Abstract
Objectives:
To evaluate the effects of 3 T magnetic field on microleakage of amalgam restorations containing three different types of silver (Ag).
Methods:
60 extracted teeth were restored with three different types of amalgam filling materials. Restored teeth were sectioned mesiodistally and divided into experimental and control groups. Experimental groups were exposed to a magnetic field of 3 T for 20 min. All samples were plunged into 2% basic fuchsin solution and examined under a digital microscope by three different observers with regard to microleakage.
Results:
Statistical analysis showed significant differences in microleakage between the groups exposed to MRI and controls, whereas differences in microleakage between amalgam types were insignificant.
Conclusions:
The primary risk of MRI systems arises from the effects of its strong magnetic field on objects containing ferromagnetic materials. An MRI of 1.5 T is known to be safe for amalgam restorations. However, our research indicates that MRI is not completely devoid of any effects on amalgam restorations.
Keywords: amalgam, MRI, microleakage, MRI safety, dental filling
Introduction
Diagnostic imaging in the field of dentistry depends, in general, on X-ray imaging that involves inherent limitations—such as reduction of three-dimensional anatomy onto a two-dimensional image—and risks—such as cancer resulting from exposure to ionizing radiation. Various methods are used in the diagnosis and treatment of oral and maxillofacial pathologies, and there is extensive literature on the benefits of intraoral radiographs, orthopantomograms, CT, cone beam CT, MRI, ultrasonography and positron emission tomography.1,2
MRI is a sophisticated imaging technique that uses strong magnets to create high-resolution images of biological tissue without ionizing radiation.3–5 MRI is often preferred as a fast and non-invasive diagnostic modality for use on the entire human body, especially for the central nervous system, musculoskeletal system, head and neck and abdominal and pelvic examinations.6,7 Also, MRI used in conjunction with spectroscopy, diffusion and perfusion images makes it possible to combine biochemical and physiological data with conventional anatomical information for more accurate diagnoses.8 MRI has three primary advantages over other imaging techniques, namely the capacity to produce very high spatial resolution images of both hard and soft tissue, to produce images in any plane and to provide images without exposure to the ionizing radiation used in X-rays and CT scans.5,6 MRI of the craniomaxillofacial area is currently used to examine salivary glands, articular pathologies of the temporomandibular joint, inflammatory changes of the orofacial region, maxillary sinuses, muscles, haematomas, space infections, head and neck masses and lymphatics, early pathological changes in bones, fractures and anatomical contiguities.4,6,7,9,10 MRI is also used for planning dental implants and locating and evaluating the loci of facial skeletal growth.9
MRI systems produce magnetic fields whose strengths are expressed in units of Tesla, with 1 T equal to 10 000 G (by comparison, the earths’ magnetic field is approximately 0.5 G). MRI scanners with homogenous and stable field strengths ranging from 0.15 T to 11 T may be used on humans, whereas units of up to 24 T may be used in animal experiments.5 In clinical practice, scanners of 3–4 T are considered to be high Tesla devices.11 Higher Tesla translates to higher MR signal rates that, with suitable software, offer advanced neuroimaging. In Turkey, 1.5 T systems are most common, although a small number of 3 T systems exist. In practice, the difference in signal generation allows for the production of superior diagnostic images in the same time frame or images of equal spatial resolution obtained at twice the speed.12 Whereas the former offers the radiologist greater flexibility for different applications, the latter is a positive feature for the patient.11,13
MRI has a number of inherent disadvantages related to the effects of a strong magnetic field, which is strong enough to pull heavy objects towards the scanner at a very high velocity in what is known as the “projectile effect”.5 Metallic objects, implants and biomedical devices are common sources of accidents, and patients with pacemakers, cochlear implants, neurostimulators and infusion pumps are considered to be in a high-risk group.6,14 MRI’s magnetic field can cause these devices to become non-functional, generating life-threatening situations, dislocation (owing to torsion) and soft-tissue burns (owing to absorbance of radiofrequency energy) in patients with fixed metal prostheses and aneurism clips. Needless to say, MRI is contraindicated in these cases.5,6,14,15
