Abstract
Study Design
Comparison of disc tissue from rat tails in six groups having different mechanical conditions imposed.
Objectives
To identify disc annulus changes associated with the supposed altered biomechanical environment in a spine with scoliosis deformity using an immature rat model that produces disc narrowing and wedging.
Background
Intervertebral discs become wedged and narrowed in a scoliosis curve, probably due in part to altered biomechanical environment.
Methods
Tail discs of 5-week-old immature Sprague-Dawley rats were subjected to an altered mechanical environment using an external apparatus applying permutations of loading and deformity for 5 weeks. Four groups of rats (A) 15 degrees Angulation, (B) Angulation with 0.1 MPa Compression, (C) 0.1 MPa Compression, and (R) Reduced mobility, together with a sham and a control group were studied. Disc height changes and matrix composition (water, DNA, GAG and HA content) were measured after 5 weeks, and proline and sulphate incorporation and mRNA expression were measured at 5 days and 5 weeks.
Results
After 5 weeks, disc space was significantly narrowed relative to internal controls in all four intervention groups. Water content and cellularity (DNA content) were not different at interventional levels relative to internal controls and not different between the concave and convex sides of the angulated discs. There was increased GAG content in compressed tissue (in Groups B and C), as expected, and compression resulted in a decrease in hyaluronic acid size. Slightly increased incorporation of tritiated-proline into the concave side of angulated discs and compressed discs was observed. Asymmetries of gene expression in Groups A and B, and some group-wise differences, did not identify consistent patterns associating the discs’ responses to mechanical alterations.
Conclusions
Intervertebral discs in this model underwent substantial narrowing after 5 weeks, with minimal alteration in tissue composition and minimal evidence of metabolic changes.
Keywords: Intervertebral disc, Growth, Deformity, Rat model, Metabolism
INTRODUCTION
A scoliosis deformity involves wedging asymmetry of both the vertebrae and intervertebral discs [1,2]. While the pathomechanism of the progressive wedging of the vertebrae is thought to involve asymmetrical loading that modulates vertebral growth (Hueter-Volkmann principle), the mechanism by which the discs become wedged is poorly understood, and the relative timing of vertebral and discal wedging is unclear [1,2]. The progression of a scoliosis deformity slows markedly or ceases at skeletal maturity when the vertebral growth plates become inactive but it is not clear why wedging of the discs would also cease.
It has been reported [3] that intervertebral discs subjected to compression, reduced mobility and angulation a young, immature rat tail model of supposed altered spinal biomechanics in scoliosis were narrowed and stiffer, with structural wedging resulting from tail angulation. The structural nature of the disc deformity was evidenced by its minimum lateral bending stiffness being at an angle close to the in vivo (deformed) value, indicating that angulated discs had remodeled to the imposed deformity [3]. This implies that wedging of intervertebral discs during progression of a scoliosis deformity may result from a combination of imposed angulation, asymmetrical compression, and reduced mobility.
The overall aim of the present study was to explore the underlying mechanism of this reported loss of disc space in immature rats by identifying how the disc tissue responded to different components of the altered mechanical environment implicated in scoliosis. Annulus tissue composition was measured after 5 weeks, and the short term (5 days) and long-term (5 weeks) effects on tissue synthesis and expression of genes associated with tissue synthesis and degradation were investigated. It was hypothesized that the loss of disc height would be associated with decreased water and GAG content, decreased HA size, reduced synthesis of collagen and GAGs, and increased expression of genes associated with disc tissue degradation and degeneration.
METHODS
Permutations of altered biomechanical conditions were imposed in different groups of the immature rat tails using the model reported previously [3]. Measurements of water content, DNA content, glycosaminoglycan (GAG) content, and hyaluronic acid (HA) size were measured after 5 weeks to identify compositional changes. Synthesis of collagen and GAG and changes in gene expressions of key matrix proteins and their degradative enzymes were assessed at 5 days and 5 weeks to determine whether metabolic changes were occurring. Rat tail discs were selected because the rat tail is easily accessible for controlled application of compression [3,4,5,6,7,8], and because of the rapid growth of these animals [9, 10]. The initial age of the animals (5 weeks) and the duration of the experiment (5 weeks) were chosen to simulate human adolescent growth. In rats, the rate of growth increases between 1 and 5 weeks, then declines until skeletal maturity, which is achieved by 11.5–13 weeks [11]. Thus peak growth velocity occurred early in the experimental period.
Animal Groups and Procedures
External rings were attached to adjacent caudal levels (Caudal 7 and Caudal 8 vertebrae) of 5-week-old (mean 140 g bodymass) Sprague-Dawley rats by two percutaneous 0.5 mm diameter pins transfixing the vertebral bodies. This was done under general anesthesia (Ketamine 80 mg/kg and Xylazine 10 mg/kg) with postoperative pain control (Buprenorphine 0.05 mg/kg). The pins were installed with fluoroscopic (x-ray) visualization of the vertebrae and apparatus. The apparatus remained in place for the duration of the experiments (5 weeks or 5 days).
