8.34 (m, 4 H). 13C NMR (CDCl3) δ 125.22, 127.05, 127.50, 128.29, 129.20, 132.14, 132.47, 133.01, 134.33, 134.38, 144.13, 150.33, 166.30, 173.27, 178.66. HRESIMS m/z 276.0894 ([C17H9NO3 + H]+, calcd 276.0661).
2-(4-Chloro-phenyl)-naphtho[2,3-d]oxazole-4,9-dione (9)
A mixture of 2-amino-3-bromo-1,4-naphthoquinone (202 mg, 0.801 mmol) and 4-chloro-benzoylchloride (2 mL) was refluxed for 5 h at high temperature. The reaction mixture was distilled under vacuum and its residue was triturated in ether:hexane (8:2) mixture. The crude product was purified via column chromatography on silica gel using hexane:CH2Cl2(50:50) to obtain a yellow solid (119 mg, 48%). Mp 274–278°C; IR (cm−1) 3065.70, 1688.56, 1666.29, 1593.20, 1475.55. 1H NMR (CDCl3) δ 7.52–7.55 (m, 2 H), 7.80–7.83 (m, 2 H), 8.23–8.29 (m, 4 H). 13C NMR (CDCl3) δ 123.67, 127.08, 127.54, 129.49, 129.65, 132.08, 132.42, 134.39, 134.46, 139.56, 144.05, 150.37, 165.31, 173.19, 178.51. HRESIMS m/z 310.0578 ([C17H8ClNO3 + H]+, calcd 310.0271)
2-(3-Chloro-phenyl)-naphtho[2,3-d]oxazole-4,9-dione (10)
A mixture of 2-amino-3-bromo-1,4-naphthoquinone (204 mg, 0.809 mmol) and 3-chloro-benzoylchloride (2 mL) was refluxed for 5 h at high temperature. The reaction mixture was cooled to room temperature, filtered under suction, washed with diethyl ether and air dried to obtain a brown solid. The solid was triturated in diethyl ether then purified via column chromatography on silica gel using 100% CH2Cl2 to obtain a yellow solid. (118 mg, 47.0%). Mp 262–265°C; IR (cm−1) 3069.37, 1683.98, 1670.37, 1582.15, 1460.43, 1200.77. 1H NMR (CDCl3) 7.49–7.53 (t, J=1.78 Hz, 1 H), 7.57–7.60 (ddd, J=8.04, 2.09, 1.12 Hz, 1 H), 7.81–7.85 (dt, J=9.02, 4.19 Hz, 2 H), 8.20–8.23 (dt, J=7.84, 1.43 Hz, 1 H), 8.24–8.32 (m, 2 H), 8.33–8.34 (t, J=1.78 Hz, 1 H). 13C NMR (CDCl3) 126.26, 126.84, 127.13, 127.58, 128.20, 130.55, 132.08, 132.45, 133.00, 134.42, 134.52, 135.51, 143.97, 150.49, 164.85, 173.23, 178.44. HRESIMS m/z 310.0564 ([C17H8ClNO3 + H]+, calcd 310.0271)
2-(2-Chloro-phenyl)-naphtho[2,3-d]oxazole-4,9-dione (11)
