Introduction
The Framingham Heart Study was established in 1948, setting into motion over six decades of dedicated study of cardiovascular disease, including congestive heart failure (CHF). Indeed, one of the seminal papers describing the epidemiology of CHF arose out of Framingham. The paper by McKee and colleagues in 1971 described criteria for CHF that continued to be used to this day in clinical and epidemiologic studies, demonstrated that hypertension was an important precursor of CHF, and characterized the poor prognosis of individuals with CHF in the community.1 This article briefly reviews the key findings from that paper, and summarizes several contemporary Framingham publications on the topic.
Congestive Heart Failure: the Framingham Criteria
Until the 1960s, there were no standardized criteria for adjudicating CHF in clinical studies. McKee and colleagues recognized the need for such criteria to facilitate efforts to document the risk factors and natural history of CHF. As they wrote, “if preventive and prophylactic programs are to be developed, the identification of factors that predispose and influence the course of the disease become important.” 1
The criteria they proposed are shown in Table 1. Included in the list were physician-assessment of neck-vein distension, rales, S3 gallop, venous pressure >16cm of water, hepatojugular reflux, and weight loss of 4.5 kg in 5 days due to diuretic therapy (major criteria). Minor criteria were ankle edema, night cough, dyspnea on exertion, hepatomegaly, tachycardia and weight loss. “Definite CHF” was defined as having at least two major criteria, or one major criterion and two minor criteria, as long as the minor criteria could not be attributed to any other condition.
Table 1. Criteria of CHF.*.
Framingham Criterion for congestive heart failure, introduced by McKee and colleagues in 1971. (Adapted with permission from: McKee PA et. al. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine 1971;285:1441–6)
| Major Criteria |
| Paroxysmal nocturnal dyspnea or orthopnea |
| Neck-vein distention |
| Rales |
| Cardiomegaly |
| Acute pulmonary edema |
| S3 gallop |
| Increased venous pressure ->16 cm of water |
| Circulation time ≥25 sec |
| Hepatojugular reflux |
| Minor Criteria |
| Ankle edema |
| Night cough |
| Dyspnea on exertion |
| Hepatomegaly |
| Pleural effusion |
| Vital capacity ↓ ⅓ from maximum |
| Tachycardia (rate of ≥ 120/min) |
| Major or Minor Criterion |
| Weight loss ≥4.5 kg in 5 days in response to treatment |
For establishing a definite diagnosis of congestive heart failure in this study, 2 major or 1 major & 2 minor criteria had to be present concurrently.
The emphasis in the criteria on symptoms and physical examination findings, rather than antecedent comorbidities or cardiac function assessment, underscored the fact that CHF is a clinical syndrome with many etiologies.2 Today, the approach to detecting the clinical manifestations of CHF is largely unchanged, despite important advances in knowledge about the biology of cardiac remodeling since the early 1970’s. Consequently, the Framingham criteria remain relevant in the 21st century, and continue to be used in epidemiologic research.
The reliance on overt symptoms and signs of CHF in the Framingham criteria has occasionally led to the criticism that the criteria lack sensitivity, particularly for milder presentations of CHF. Sometimes, individuals will fail to fulfill an adequate number of major or minor criteria and be regarded as having “probable” or “questionable” CHF. It is important to recognize the value in research of using diagnostic criteria that are highly specific, even at the expense of sensitivity. The number of CHF cases in any epidemiologic cohort will be far lower than the number of controls. As a consequence, misclassification of cases (as might occur with criteria lacking specificity) will cause greater problems than misclassification of controls (as might occur with criteria lacking sensitivity).
Epidemiology and natural history of CHF
Applying these new criteria, McKee and colleagues characterized the epidemiology of CHF in the Framingham cohort. They followed 5,209 men and women from the Framingham cohort for up to sixteen years. Eliminated from the analysis were 17 subjects who had a diagnosis of CHF at the time of Framingham recruitment. Subjects were assessed every two years with vital signs, ECG, chest x-ray, urinalysis, vital capacity on pulmonary function testing, and blood work. Only 2% of subjects were completely lost to follow up.3 A total of 142 individuals developed ‘definite’ CHF according to the Framingham criteria. The rate of CHF per person-year rose more than 10 times between the age of 29–39 years (0.6–0.8 cases/1000 years) and 70–74 years (8.7 cases/1000 years).