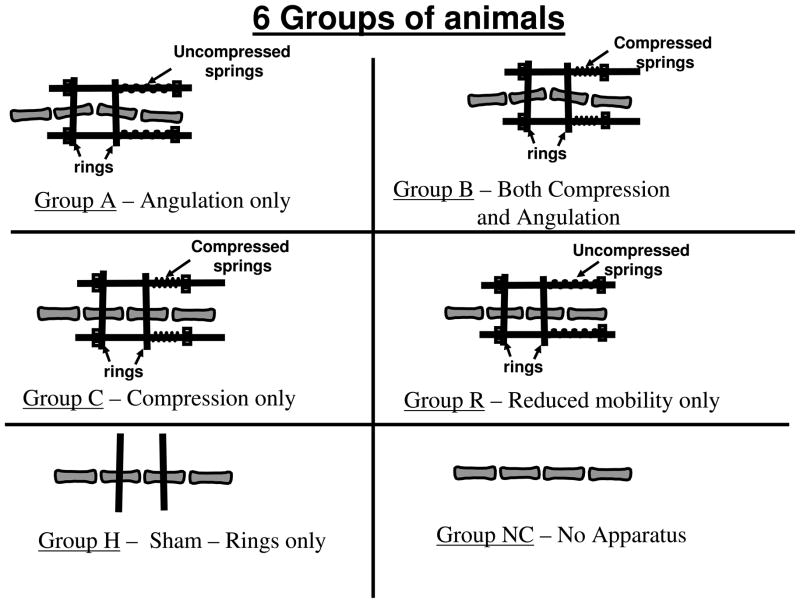
The method and apparatus described by MacLean et al. [7] was modified for this study so that rings could be installed either parallel or with an initial 15-degree relative angulation (by rotating the rings each by 7.5 degrees to the transverse plane of the vertebrae). Angulation of 15 degrees has been shown to cause substantial disc space loss [3], and is comparable to the apical wedging in a clinically significant scoliosis curve. Threaded rods linking the rings were used to control the angulation between the rings, along with springs that could be mounted on the rods to apply a sustained compressive force (Figure 1). The connecting rods and springs were adjusted to align the rings provisionally parallel to each other, or at a small angle to compensate for misalignment measured from post-surgery CT images.. Compression was imposed by compressing the calibrated springs to produce a force corresponding to the desired stress [12]. The lengths of the springs were measured and adjusted weekly. The stress was the applied force divided by vertebral metaphyseal cross sectional areas as reported in Stokes et al. [12].
Figure 1.
Diagrammatic representation of tail instrumentation for the six groups of animals studied. In Groups A and B the imposed angulation was 15 degrees.
Four ‘intervention’ groups of animals having different permutations of imposed mechanical conditions, a sham group and control group were compared (Figure 1). In Group A (Angulation) a 15 degree angulation was imposed by adding springs and locknuts to the rods but not compressing the springs. In Group B, a 0.1 MPa compression stress was imposed by compressing the calibrated springs, along with the 15-degree angulation. In Group C the 0.1 MPa compression was imposed, without angulation. In Group R there was no imposed angulation and the springs were not compressed. This was termed the Reduced Mobility Group since the apparatus was observed to limit tail motion in the instrumented region. In the Sham Group (Group H) the rings were attached to the adjacent vertebrae without angulation, but no rods or springs were employed. In the control group (Group NC) no apparatus was applied.
There were five sets of animals in each of the four ‘intervention’ groups (A,B,C and R) for (1) PCR at 5 days, (2) proline and sulphate incorporation at 5 days, (3) PCR at 5 weeks, (4) proline and sulphate incorporation at 5 weeks, and (5) composition (water, GAG and DNA content, and HA size) at 5 weeks. There were initially 11 animals per set (10 in set 2) for a total of 216 intervention animals. This number of animals was based on a power analysis to identify group differences greater than twice the variance of mean measurements with 80% power and probability < 0.05. There were 11 sham animals (Group H) and 8 control animals (Group NC), used for disc space and compositional studies only. Disc dimensions were monitored by micro CT scans with animals under sedation 2 days after installation of the apparatus and again immediately prior to euthanasia at 5 weeks (sets 3 and 5). Radioactive tracers (35S sodium sulphate and tritiated proline (PerkinElmer, Waltham, MA, USA) 0.5 mCi/kg and 1mCi/kg respectively) were injected intra-peritoneally (sets 2 and 4) after installation of the tail apparatus. All live animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
After euthanasia, sections of the tails including four vertebrae and 3 discs (the intervention level disc (level ‘L’) and adjacent control discs (levels ‘C’) were removed for analysis. In Groups A and B, the discs were cut into two halves (corresponding to the convex and concave sides of the tail) and processed separately. In the data analyses, values from the two adjacent control discs (levels ‘C’) were averaged.
Analysis of Composition
Discs were dissected free of adjacent tissues, the nucleus pulposus was removed and the remaining annulus tissue was immediately weighed, lyophilized and reweighed to obtain dry weight and hydration. Tissue was then rehydrated and digested in 0.5 ml 1 mg/ml alpha-chymotrypsin in 50 mM Tris-HCl containing 1 mM EDTA, 1 mM iodoacetamide and 10 mg/ml pepstatin-A overnight at 37 C, then in 1 ml 1 mg/ml proteinase-K in the same buffer for 1 week at 56 C. Samples were then centrifuged and the supernatant removed. Aliquots of this digest were used to measure GAG content by the DMMB dye-binding method [13] and DNA content as a surrogate for cellularity by the Hoechst 33258 assay.