A mixture of 2-amino-3-bromo-1,4-naphthoquinone (210 mg, 0.833 mmol) and 2-chloro-benzoylchloride (2 mL) was refluxed for 5 h at high temperature. The reaction mixture was distilled under vacuum. Its residue was triturated in ether:hexane (50:50) and purified by column chromatography on silica gel using hexane: ethyl acetate (75:25) to obtain a yellow solid. (31.6 mg, 12.2%). Mp 202–206°C. IR (cm−1) 3066.73, 3025.33, 1689.03, 1673.26, 1472.52, 1585.72, 1203.79. 1H NMR (CDCl3) 7.42–7.46 (t, J=7.38 Hz, 1 H), 7.50–7.54 (t, J=7.16 Hz, 1 H), 7.58–7.60 (d, J=7.94 Hz, 1 H), 7.80–7.83 (m, 2 H), 8.23–8.30 (m, 3 H). 13C NMR (CDCl3) 124.34, 127.10, 127.13, 127.54, 131.68, 132.12, 132.35, 132.54, 133.26, 134.05, 134.34, 134.47, 143.53, 150.53, 164.28, 173.23, 178.33. HRESIMS m/z 310.0481 ([C17H8ClNO3 + H]+, calcd 310.0271)
2-(4-Fluoro-phenyl)-naphtho[2,3-d]oxazole-4,9-dione (12)
A mixture of 2-amino-3-bromo-1,4-naphthoquinone (202 mg, 0.801 mmol) and 4-fluoro-benzoylchloride (2 mL) was refluxed for 5 h at high temperature. The reaction mixture was distilled under vacuum. Its residue was triturated in ether:hexane (8:2) and purified via column chromatography on silica gel using hexane:ethyl acetate (75:25). Further purification by column chromatography on silica gel with 100% CH2Cl2 produced a yellow solid. (93 mg, 39.6%). Mp 304–305°C. IR (cm−1) 3071.08, 1688.26, 1602.80, 1456.70. 1H NMR (CF3CO2D: CDCl3) 7.30–7.34 (t, J=8.56 Hz, 2 H), 7.93–7.96 (dt, J=8.96, 4.61 Hz, 2 H), 8.25–8.35 (m, 4 H). 13C NMR (CF3CO2D: CDCl3) 117.57 (d, 2JF,C=22.9 Hz, C-C-F), 120.22, 128.53, 128.78, 131.51 (d, 3JF,C=9.9 Hz, C-C-C-F), 131.93, 132.37, 136.13, 136.37, 144.05, 150.54, 167.34 (d, 1JF,C= 258.4 Hz, C-F), 167.78, 175.67, 180.05. HRESIMS m/z 294.0854 ([C17H8FNO3 + H]+, calcd 294.0566).
X-ray crystallography
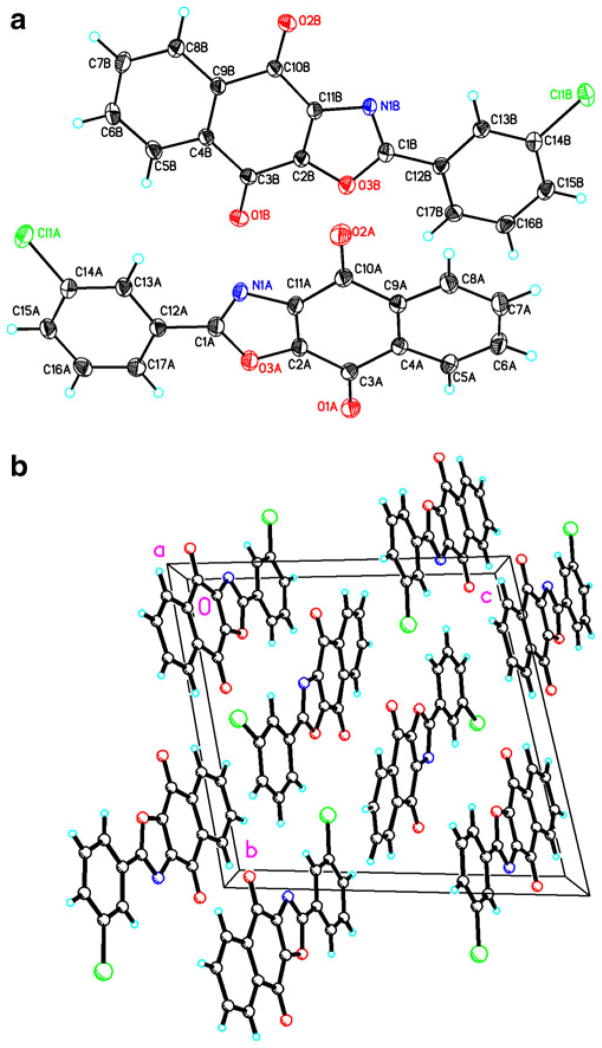
Crystals of compound 10 was obtained from a mixture of ethanol/dichloromethane via slow evaporation of the solvent at room temperature. Analysis was done with the Oxford Diffraction Gemini-R diffractometer. The crystal data and structure refinement parameters are available [18]. The overall molecular geometry with the atomic number scheme is shown in Fig. 1(a), while the molecular packing is shown in Fig. 1(b). Figure 1 (a) showed that 10 is a distorted planar structure. This distortion is due to the rotation about the C1-C12 single bond. The packing pattern, in Fig. 1(b) illustrates the molecules inversely stacked.