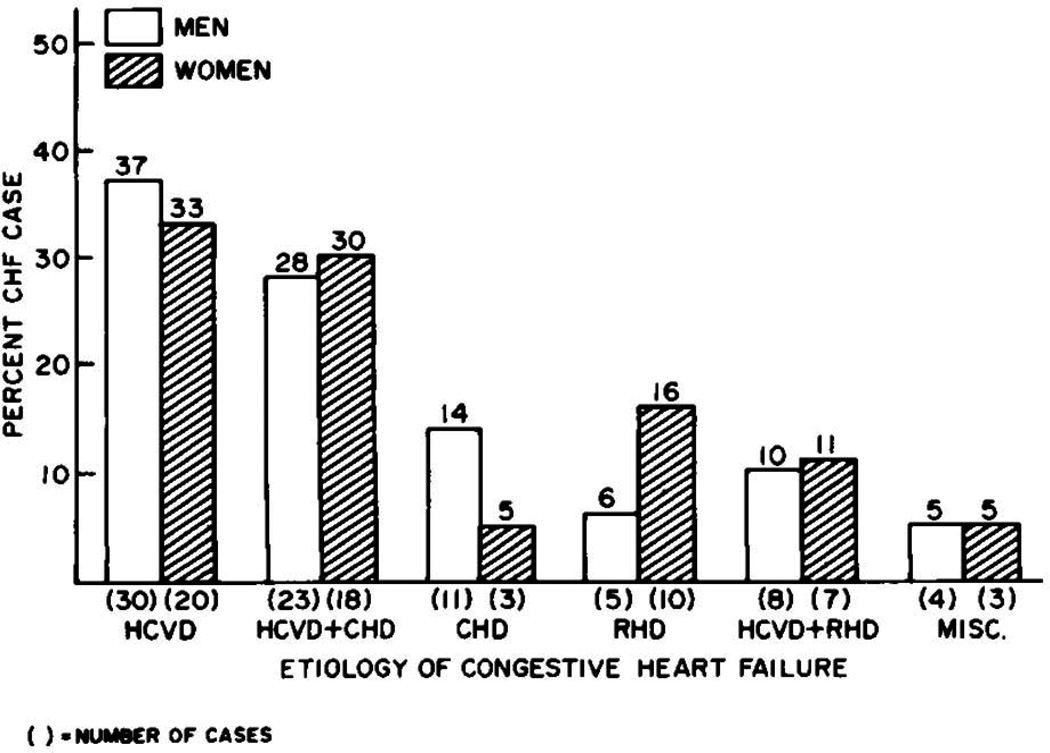
The longitudinal design of the cohort facilitated the characterization of antecedent comorbidities in the 142 individuals with CHF (Figure 1). Definite hypertension, defined using criteria employed at the time (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 95 mm Hg), was present in 75% of cases. This was typically accompanied by evidence of cardiomegaly on chest X-ray or ECG. Coronary heart disease was present in approximately half of the individuals with hypertension. Conversely, coronary heart disease without hypertension was present in only 10% of individuals with CHF.
Figure 1.