Analysis of Hyaluronic Acid Size
The disc digests used for composition analysis were also used for analysis of HA size differences. Digests were pooled (0.5 ml total) from 5 animals and heated at 100 C for 5 min to inactivate proteinases. The sample was then mixed with 0.5 ml 50 mM Tris/HCl, 150 mM NaCl, pH7.4, containing 0.5 % BSA and fractionated by gel filtration chromatography through Sephacryl S1000 (95 cm × 1 cm column) in 50 mM Tris/HCl, 150 mM NaCl, pH 7.4, at 6 ml/h. Fractions (1 ml) were collected and analyzed for HA content by a competitive binding assay using a biotinylated HA binding protein [14]. To compensate for differences in HA content between the samples and to facilitate comparison of the chromatograms, results were expressed as a percentage of the fraction with the highest HA content.
Incorporation of Sulphate and Proline
The annulus tissue was digested in 0.5 ml of proteinase K (1.0 mg/ml; at 58–60 C) until digestion was complete and dialysed (8–10 kDa size Spectra/Por Float-A-Lyzer (VWR 28170-342)) to remove unincorporated isotopes. Four-times the sample volume of scintillation fluid, (Research Products Int. 111195/T) was added and 2 repeated counts of 600 seconds duration were performed, using a Wallac (model 1409) scintillation counter.
Real-time RT-PCR
Gene expression evaluated by mRNA levels for matrix proteins (Collagen I, Collagen II, Aggrecan) and degradative enzymes and their inhibitors (MMP13, MMP3, TIMP1, TIMP3, ADAMTS4 and ADAMTS5) was assayed by real time RT-PCR. RNA isolation, probes, primers, reverse transcription and amplification as described by MacLean et al. [7] were employed. The relative amount of mRNA in the instrumented disc compared with the internal control discs for each of the nine genes was computed according to the comparative Ct method [7,15]. For each sample the number of PCR thermal cycles producing fluorescence that reached a predetermined threshold level was recorded as the Ct value. The relative gene expression for each of the nine genes in tissue from the instrumented level was computed by subtracting the Ct value of an endogenous control gene (18SrRNA) from the Ct value of the experimental gene, yielding the ΔCt value. Then ΔCt values of the instrumented samples were further normalized to the ΔCt values of the internal control discs, yielding the ΔΔCt value. Finally, assuming that each strand of cDNA in the sample is copied exactly once per PCR cycle (giving an exponential relation between cycle number and quantity of cDNA), the base-2 antilog of the ΔΔCt value was used in statistical analyses.
Statistical analyses
Group-wise and level-wise comparisons were made by analyses of variance of individual measurements of intervertebral discs, or of paired differences (between sides, or levels) with post hoc t-tests (Bonferroni correction). Analyses were made with SPSS software (SPSS Inc., Chicago, Illinois). The p-level 0.05 was considered significant.
RESULTS
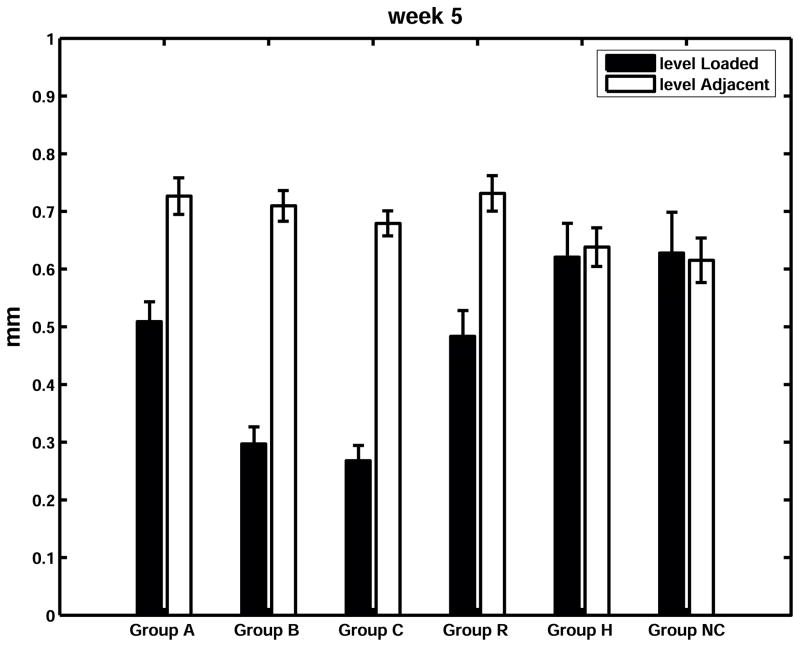
Animals’ body mass averaged 140 g when instrumentation was installed, and averaged 440 g when they were euthanized after 5 weeks. There was a substantial and highly significant narrowing of the disc space in all ‘intervention’ animals. (Figure 2). The loaded disc values as a percentage of the control disc values were 70%, 42%, 39% and 66% in Groups A (angulated), B (angulated and compressed), C (compressed) and R (reduced mobility) respectively, while the corresponding percentages for the sham and control groups were 97% and 102% (Figure 3).
Figure 2.