Fig. 1.
Shows (a) the molecular structure and (b) the packing patterns of compound 10
Results and discussion
Naphthoquinones with fused five-membered rings have been shown to reduce multidrug resistance and potentially increase cytotoxicity [19]. In order to obtain a series of 2-arylnaphtho[2,3-d]oxazole-4,9-dione (oxazolo-1,4-naphthoquinone) derivatives for bioactivity studies, a one-pot synthesis of 2-aryl substituted-oxazolo-1,4-naphthoquinone was accomplished as outlined in Scheme 1. The reaction of benzoyl chloride derivatives with 2-amino-3-bromo-1,4-naphthoquinone at elevated temperatures furnished the oxazolo-1,4-naphthoquione derivatives as brown/yellow solids with an average yield of 38%. This reaction is believed to proceed via the initial formation of the 2-amido-3-bromo-1,4-naphthoquinone derivative 7 (Scheme 1). A subsequent in situ intramolecular cyclization of the amido-derivative at reflux temperature then yielded the final target product. Formation of the oxazole product (8–12) under these conditions is temperature dependent as only the imido- and/or amido- substituted derivatives are obtained at temperatures below 200°C. Purification of the crude product was accomplished by trituration followed by column chromatography on silica gel with appropriate solvent. Structure determination was carried out by studying their infrared, 1H- & 13C- NMR, and high resolution electrospray ionization mass spectrometric characteristics. These analogs underwent in vitro cytotoxicity studies on PC3 and LNCaP prostate cancer cell lines. Structure-activities-relationship (SAR) studies revealed how substitution at the ortho-, meta-and para- positions of the oxazole substituent affected potency. Cytotoxicity studies on the fluoro- analog 12 also allowed us to compare the effect of more electronegative fluorine to that of the para-chloro- analog 9.
Scheme 1.
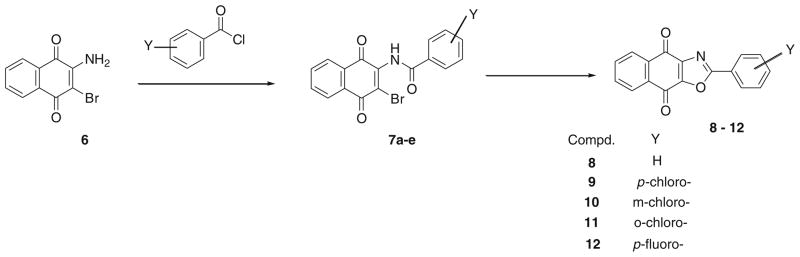
Synthesis of 2-arylnaphtho[2,3-d]oxazole-4,9-dione derivatives
MTT assay was used to determine the anti-carcinogenic activities of these compounds on both androgen-dependent LNCaP and androgen-independent PC3 prostate cancer cell lines. The bio-activity screening revealed significant dose-dependent and time-dependent cytotoxicity of the oxazolonaphthoquinones (Tables 1 and 2). After 5 days of exposure, these compounds showed the most potency for the androgen-dependent LNCaP with the ortho-chloro-substituted analog 11 displaying the strongest cytotoxicity (IC50=0.01 μM) followed by the meta-chloro- analog 10 (IC50=0.03 μM). For androgen-independent PC3 prostate cancer cell lines, the most potent compounds appear to be the meta-chloro- analog 10 (IC50=0.08 μM) and the para-chloro- analog 9 (IC50=0.08 μM). It thus appears that the meta-chloro- analog consistently displayed highly potent cytotoxicity regardless of whether the cell line is androgen-dependent (LNCaP) or androgen-independent (PC3). The para-chloro- analog 9 appears to display the lowest potency for LNCaP with an IC50 of 0.40 μM after 120 