Every object in a magnetic field exhibits a degree of magnetism that depends primarily upon material composition, although it should be noted that all materials become magnetized when placed in an external magnetic field, regardless of their inherent level of ferromagnetism. Metallic objects, such as crowns, onlay restorations, inlay restorations, fixed bridges, orthodontic brackets and arches, fixed splints, implants and reconstruction materials (miniplates, miniscrews, stainless steel wires etc.) composed of precious (Au, Ag, Pt) and non-precious (Cr, Co, Mo, Ni, Ti) metals, alloys, amalgams, pure gold fillings, are often located in the orofacial region, and in vitro tests have been designed to characterize the properties of these materials to ensure patient safety.4,16–19 Although fixed dental prostheses, amalgam restorations and orthodontic appliances can produce image distortions on MRI scans of the face, they exhibit minimal deflection in a static magnetic field and are thus regarded as safe for MRI.15
Dental amalgam has been an accepted part of dental treatment for nearly two centuries.20,21 The amalgam alloy currently in use is composed of silver (40–70%), tin (12–30%) and copper (12–24%), and may also include small amounts of palladium (0–0.5%), indium (0–4%) and zinc (0–1%).22 The material possesses many advantages, including simplicity of manipulation, durability, low cost and restoration longevity.23 However, marginal adaptation of a dental restorative material to the cavity walls is critical in terms of longevity, and because amalgam restoration material affords no chemical adhesion to the tooth structure, it is inevitable that microleakage will begin immediately after insertion.24 Microleakage, defined as the passage of fluid, bacteria, molecules or ions and air between a restorative material and the tooth cavity wall, has been identified as a significant problem with amalgam that can result in tooth discoloration, pulp irritation and secondary caries.25
Although 1.5 T MRI systems are common, 3 T MRI systems that exert a stronger static magnetic field, stronger and faster gradient fields and more powerful radiofrequencies have also come into extensive use worldwide, calling into question the safety of metallic objects.11,12,14 Many studies have looked at the interactions of various metal objects with 1.5 T systems, not all those mentioned as safe with 1.5 T systems have been cleared for 3 T systems. Therefore, this study aimed to evaluate potential adverse effects of 3 T magnetic fields on amalgam restorations containing different proportions of silver in terms of microleakage.
Materials and methods
This study was conducted with the approval of the Ethics Committee of the Kirikkale University School of Medicine (no. 12/31). 60 non-carious molar teeth extracted at the Oral and Maxillofacial Surgery Department for various reasons were used in this study.
Sample preparation
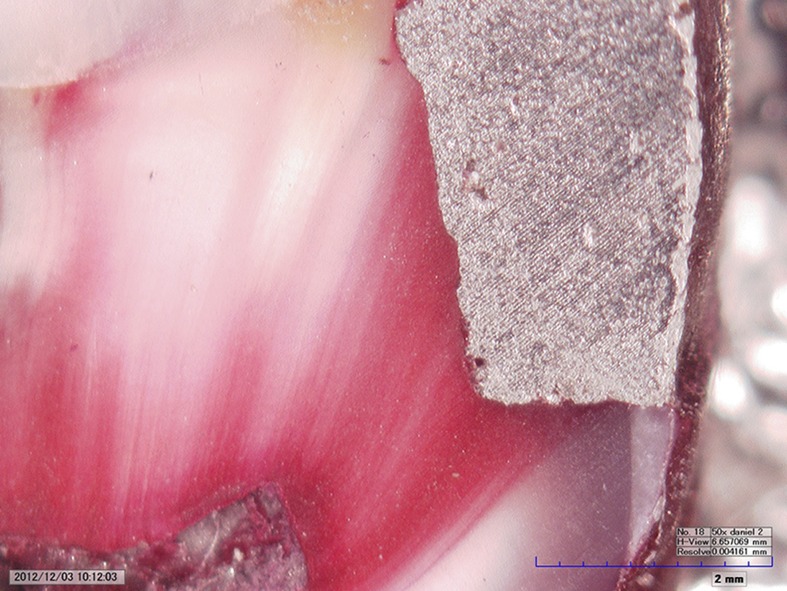
Teeth were stored in isotonic saline solution after surface debridement. Class V rectangular cavities measuring 5 mm mesiodistally, 3 mm occlusogingivally and 2 mm in depth were prepared within the enamel borders using no. 837L diamond burs (DMA Dilman™, Ankara, Turkey) with a high-speed handpiece underwater spray. Cavity dimensions were calibrated using a periodontal probe, and burs were changed after a maximum of five preparations. Following preparations, teeth were randomly divided into three groups (n = 20) according to the composition of the amalgam restoration material used as follows: Group 1, 40% Ag; Group 2, 50% Ag; and Group 3, 70% Ag. Amalgam was condensed in the direction of the cavity walls and restorations were burnished and polished following standard procedures. Teeth were stored in saline solution throughout the experiment (approximately 3 months). The teeth were sectioned mesiodistally using 125 mm diameter diamond-coated wafering blades (Metkon Instruments™ Ltd, Bursa, Turkey) in a linear precision cutting device (Micra Cut 125, Metkon, Turkey). Buccal and lingual sections of each tooth were randomly distributed into experimental and control subgroups and all teeth were coated with a thin layer of nail varnish up to 1 mm around the restoration margins. In our preliminary testing, we observed pulpal basic fuchsin staining that disabled microleakage evaluation (Figure 1). Therefore, the inner surfaces were sealed with a thin layer of cyanoacrylate glue to prevent undesirable dye penetration.