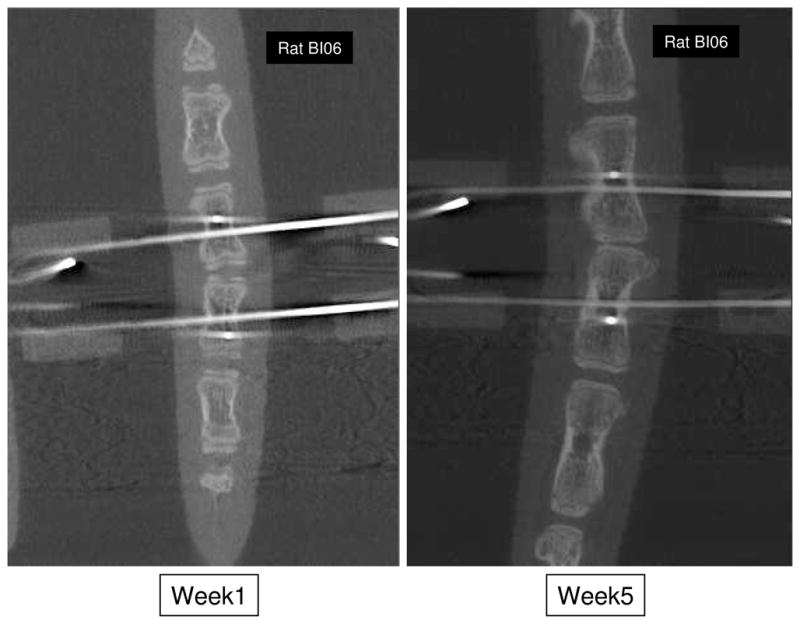
Mid-coronal plane sections from micro CT scans showing instrumented level of an animal in the ‘B’ group at weeks 1 and 5.
Figure 3.
Disc space measurements in mm at week 5 (Mean and SEM). For each group – left (filled) column: intervention level; right (unfilled) column: average of two adjacent internal control levels.
Composition
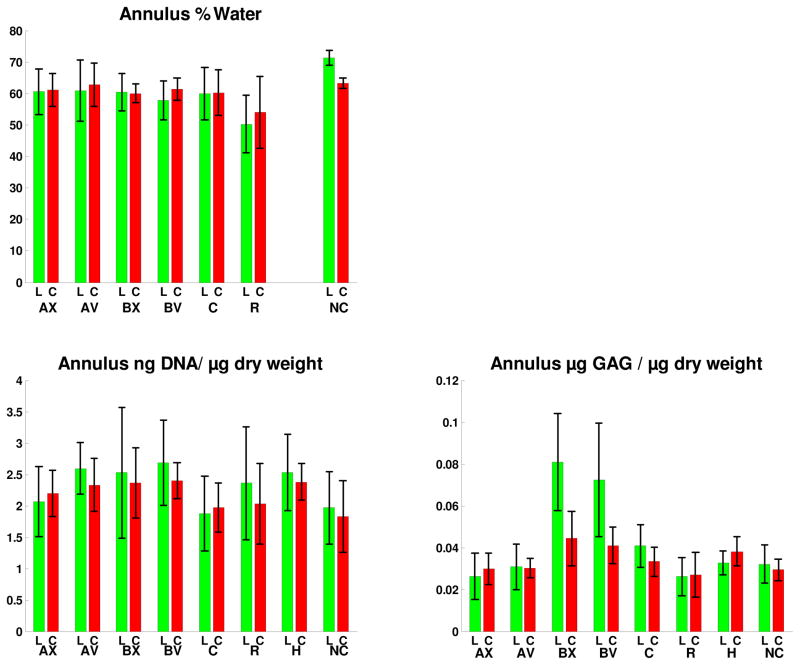
Both hydration (water content) and DNA content (as a surrogate for cellularity) of discs at the intervention levels were not different from those of the internal control or of the sham (Group H) animals at 5 weeks. There was no evidence of any difference between disc tissue hydration or DNA content on the convex and concave sides of angulated tails (Groups A and B) (Figure 4). GAG content was increased in compressed discs compared with control discs in Groups B and C (Figure 4). The intervention level contained significantly more GAG than control levels in Group C and in Group B on both concave and convex sides of the tail. However, there were no significant convex-concave side differences in the angled discs (Groups A and B).
Figure 4.
Annulus hydration (mean and SD - dry weight as a percentage of wet weight), annulus tissue DNA content (mean and SD, by Hoechst assay), and GAG content (mean and SD by DMMB dye binding method) for the four intervention groups A, B, C and R, and the Sham (H) and Control (NC). Measurements were made after 5 weeks for all groups. For Groups A and B, the labels AX, AV, BX and BV identify the Group and the side of the disc - X= convex side of the tail, V= concave side of the tail. Label ‘L’ is for the Intervention (loaded) disc. Label C corresponds to the mean value of adjacent (internal control) discs. Hydration data not presented for the Sham group because of numerous missing values.