Risk factors seen in patients who developed congestive heart failure during the 16 years of follow-up in the McKee study. HCVD = hypertensive cardiovascular disease, CHD = coronary heart disease, RHD = rheumatic heart disease. (Adapted with permission from: McKee PA et. al. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine 1971;285:1441–6)
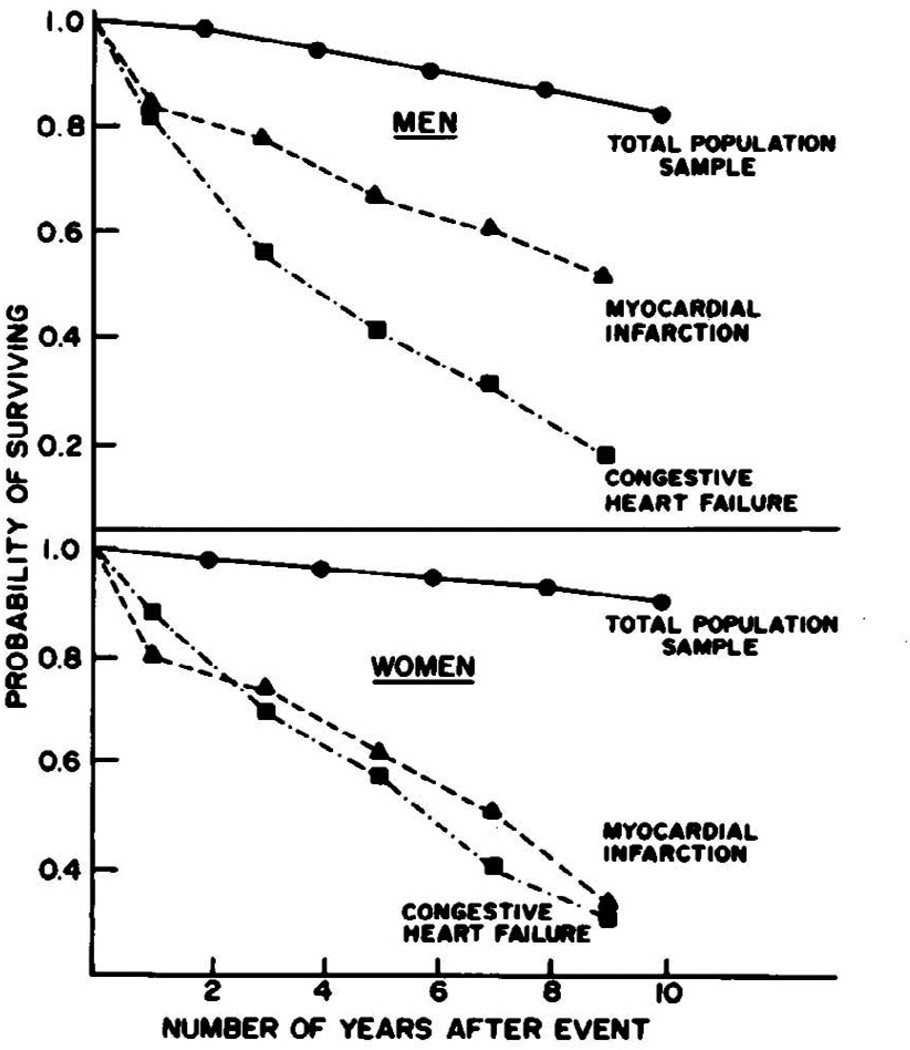
Prior to the advent of modern therapies, treatment of CHF primarily involved diuresis and digoxin. Clinical perceptions of survival were based on relatively limited experiences in the hospital setting. Thus, one of the key contributions of McKee and colleagues was to document the poor prognosis of individuals with CHF in the community. They examined the outcomes of individuals meeting criteria for definite, probable, or questionable CHF. As seen in Figure 2, approximately 1 in 2 subjects with CHF died within 5 years of the initial diagnosis. The mortality rate was substantially higher than that observed in Framingham individuals with myocardial infarction (approximately 30% at 5 years). This difference was even more pronounced a decade after the index event: roughly 4 out of every 5 individuals with CHF had died, compared with 1 out of every 2 individuals with myocardial infarction.
Figure 2.
Survival probability seen in McKee study comparing subjects who developed congestive heart failure to those who had myocardial infarctions, or neither cardiac event. (Adapted with permission from: McKee PA et. al. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine 1971;285:1441–6)
The highest rate of death was observed in the first year after diagnosis. Unexpectedly, women who were diagnosed with heart failure had a slightly higher relative risk of death than men, compared with age and sex-matched controls. This elevated relative risk persisted even 9 years after diagnosis.