Figure 1.
Staining by the improper preparation of specimens in our preliminary study
MRI protocol
Samples in the experimental subgroups were exposed to a 3 T magnetic field (Siemens MAGNETOM Trio™, Siemens Medical Systems, Erlangen, Germany). As in a T1 anatomical imaging protocol, the specimens were setted in gantry and 20 min of MRI was performed. The control group was located outside of the magnetic field and experiment room.
Microleakage evaluation
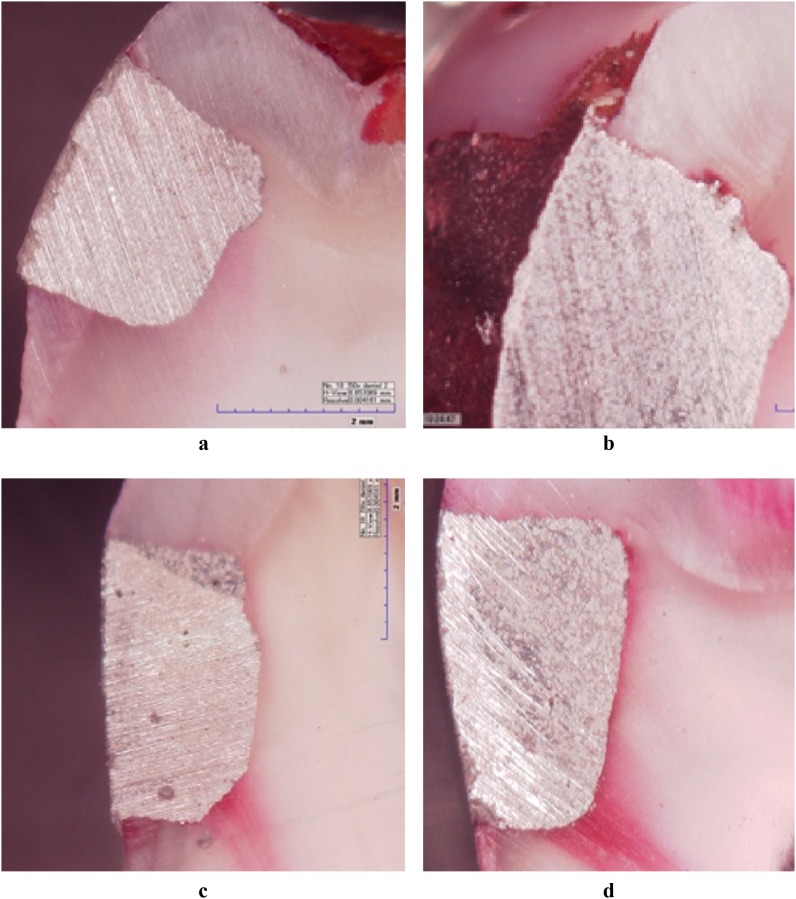
All specimens were immersed in 2% basic fuchsin solution at room temperature subsequent to the MRI protocol. The specimens were removed after 24 h, cleaned with water and pumice slurry and then sectioned buccolingually through the center of the restoration using a diamond saw in a linear precision cutting device under watercooling. No fracturing or detachment of restorations was observed during the sectioning procedures. Samples were examined for microleakage under a digital microscope (KH-7700 Hirox Digital microscope, Tokyo, Japan) at ×50 magnification in a random order by three observers (Observers 1, 2 and 3) blinded to the groups. The microleakage was scored as follows: 0, no dye penetration; 1, dye penetration in the enamel; 2, dye penetration in the dentin and enamel but not in the axial wall of the restoration; and 3, dye penetration along the axial wall (Figure 2).
Figure 2.
Microleakage scores of dye penetration. (a) An example of no dye penetration (Score 0) in the control group. (b) Dye penetration (Score 1) along the enamel in 50% Ag control group. (c) Shows microleakage (Score 2) in 70% MRI group. (d) A sample of dye penetration (Score 3) in 40% Ag MRI group
Statistical analysis
Wilcoxon and Kruskal–Wallis tests were used to compare microleakage in the experimental and control groups. Cronbach’s Alpha test was used to evaluate the interobserver reliability of microleakage scores. Mean values of the different observer scores were calculated and used as a basis for statistical analysis.