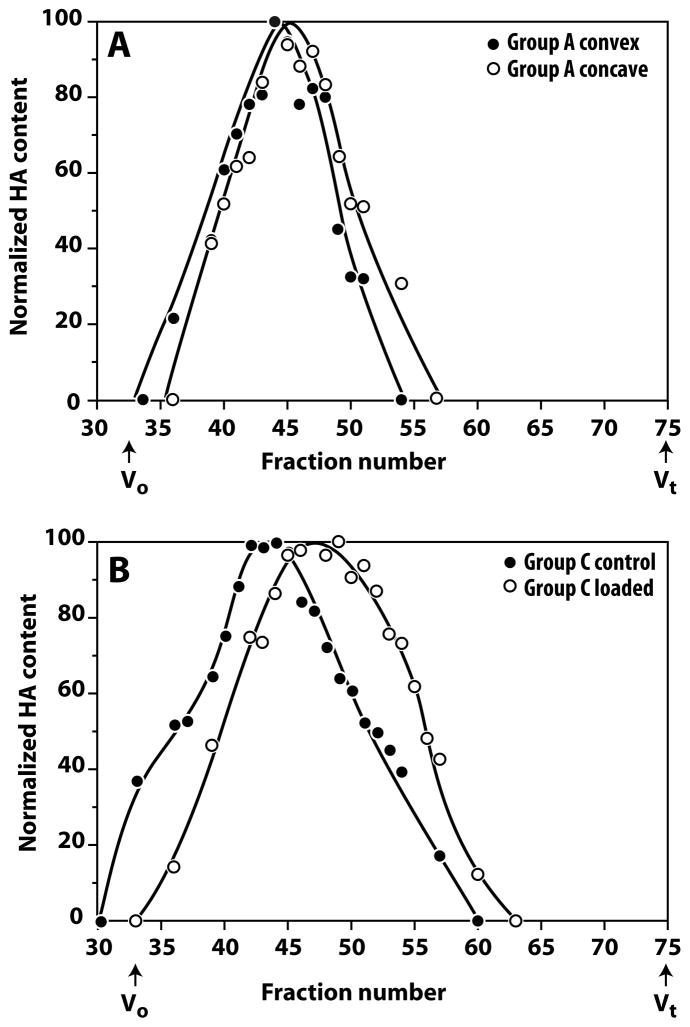
Hyaluronic Acid Size
In the instrumented angulated discs (Group A) there was a small decrease in HA size in the concave side relative to the convex side (Figure 5A). In the compression group (Group C), decreased HA size was observed in the instrumented discs relative to the distal control discs (Figure 5B). The magnitude of this difference was greater than between the sides of the angulated discs. In both cases, the decrease in HA size appears to be related to increased disc compression.
Figure 5.
Hyaluronic acid size variation in angulated and compressed rat discs. Pooled extracts from angulated and compressed discs were analyzed for HA size variation by chromatography through Sephacryl S1000. Panel A compares the convex and concave sides of the instrumented (loaded) angulated disc (Group A). There is a small decrease in HA size in the concave side relative to the convex side. Panel B compares the instrumented (loaded) and distal control discs of the compression group (Group C). There is a major decrease in HA size in the loaded disc. Vo and Vt represent the void and total volumes of the column, respectively.
Incorporation of Sulphate and Proline
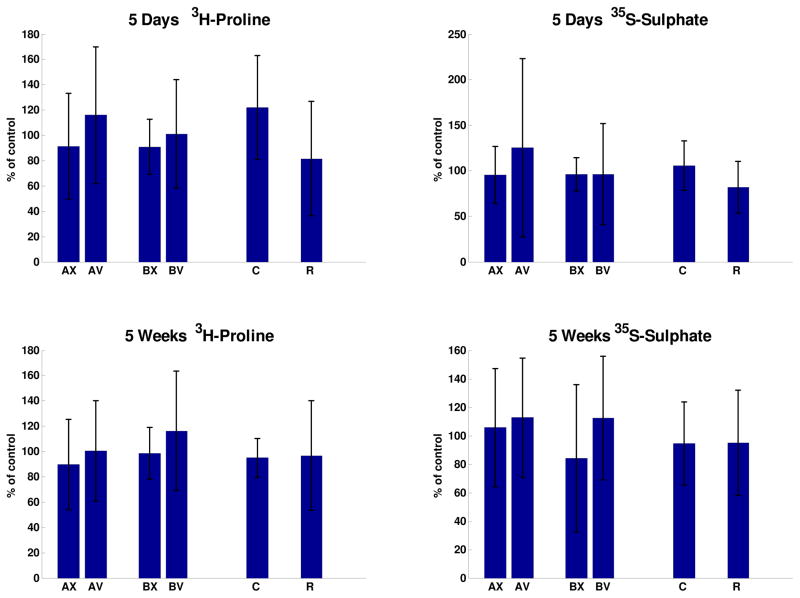
Slightly increased incorporation of tritiated-proline into disc tissue of compressed discs and into the concave side of angulated tails was observed (Figure 6), but differences were not statistically significant. Values of incorporated isotope at the intervention level expressed as a percentage of the control level were not significantly different in any of the Groups. In Groups A and B, none of the convex-concave side differences were significant. In the case of sulphate incorporation, there were significant no differences between loaded and control discs and between the concave-convex sides in discs from angulated tails (Figure 6).
Figure 6.
Incorporation of tritiated proline and of Na235SO4 after 5 days and 5 weeks in four groups of animals (A, B, C and R). For Groups A and B: X= disc tissue on convex side and V = disc tissue on concave side of the tail. Values for each animal were normalized to the mean value of adjacent (internal control) discs, and expressed as a percentage.