h of exposure. The apparent loss of potency for compound 9 on LNCaP between 72 h and 120 h (Table 2) was not observed on PC3 cell line (Table 1) in which there was a consistent time dependent cytotoxicity for all the five drugs studied. Further comparison of compound 9 with the para-fluoro-analog 12 revealed better activity of 12 (IC50=0.06 μM) on the androgen-dependent LNCaP prostate cancer cell line. On the other hand, comparison of the cytotoxicities of these two para-substituted analogs on the androgen-independent PC3 revealed a better potency for the p-chloro- analog 9 (IC50=0.08 μM) than the p-fluoro- analog 12 (IC50= 0.20 μM) after 5 days of exposure. Hence, both position and electronegativity of the substituent on the aryloxazole group appears to be important in modulating the cytotoxicity of this class of compounds.
Table 1.
IC50 (μM) of 2-aryl-naphtho[2,3-d]oxazole-4,9-dione derivatives (8–12) on PC-3 prostate cancer cell line after 72 and 120 h exposure
| Compd | 72 h Exposure | 120 h Exposure |
|---|---|---|
| 8 | 0.38±0.02 | 0.36±0.025 |
| 9 | 0.22±0.03 | 0.08±0.01 |
| 10 | 1.26±0.11 | 0.08±0.007 |
| 11 | 0.42±0.04 | 0.16±0.014 |
| 12 | 0.24±0.02 | 0.20±0.012 |
Table 2.
IC50 (μM) of 2-aryl-naphtho[2,3-d]oxazole-4,9-dione derivatives (8–12) on LNCaP prostate cancer cell line after 72 and 120 h exposure
| Compd | 72 h Exposure | 120 h Exposure |
|---|---|---|
| 8 | 1.0±0.08 | 0.19±0.025 |
| 9 | 0.035±0.005 | 0.40±0.056 |
| 10 | 0.1±0.01 | 0.03±0.006 |
| 11 | 0.5±0.065 | 0.01±0.002 |
| 12 | 0.03±0.004 | 0.06±0.005 |
In summary, the aryl-substituted oxazolonaphthoquinones in this study displayed potent cytotoxicity on both androgen-dependent LNCaP and androgen-independent PC3 prostate cancer cell lines with IC50 range of 0.01–0.40 μM and 0.08–0.36 μM, respectively after 5 days of drug exposure. The meta-chloro- analog 10 appears to display very potent cytotoxicity irrespective of the cell line. In addition, it appears there is generally a slightly stronger cytotoxicity on the androgen-dependent LNCaP than on the androgen-independent PC3 prostate cancer cell lines. We believe that these quinones exercise their cytotoxicity via a combination of mechanistic pathways and are currently investigating some of these in addition to developing other analogs for further SAR studies. An anticancer drug that exerts its effect via multiple mechanistic pathways might provide the answer for new generation of drugs that are able to overcome multidrug resistant cancer cells. These drugs may be able to inhibit cross communication among the different pathways that allow for cancer cell survival.
Acknowledgments
This work was supported in part by grant number 5-U54-CA914-31 (Howard University/Johns Hopkins Cancer Center Partnership).
Contributor Information
Yakini Brandy, Department of Chemistry, Howard University, Washington, DC 20059, USA.
Innocent Ononiwu, Email: imononiwu@mail.ecsu.edu, Department of Pharmacy & Health Professions, Elizabeth City State University, Elizabeth City, NC 27909, USA.