Results
We evaluated 240 inner surfaces of Class V amalgam restorations on 60 extracted teeth. Any fracturing or dislodging of restoration and pulpal overstaining was observed during the experiment. We evaluated interobserver consistency in scoring microleakage forms, and it was revealed that the interobserver consistency was not reliable (p = 0.087). Therefore, mean values of the three observer scores were calculated and used as a basis for statistical analysis. Specimens exposed to MRI exhibited significantly higher microleakage values than control specimens (40%, p = 0.014; 50%, p = 0.007; 70%, p = 0.003; Table 1). The diversity of dye penetration between the control group and the MRI experimental group is shown in Figure 2a (no dye penetration with Score 0) and 2d (dye penetration Score 3). Meanwhile, no differences were observed between the silver ratios of subgroups in both control and MRI experimental groups (p = 0.072; Table 2). Dye penetration in MRI subgroups of 70% Ag and 40% Ag is shown in Figure 2c,d. Also different patterns of dye penetration in the samples of 50% Ag control group and 50% Ag MRI group can be seen in Figures 2b and 3.
Table 1.
Comparison of microleakage scores between MRI experiment and control groups
| Variables | A | B | C |
| Subject number | 20 | 20 | 20 |
| MRI and control groups | A1, A2 | B1, B2 | C1, C2 |
| Number | 20, 20 | 20, 20 | 20, 20 |
| Mean rank | 16.03, 24.98 | 15.63, 25.38 | 18.10, 26.88 |
| p-value (Wilcoxon test) | 0.014 | 0.007 | 0.003 |
A1 is the control group of 40% Ag amalgam restorated teeth, A2 is the MRI experiment group of 40% amalgam, B1 is the control group of specimens that restorated with 50% Ag amalgam, B2 is the MRI experiment group of specimens that restorated with 50% Ag amalgam, C1 is the control group of specimens that restorated with 70% Ag amalgam and C2 is the MRI experiment group of specimens that restorated with 70% Ag amalgam.
Table 2.
Comparison between different silver subgroups of the MRI experiment group
| Silver group | Mean rank |
| A | 28.30 |
| B | 28.08 |
| C | 35.13 |
| p-value (Kruskal–Wallis test) | 0.072 |
A, specimens that restorated with 40% Ag amalgam; B, specimens that restorated with 50% Ag amalgam; C, specimens that restorated with 70% Ag amalgam restorations.
Figure 3.
Dye penetration in half of the 50% Ag MRI group
Discussion
To the best of our knowledge, the literature includes only one similar study evaluating the effects of magnetic fields on amalgam restorations.3 In that study, Shahidi et al3 compared the response of three different brands of amalgam with a 1.5 T magnetic field: CinaLux (non-gamma 2 spherical amalgam; Faghihi Dental, Tehran, Iran), GS-80 (non-gamma 2 admix amalgam; SDI, Victoria, Australia) and Vivacap (non-gamma 2 spherical amalgam; Ivoclar Vivadent, Liechtenstein, Germany). Silver is the main component in amalgam, accounting for approximately 60% of the material, with tin, copper and zinc the other main components. Silver is diamagnetic but becomes paramagnetic when it reacts with mercury during setting.26,27 Therefore, in the present study, the effects of a 3 T magnetic field on amalgam restoration materials with different ratios of silver content (40%, 50% and 70%) was evaluated.
We could not find any investigation on the subject of the effects of 3 T MRI on the currently used improved amalgam restorative materials. There are only a few studies that are about mercury release by the MRI process.14
In this study, preliminary testings demonstrated that, despite a coating of nail varnish around restoration margins and the pulpal surface, excessive pulpal staining with basic fuchsin dye inhibited microleakage examination; therefore, cyanoacrylate glue was used to coat the inner surfaces of specimens to provide sufficient isolation for microleakage evaluation (see Figure 1).
Dye penetration patterns in this study differed noticeably from those with Shahidi et al3. Specifically, in the present study, a linear pattern of dye penetration along dentinal tubules was observed in contrast to the blot-like staining seen in Shahidi et al (Figure 2).
Shahidi et al3 found significant differences between microleakage scores of GS-80 and Vivacap specimens exposed to a 1.5 T magnetic field but found no difference in microleakage scores of CinaLux specimens subjected to MRI and CinaLux controls. The present study subjected specimens placed to a much stronger 3 T magnetic field, and significant differences in microleakage were observed between all the MRI and control groups. However, no microleakage differences were observed between the silver ingredients of both MRI and control subgroups.