Contemporary studies of CHF mortality in Framingham
Several studies updating the observations of McKee and colleagues have been published in the past several decades. In 1993, Ho and colleagues studied 652 individuals from the Framingham original and offspring cohorts with new, definite CHF.4 The investigators demonstrated that there had been no reduction in mortality after CHF since the McKee report. CHF continued to be a lethal condition with short median survival. The median survival after the onset of congestive heart failure was only 1.66 years in men, and 3.17 years in women. They did note, however, an 11% reduction in CHF incidence in men, and 17% reduction in women (p<0.05).5
In 2002, Levy and colleagues assessed a half century of Framingham data between 1950 and 1999, encompassing 1,075 individuals who developed heart failure.6 The temporal trend for CHF mortality revealed a 10 to 11% reduction in the risk of death in both genders, after adjusting for differences in age (p<0.003). Individuals with CHF diagnosed in 1990–99 had a one-third lower mortality after CHF than those diagnosed in 1950–69, after adjusting for cardiovascular risk factors (HR 0.69 with 95% CI 0.50–0.95 in men, and HR 0.68 with CI 0.48–0.98 in women). Nonetheless, in spite of this favorable trend, the authors noted that CHF in the community continued to carry a poor prognosis, with 3 out of 5 men and 2 out of 5 women dying within 5 years of diagnosis.
Risk factors for CHF
The comprehensive characterization of Framingham participants over the course of decades provides a unique opportunity to study conditions that may predispose to CHF. As noted above, McKee and colleagues provided one of the first systematic descriptions of the potential contribution of hypertension to CHF incidence. A follow-up study by Kannel and colleagues demonstrated that hypertensive men had a nearly 8-fold risk of developing CHF compared with normotensive men.3 Similarly, hypertensive women had a 4-fold risk compared with normotensive women. A later study by Levy and colleagues used an updated definition of hypertension, and found that more than 90% of Framingham participants who developed CHF had antecedent hypertension.7 Using multivariable models, they estimated the population attributable risk for CHF from hypertension to be nearly 40% in men and 60% in women.
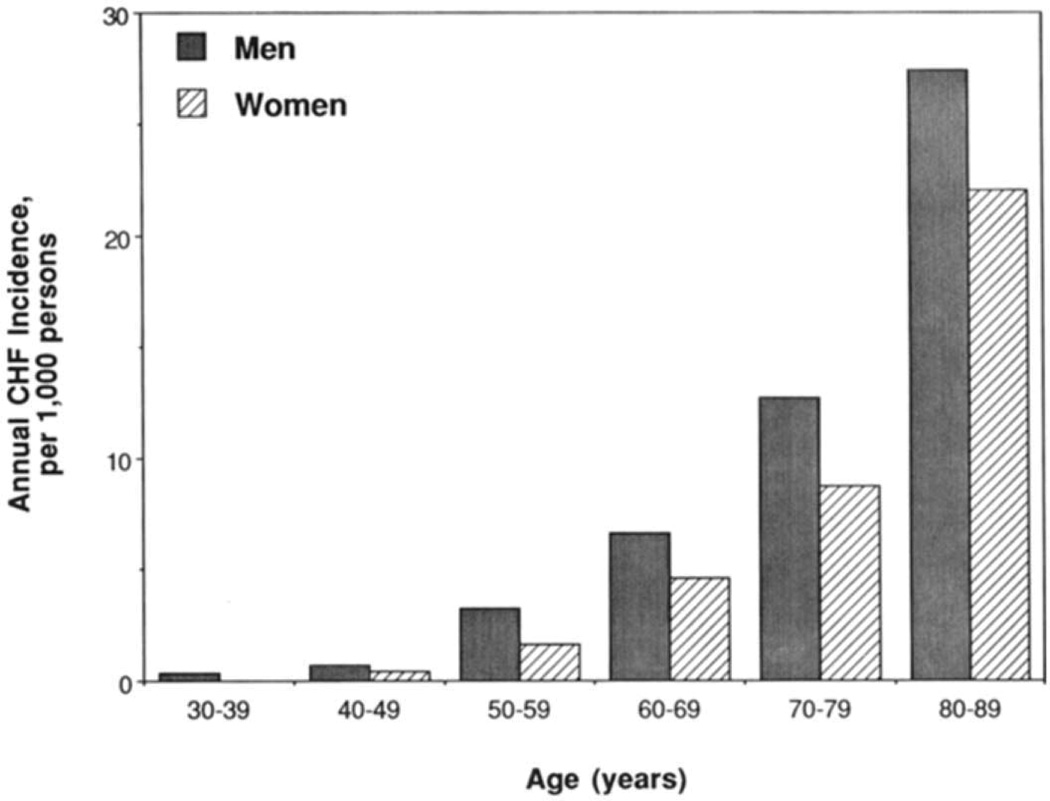
The study by McKee and colleagues was also among the first to document the age- and gender-related variation in CHF incidence. This observation was updated in the study by Ho and colleagues 20 years later. As shown in Figure 3, incidence rates of CHF rose markedly with age and was higher in men than in women at all ages.5