Real-time RT-PCR
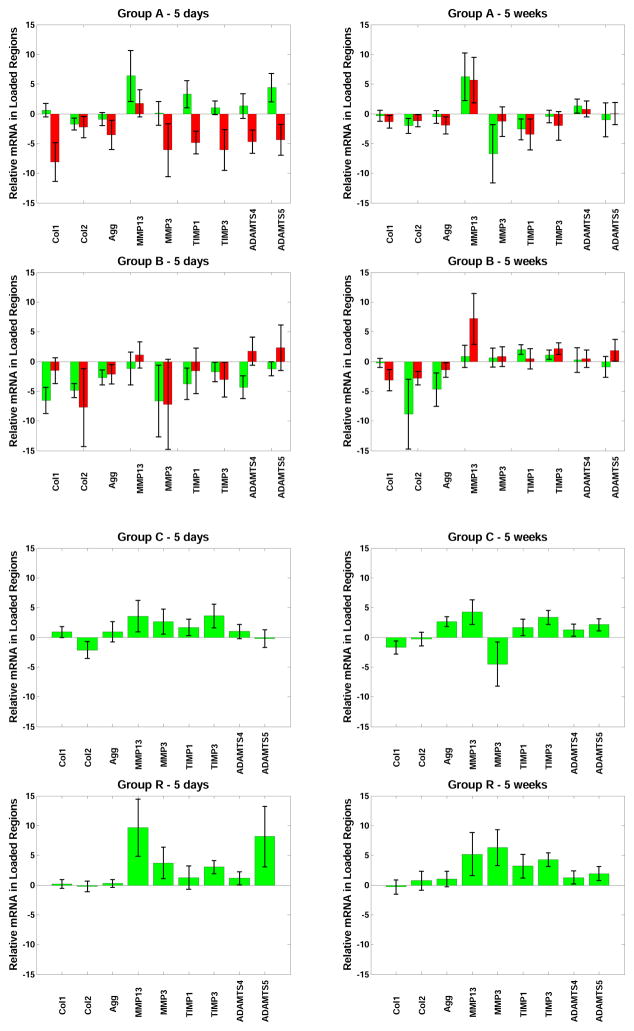
Reduced expression of most genes was observed on the concave side of angulated discs in Group A animals at 5 days (convex-concave side differences significant for Collagen-1, TIMP-1, TIMP-3 and ADAMTS5), but no long-term convex-concave side differences were observed. In Group B reduced gene expression was observed bilaterally for most genes at 5 days and this was statistically significant for Collagen-1, Collagen-2 and Aggrecan. There was significant increase in expression of TIMP-3 at 5 weeks in Groups B, C and R, and in Group R at 5 days. Other degradative enzymes and their inhibitors were observed to have increased expression in Group R at both 5 days and 5 weeks, but this was only significant for TIMP-3 (Figure 7). In analyses of differences between Groups, Collagen-1 expression was significantly reduced in Groups A and B relative to Group C; TIMP-3 was less expressed in Group A than in Group C; Aggrecan was less expressed in Group B than Group C; and both MMP-3 and TIMP-1 were less expressed in Group A than in Group R.
Figure 7.
Gene expression for Collagen 1, Collagen 2, Aggrecan, MMP13, MMP3, TIMP1, TIMP3, ADAMTS4, and ADAMTS5 by real time RT-PCR after 5 days and after 5 weeks in Groups A, B, C and R (mean values and standard deviations). Values are expressed exponentially as fold-change, relative to within-animal controls, hence on the vertical; axis, 0 = no difference, 1 = a doubling of mRNA, 2 = 4 times the mRNA as in control, etc. For Groups A and B, the left column corresponds to disc tissue on the convex side of the tail, and the right column corresponds to tissue on the concave side of the tail.
DISCUSSION
All experimental interventions produced substantial narrowing of the intervertebral disc space of these growing animals over the five-week period that corresponds to a large proportion of the post-natal growth of the animals. Although the growth curve of rats is not the same as human, this experimental period is considered to be analogous to the human adolescent phase. Despite the substantial narrowing of disc space, measurements of annulus tissue composition after 5 weeks of loading were surprisingly similar at the intervention levels relative to the internal controls, and symmetrical where angulation had been applied. Lack of statistically significant differences or consistent trends in the results implies that only small effects were present, and that there had been substantial remodeling of the tissue to restore normal composition, hydration and cellularity.
The changes reported in HA size are consistent with altered disc remodeling in response to the different types of loading, and suggest that compression is associated with HA depolymerization. A decrease in HA size could be due to a load-induced increase in oxidative stress resulting in free radical production or increase in hyaluronidase expression. However, the observed deploymerization is not extensive, and it is not known whether such a change in HA size could affect disc function.
The synthesis of collagens and aggrecan also indicated a surprising lack of alteration in experimental discs at both 5 days and 5 weeks after loading. While there were some significant asymmetries of gene expression in angulated groups and some group-wise differences, these also did not identify consistent patterns associating how discs responded to either compression, angulation, or reduced mobility. The expected association of loss of disc height with decreased water and GAG content, with reduced synthesis of collagen and GAGs, and with increased expression of genes associated with disc tissue degradation and degeneration was not supported.
The unexpected lack of disc annulus tissue changes in this rat tail model, despite substantial disc space loss at the intervention levels, may reflect rapid adaptive changes, and also may be associated with differences in the cell population of young rodents compared with human discs [16]. There are limitations in using the rat disc as a model for human discs, notably the presence of notochordal cells and the ossified endplate that may alter the nutritional status of the disc, although annulus tissue only was studied here. The young rat disc may be able (because of different disc cell phenotypes) to avoid tissue degenerative changes that occur in adolescent humans and older rats. However our young rats did undergo substantial loss of disc space and wedging, and the intent of the present study was to identify underlying mechanisms. In the tails with imposed angulation, the within-animal control discs adjacent to the intervention level were used for comparison and included compensating curves and wedging that could influence assymmetry.