Dolapo Adedeji, Department of Pharmacy & Health Professions, Elizabeth City State University, Elizabeth City, NC 27909, USA.
Vonetta Williams, Department of Chemistry, Howard University, Washington, DC 20059, USA.
Claudia Mouamba, Department of Chemistry, Howard University, Washington, DC 20059, USA.
Yasmine Kanaan, Department of Microbiology and Cancer Center, College of Medicine, Howard University, Washington, DC 20059, USA.
Robert L. Copeland, Jr, Department of Pharmacology and Cancer Center, College of Medicine, Howard University, Washington, DC 20059, USA. Department of Pharmacology, Howard University, Washington, DC 20059, USA.
Dwayne A. Wright, Department of Chemistry, Howard University, Washington, DC 20059, USA
Ray J. Butcher, Department of Chemistry, Howard University, Washington, DC 20059, USA
Samuel R. Denmeade, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, The Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA
Oladapo Bakare, Email: obakare@howard.edu, Department of Chemistry, Howard University, Washington, DC 20059, USA.
References
- 1.Ashe C. Antitumor quinones. Mini-RevMed Chem. 2005;5:449–467. doi: 10.2174/1389557053765556. [DOI] [PubMed] [Google Scholar]
- 2.Garuti L, Roberti M, Pizzirani D. Nitrogen-containing heterocyclic quinones: a class of potential selective antitumor agents. Med Chem. 2007;7:481–489. doi: 10.2174/138955707780619626. [DOI] [PubMed] [Google Scholar]
- 3.Hadden MK, Hill SA, Davenport J, Matts RL, Blagg BS. Synthesis and evaluation of Hsp90 inhibitors that contain the 1, 4- naphthoquinone scaffold. Bioorg Med Chem. 2009;17:634–640. doi: 10.1016/j.bmc.2008.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavergne O, Fernandes A, Brehu L, Sidhu A, Brezak M, Prevost G, Ducommun B, Contour-Galcera M. Synthesis and biological evaluation of novel heterocyclic quinines as inhibitors of the dual specificity protein phosphatase CDC25C. Bioorg Med Chem. 2006;16:171–175. doi: 10.1016/j.bmcl.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Brun M, Braud E, Angotti D, Mondesert O, Quaranta M, Montes M, Miteva M, Gresh N, Ducommun B, Garbay C. Design, synthesis, and biological evaluation of novel naphthoquinone derivatives with CDC25 phosphatase inhibitory activity. Bioorg Med Chem. 2005;13:4871–4879. doi: 10.1016/j.bmc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Shen H, Carr BI, Wipf P, Lazo JS, Pan S. NAD(P) H: quinone Oxidoreductase-1-Dependent AND –independent cytotoxicity of potent quinone Cdc25 phosphatase inhibitors. JPET. 2004;309:64–70. doi: 10.1124/jpet.103.059477. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Malachowski WP, DuHadaway JB, LaLonde JM, Carroll PJ, Jaller D, Metz R, Prendergast GC, Muller AJ. Indoleamine 2,3-Dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J Med Chem. 2008;51:1706–1718. doi: 10.1021/jm7014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Cheong J, Park Y, Choi YH. Down-regulation of cyclooxygenase-2 and telomerase activity by β-lapachone in human prostate carcinoma cells. Pharm Res. 2005;51:553–560. doi: 10.1016/j.phrs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Lu Q, Liu W, Ding J, Cai J, Duan W. Shikonin derivatives: synthesis and inhibition of human telomerase. Bioorg Med Chem Lett. 2002;12:1375–1378. doi: 10.1016/s0960-894x(02)00158-0. [DOI] [PubMed] [Google Scholar]