Figure 3.
Comparison of congestive heart failure annual incidence per decade between men and women, as seen by Ho and colleagues. (Adapted with permission from: Ho KKL et. al. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology 1993;22:A6–A13)
More recent studies from Framingham have looked comprehensively at predictors of new-onset CHF. The goal of these studies is to provide general practitioners with a cost-effective method to identify patients at high 4-year risk of developing heart failure, to enable better targeting of preventive measures. In 1999, Kannel and colleagues used 38 years of Framingham follow-up data to describe a clinical risk score for predicting CHF. Incorporated in the score were the following variables, all of which can be assessed in the outpatient clinic: age, gender, ECG left ventricular hypertrophy, heart rate, systolic blood pressure, diabetes mellitus, prior myocardial infarction, valvular disease by examination, and hypertension (Table 2).8 60% of new CHF events in men and 73% in women occurred in individuals in the top quintile of multivariable risk according to the risk score.
Table 2.
Four year probability for developing congestive heart failure among men 45 to 94 years of age. (Adapted with permission from: Kannel WB et. al. Profile for estimating risk of heart failure. Archives of Internal Medicine 1999;159:1197.)
| Points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | |
| Variables | ||||||||||
| Age (years) | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | 90–94 |
| Systolic blood pressure (mm Hg) |
<120 | 120–139 | 140–169 | 170–189 | 190–219 | >219 | ||||
| Heart rate (bpm) | <55 | 55–64 | 65–79 | 80–89 | 90–104 | >104 | ||||
| LVH on ECG | No | Yes | ||||||||
| Coronary Heart Disease | No | Yes | ||||||||
| Valve Disease | No | Yes | ||||||||
| Diabetes | No | Yes | ||||||||
| Points | 4-Year Probability of Congestive Heart Failure |
Points | 4-Year Probability of Congestive Heart Failure |
|---|---|---|---|
| 5 | 1 | 24 | 30 |
| 10 | 2 | 25 | 34 |
| 12 | 3 | 26 | 39 |
| 14 | 5 | 27 | 44 |
| 16 | 8 | 28 | 49 |
| 18 | 11 | 29 | 54 |
| 20 | 16 | 30 | 59 |
| 22 | 22 |
Framingham investigators have also examined newer predictors of CHF, including circulating biomarkers. For instance, in 2004, Wang and colleagues published the first large, longitudinal study showing that levels of plasma natriuretic peptides predicted future CHF in ambulatory individuals.9 Individuals with B-type natriuretic peptide concentrations in the top quintile (>20pg/mL in men, and >23.3 pg/mL in women) had a 3-fold increased risk of developing CHF over 5-years.