Changes in this rat tail model are induced secondary to an imposed spinal deformity, and the imposed altered mechanical environment did not include any non-mechanical factors that might be present in human scoliosis. The average amount of force imposed on the tails of Group C animals was typically 1 N at week 1, increasing to 2.2 N at week 5 (to maintain the nominal 0.1 MPa stress on the disc). Thus it was just less than bodyweight (125 g) at week 1 and about half bodyweight (440 g) at week 5.
Disc narrowing [6] along with alterations in composition [5] in older animals have been reported in axially loaded (compressed) rat discs, and complete immobilization has been associated with disc degeneration in older rats [5,7] and in fused canine spines [17, 18, 19] and in articular cartilage [20]. This suggests that reduced mobility (rather than complete immobilization) may be a factor in disc degeneration in human scoliosis, and may account for why expansion thoracoplasty has been associated with disc degeneration in young patients [21].
In humans, altered disc composition was reported in patients with idiopathic scoliosis, as well as those having scoliosis associated with cerebral palsy, and was thought to be secondary to the spinal curvature, not the cause of it [22]. Differences in hydration and biosynthetic activity in discs of humans with scoliosis was attributed to ineffective response to a pathological mechanical environment [23]. In human adolescents with scoliosis, MR imaging revealed widespread changes in discs with minimal wedging [24]. Excised tissue had decreased water content and decreased cell density, correlating with increasing severity of curves, and endplate damage and alterations in diffusion were observed earlier than MRI changes, suggesting that degenerative changes were primarily due to nutritional factors. Endplate calcification has been observed in discs of humans with scoliosis [25] and in a porcine model of induced scoliosis [26], and may contribute to nutritional deficits.
MacLean et al. [7, 27] reported altered gene expression in rats older than 12 months. In the annulus, both full immobilization (for 72 hours) and dynamic compression (1 MPa at 0.2 Hz) down-regulated anabolic genes and up-regulated catabolic genes [7], with immobilization apparently contributing the largest effect. Dynamic loading at 0.2 MPa had little effect on gene expression [27]. The compression magnitude in the present study was 0.1 MPa, but sustained over a 5-week period. Thus, discs in the present study that were subjected to much lesser stress, but sustained for longer duration and in younger animals (with presumably higher metabolic activity) compared to discs studied by MacLean et al. [7,27], did not show evidence of catabolic response despite their large amount of disc narrowing and the vertebral wedging. Disc narrowing has also been reported in loaded rabbit discs [28], in ‘immobilized’ rat tail discs [5], and mechanically stressed (over-active) rats [29]. Apoptosis and disc narrowing was reported in compressed skeletally mature mouse tail discs [30], and imposed bending produced cellular changes preferentially on the concave side [31].
This model of altered biomechanics of the immature intervertebral disc was intended to identify which components of the altered biomechanical environment were responsible for structural changes in the discs, especially tissue loss (disc narrowing). Since there were disc changes associated with all experimental interventions and ‘reduced mobility’ was present in all interventions, reduced mobility could be a major source of disc changes and may also be a factor in progressive disc deformity in human scoliosis. However, the sparse evidence of compositional, biosynthetic and gene expression differences suggests that underlying metabolic processes were highly active in experimental discs, countering the experimental interventions in this model. The exact relationship of this animal model to intervertebral discs in human scoliosis, both in terms of the biomechanical and metabolic differences, remains to be fully explored.
Keypoints.
Wedging of intervertebral discs in scoliosis is thought to be associated with altered mechanical loading.
Angulation and compression were imposed on immature rat tail discs for five weeks to simulate the supposed altered mechanical environment in scoliosis. Disc annulus composition, synthesis and gene expression were measured.
Intervertebral discs in this model underwent substantial narrowing after 5 weeks, with minimal alteration in tissue composition and minimal evidence of metabolic changes.
Acknowledgments
This work was made possible by a grant from the National Institutes of Health (NIH R01 AR 053132). Some technical support was provided by Haddon Pantel.
Footnotes
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Modi HN, Suh SW, Song HR, Yang JH, Kim HJ, Modi CH. Differential wedging of vertebral body and intervertebral disc in thoracic and lumbar spine in adolescent idiopathic scoliosis - A cross sectional study in 150 patients. Scoliosis. 2008;3:11. doi: 10.1186/1748-7161-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Will RE, Stokes IA, Qiu X, Walker MR, Sanders JO. Cobb angle progression in adolescent scoliosis begins at the intervertebral disc. Spine. 2009;34(25):2782–6. doi: 10.1097/BRS.0b013e3181c11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stokes IAF, McBride C, Aronsson DD, Roughley PJ. Intervertebral disc changes with angulation, compression and reduced mobility simulating altered mechanical environment in scoliosis. Eur Spine J. 2011;20(10):1735–1744. doi: 10.1007/s00586-011-1868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech (Bristol, Avon) 2003;18(3):182–9. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 5.Iatridis JC, Mente PL, Stokes IAF, Aronsson DD, Alini M. Compression induced changes to intervertebral disc properties in a rat tail model. Spine. 1999;24(10):996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Lai A, Chow DH, Siu SW, Leung SS, Lau EF, Tang FH, Pope MH. Effects of static compression with different loading magnitudes and durations on the intervertebral disc: an in vivo rat-tail study. Spine. 2008;33(25):2721–7. doi: 10.1097/BRS.0b013e318180e688. [DOI] [PubMed] [Google Scholar]
- 7.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003 May 15;28(10):973–81. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 8.Wuertz K, Godburn K, MacLean JJ, Barbir A, Donnelly JS, Roughley PJ, Alini M, Iatridis JC. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27(9):1235–42. doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulse Neufeld J, Haghighi P, Machado T. Growth related increase in rat intervertebral disc size: a quantitative radiographic and histologic comparison. Lab Anim Sci. 1990;40:303–7. [PubMed] [Google Scholar]
- 10.Harlan Laboratories. [Accessed November 2012];Sprague Dawley® Outbred Rat. http://www.harlan.com/products_and_services/research_models_and_services/research_models/sprague_dawley_outbred_rat.hl.