- 10.Acharya BR, Bhattacharyya B, Chakrabarti G. The natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin binding. Biochemistry. 2008;47:7838–7845. doi: 10.1021/bi800730q. [DOI] [PubMed] [Google Scholar]
- 11.Acharya BR, Choudhury D, Das A, Chakrabarti G. Vitamin K3 disrupts the microtubule networks by binding to tubulin: a novel mechanism of its antiproliferative activity. Biochemistry. 2009;48:6963–6974. doi: 10.1021/bi900152k. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Hassani M, Karki R, Walter ED, Koelsch KH, Seradj H, Lineswala JP, Mirzaei H, York JS, Olang F, Sedighi M, Lucas JS, Eads TJ, Rose AS, Charkhzarrin S, Hermann NG, Beall HD, Behforouz M. Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumore agents. Bioorg & Med Chem. 2010 doi: 10.1016/j.bmc.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tudor G, Gutierrez P, Aguilera_Gutierrez A, Sausville E. Cytoxocitiy and apoptosis of benzoquinones: redox cycling, cytochrome c release, and BAD protein expression. Biochem Pharmacol. 2002;65:1061–1075. doi: 10.1016/S0006-2952(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 14.Bakare O, Ashendel CL, Peng H, Zalkow LH, Burgess EM. Synthesis and MEK1 inhibitory activities of imido-substituted 2- Chloro-1, 4-naphthoquinones. Bioorg Med Chem. 2003;11:3165–3170. doi: 10.1016/s0968-0896(03)00267-0. [DOI] [PubMed] [Google Scholar]
- 15.Berhe S, Kanaan Y, Copeland RL, Wright DA, Zalkow LH, Bakare O. Microwave-assisted synthesis of imido-substituted 2-chloro-1, 4-naphthoquinone derivatives and their cytotoxic activities on three human prostate cancer cell lines. Lett Drug Des Discovery. 2008;5(8):485–488. [Google Scholar]
- 16.Copeland RL, Jr, Das JR, Bakare O, Enwerem NM, Berhe S, Hillaire K, White D, Beyene D, Kassim K, Kanaan Y. Cytotoxicity of 2, 3-dichloro-5, 8-dimethoxy-1, 4-naphthoquinone in androgen-dependent and –independent prostate cancer cell lines. Anticancer Res. 2007;27:1537–1546. [PubMed] [Google Scholar]
- 17.Kanaan Y, Das JR, Bakare O, Enwerem NM, Berhe S, Beyene D, Williams V, Zhou Y, Copeland RL., Jr Biological evaluation of 2, 3-dichloro-5, 8-dimethoxy-1, 4-naphthoquinone as an antibreast cancer agent. Anticancer Res. 2009;29:191–200. [PubMed] [Google Scholar]
- 18.Empirical formula, C17H8ClNO3; Temperature, 295(2) K; CuKα, Space group, P −1; a=8.5956(3) Å, b=12.8824(5) Å, c=13.2479(5) Å, α=74.485(3)°, β=74.706(3)°, γ=71.518(3)°; Volume, 1314.65 (8) Å3; Z=4; Theta range for data collection, 4.57 to 77.56°; Reflections collected, 10515; Independent reflections, 5481 [R(int)= 0.0229]; Semi-empirical Absorption correction, Tmax, Tmin, 1.00000 and 0.66678; Full-matrix least-squares refinement on F2; Data/restraints/parameters, 5481/0/397; Goodness-of-fit on F2, 1.072; Final R indices [I>2sigma(I)], R1=0.0561, wR2=0.1768, R indices (all data), R1=0.0617, wR2=0.1806; Largest diff. peak and hole, 0.370 and −0.271 e.Å−3
- 19.Dzieduszycka M, Bontemps-Gacz MM, Stefanska B, Martell S, Piwkowska A, Arciemiuk M, Borowski E. Synthesis of 7- oxo-7H-naphthol[1, 2, 3-de]quinoline derivatives as potential anticancer agents active on multidrug resistant cell lines Bioorg. Bioorg Med Chem. 2006;14:2880–2886. doi: 10.1016/j.bmc.2006.01.008. [DOI] [PubMed] [Google Scholar]