Studies from Framingham have also defined echocardiographic predictors of future heart failure.10 For instance, Vasan and colleagues demonstrated the higher left ventricular diastolic dimensions in individuals free of myocardial infarction predicted future heart failure (adjusted hazard ratio, 1.5, 95% confidence interval 1.25 to 1.73, per standard deviation increment in end-diastolic dimension).10 More recently, Wang and colleagues showed that individuals with asymptomatic left ventricular systolic dysfunction (ejection fraction ≤50%) had a nearly 5-fold increased risk for developing heart failure (adjusted hazard ratio, 4.7, 95% confidence interval, 2.7 to 8.1).11 Recent guidelines have embraced the concept that subclinical abnormalities of cardiac structure and function (“stage B heart failure”) are important precursors of overt heart failure.12 Ongoing studies in other cohorts are investigating the cost-effectiveness of targeted echocardiographic screening programs.13
Relevance to lower and middle income countries
Over the past decades, global socioeconomic development has resulted in an ‘epidemiological transition,’ with shifts in the leading causes and death. As of 2008, 4 out of every 5 deaths globally from cardiovascular disease occurred in developing nations.14 A paucity of data exists on the global prevalence of heart failure, although some estimate that more than 23 million people worldwide are living with the condition.15 A limitation of the original Framingham cohort is that the volunteers were almost entirely Caucasian, although the new Omni cohorts of the Framingham study reflect a more diverse US population.
Nevertheless, multiple observations from the study of McKee and colleagues are relevant to the global problem of heart failure. As in the U.S., hypertension has been identified as a key risk factor for heart failure in the other countries; for instance, it accounts for an estimated 45% of all acute heart failure cases in sub-Saharan Africa.16 Similarly, the powerful association between age and heart failure risk has important implications in the developing world, where life expectancy has risen from 52 to 57 years in lower income nations, and from 63 to 68 years in middle income nations, between 1990 and 2009.17 In India, it is estimated that the number of individuals over the age of 60 years will have risen to 113 million by 2016, nearly twice the number in this age group in 1996.18
Conclusion
The landmark publication by McKee and colleagues defined the epidemiology and natural history of CHF using a set of standardized criteria for the first time in a community-based study. The study underscored the high mortality associated with CHF, but also highlighted potential opportunities for prevention based on the modification of risk factors such as hypertension. Their observations have been subsequently validated in numerous studies from both Framingham and other epidemiologic cohorts, and continue to provide a foundation for future investigations aimed at reducing the burden of CHF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. The New England journal of medicine. 1972;287:781. doi: 10.1056/NEJM197210192871601. [DOI] [PubMed] [Google Scholar]
- 4.Ho K, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 5.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993;22:A6–A13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. New England Journal of Medicine. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA: the journal of the American Medical Association. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 8.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PWF, Levy D. Profile for estimating risk of heart failure. Archives of Internal Medicine. 1999;159:1197. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New England Journal of Medicine. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. New England Journal of Medicine. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 12.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Stainback RF, Redberg RF, Schiller NB, Cohen NH, Foster E. Transesophageal echocardiography predicts mortality in critically III patients with unexplained hypotension. Journal of the American College of Cardiology. 1995;26:152–158. doi: 10.1016/0735-1097(95)00129-n. [DOI] [PubMed] [Google Scholar]
- 14.Global status report on noncommunicable diseases. World Health Organization; 2010. Apr, 2011. [Google Scholar]
- 15.McMurray JJ, Petrie MC, Murdoch DR, Davie AP. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19:9–16. [PubMed] [Google Scholar]
- 16.Damasceno A, Mayosi BM, Sani M, et al. The Causes, Treatment, and Outcome of Acute Heart Failure in 1006 Africans From 9 Countries: Results of the Sub-Saharan Africa Survey of Heart Failure. Arch Intern Med. 2012;3:1–9. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 17.World Health Statistics. France: World Health Organization; 2012. [Google Scholar]
- 18.Huffman MD, Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India. 2010;23:283–288. [PMC free article] [PubMed] [Google Scholar]