- 11.Roach HI, Mehta G, Oreffo RO, Clarke NM, Cooper C. Temporal analysis of rat growth plates: cessation of growth with age despite presence of a physis. J Histochem Cytochem. 2003;51(3):373–83. doi: 10.1177/002215540305100312. [DOI] [PubMed] [Google Scholar]
- 12.Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res. 2006;24(6):1327–34. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 14.Durigova M, Roughley PJ, Mort JS. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthritis Cart. 2008;16:98–104. doi: 10.1016/j.joca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Barbir A, Godburn KE, Michalek AJ, Lai A, Monsey RD, Iatridis JC. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine. 2011;36(8):607–14. doi: 10.1097/BRS.0b013e3181d9b58b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki T, Kobayashi S, Takeno K, Meir A, Urban J, Baba H. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15(12):3835–46. doi: 10.1089/ten.tea.2009.0250. [DOI] [PubMed] [Google Scholar]
- 17.Bushell GR, Ghosh DP, Taylor TK, Sutherland JM, Braund KG. The effect of spinal fusion on the collagen and proteoglycans of the canine intervertebral disc. J Surg Res. 1978;25(1):61–9. doi: 10.1016/0022-4804(78)90159-2. [DOI] [PubMed] [Google Scholar]
- 18.Cole TC, Ghosh P, Hannan NJ, Taylor TK, Bellenger CR. The response of the canine intervertebral disc to immobilization produced by spinal arthrodesis is dependent on constitutional factors. J Orthop Res. 1987;5(3):337–47. doi: 10.1002/jor.1100050305. [DOI] [PubMed] [Google Scholar]
- 19.Cole TC, Burkhardt D, Ghosh P, Ryan M, Taylor T. Effects of spinal fusion on the proteoglycans of the canine intervertebral disc. J Orthop Res. 1985;3(3):277–91. doi: 10.1002/jor.1100030304. [DOI] [PubMed] [Google Scholar]
- 20.Videman T. Connective tissue and immobilization. Key factors in musculoskeletal degeneration? Clin Orthop Relat Res. 1987;(221):26–32. [PubMed] [Google Scholar]
- 21.Yilgor C, Demirkiran G, Ayvaz M, Yazici M. Is expansion thoracoplasty a safe procedure for mobility and growth potential of the spine? Spontaneous fusion after multiple chest distractions in young children. J Pediatr Orthop. 2012;32(5):483–9. doi: 10.1097/BPO.0b013e318257d3a9. [DOI] [PubMed] [Google Scholar]
- 22.Oegema TR, Jr, Bradford DS, Cooper KM, Hunter RE. Comparison of the biochemistry of proteoglycans isolated from normal, idiopathic scoliotic and cerebral palsy spines. Spine. 1983;8(4):378–84. doi: 10.1097/00007632-198305000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Antoniou J, Arlet V, Goswami T, Aebi M, Alini M. Elevated synthetic activity in the convex side of scoliotic intervertebral discs and endplates compared with normal tissues. Spine. 2001;26(10):E198–206. doi: 10.1097/00007632-200105150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Rajasekaran S, Vidyadhara S, Subbiah M, Kamath V, Karunanithi R, Shetty AP, Venkateswaran K, Babu M, Meenakshi J. ISSLS prize winner: A study of effects of in vivo mechanical forces on human lumbar discs with scoliotic disc as a biological model: results from serial postcontrast diffusion studies, histopathology and biochemical analysis of twenty-one human lumbar scoliotic discs. Spine. 2010;35(21):1930–43. doi: 10.1097/BRS.0b013e3181e9a156. [DOI] [PubMed] [Google Scholar]
- 25.Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747–57. doi: 10.1002/jor.1100110517. [DOI] [PubMed] [Google Scholar]
- 26.Laffosse JM, Odent T, Accadbled F, Cachon T, Kinkpe C, Viguier E, Sales de Gauzy J, Swider P. Micro-computed tomography evaluation of vertebral end-plate trabecular bone changes in a porcine asymmetric vertebral tether. J Orthop Res. 2010;28(2):232–40. doi: 10.1002/jor.20974. [DOI] [PubMed] [Google Scholar]
- 27.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine (Phila Pa 1976) 2002;27(23):2684–90. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Neufeld JH. Induced narrowing and back adaptation of lumbar intervertebral discs in biomechanically stressed rats. Spine. 1992;17(7):811–6. doi: 10.1097/00007632-199207000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23(23):2493–506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1(4):239–45. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]