Despite numerous studies in the literature reporting on the potential adverse effects of dental amalgam, Uçar and Brantley28 have stated that the existing scientific data do not justify replacing amalgam with alternative restorative dental materials. Exposure of amalgam restorations, orthodontic braces and wires, implants and other metal comprising common dental prosthetics to an MRI is not contraindicated.15 With regard to the microleakage, observed following exposure to a 3 T magnetic field, a long-term observation is necessary.
In the current study, weak intraobserver reliability was observed in the evaluation of microleakage using the same scale and a digital microscope. This may be owing to a lack of standardization of the views with the aid of a visual microleakage scale. As noted by Shahidi et al, more sensitive techniques such as measurement of ammoniac silver nitrate penetration between the amalgam and the tooth structure could provide more accurate evaluation of microleakage.
In conclusion, we found higher microleakage scores in amalgam restorated teeth in vitro, which were exposed to 3 T MRI procedure regardless of their silver content. 3 T MRI is not contraindicated for patients who have amalgam restorations; however, it needs to be evaluated from different perspectives.
Acknowledgments
This research was supported by the Scientific Research Projects Coordination Unit of Kirikkale University. We would like to extend our sincere thanks to Prof Dr İsmail Karaca for his kind help.
References
- 1.Morimoto Y, Tanaka T, Yamamoto N, Kodama M, Seta Y, Habu M. New trends and advances in oral and maxillofacial imaging. Curr Med Imaging Rev 2009; 5: 226–237 [Google Scholar]
- 2.Idiyatullin D, Corum C, Moeller S, Prasad HS, Garwood M, Nixdorf DR. Dental magnetic resonance imaging: making the invisible visible. J Endod 2011; 37: 745–752 10.1016/j.joen.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahidi SH, Bronoosh P, Alavi AA, Zamiri B, Sadeghi AR, Bagheri MH, et al. Effect of magnetic resonance imaging on microleakage of amalgam restorations: an in vitro study. Dentomaxillofac Radiol 2009; 38: 470–474 10.1259/dmfr/30077669 [DOI] [PubMed] [Google Scholar]
- 4.Hubálková H, La Serna P, Linetskiy I, Dostálová T. Dental alloys and magnetic resonance imaging. Int Dent J 2006; 56: 135–141 [DOI] [PubMed] [Google Scholar]
- 5.Huettel SA, Song AW, McCarthy G. Functional magnetic resonance imaging. 2nd edn Sunderland, MA: Sinauer Associates, Inc; 2008 [Google Scholar]
- 6.Oyar O. The clinical use and indications of magnetic resonance imaging. Harran Univ Tıp Fak Derg 2008; 5: 31–40 [Google Scholar]
- 7.Tutton LM, Goddard PR. MRI of the teeth. Br J Radiol 2002; 75: 552–562 [DOI] [PubMed] [Google Scholar]
- 8.Sentürk S, Oğuz KK, Cila A. Dynamic contrast-enhanced susceptibility-weighted perfusion imaging of intracranial tumors: a study using a 3T MR scanner. Diagn Interv Radiol 2009; 15: 3–12 [PubMed] [Google Scholar]
- 9.Tanasiewicz M. Magnetic resonance imaging in human teeth internal space visualization for requirements of dental prosthetics. J Clin Exp Dent 2010; 2: 6–11 [Google Scholar]
- 10.Nasel CJ, Pretterklieber M, Gahleitner A, Czerny C, Breitenseher M, Imhof H. Osteometry of the mandible performed using dental MR imaging. AJNR Am J Neuroradiol 1999; 20: 1221–1227 [PMC free article] [PubMed] [Google Scholar]
- 11.Dinçer A. Advanced MRI applications and 3 Tesla in neuroradiology [Nöroradyolojide İleri MR Uygulamaları ve 3 Tesla MR]. Klin Gelisim 2010; 23: 1–5 [Google Scholar]
- 12.Jerrolds J, Keene S. MRI safety at 3T versus 1.5T. Internet J World Health Soc Politics 2009; 6: 1 [Google Scholar]
- 13.Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging 2002; 16: 721–732 10.1002/jmri.10207 [DOI] [PubMed] [Google Scholar]
- 14.Mortazavi SM, Daiee E, Yazdi A, Khiabani K, Kavousi A, Vazirinejad R. Mercury release from amalgam restorations after magnetic resonance imaging and following mobile phone use. Pak J Biol Sci 2008; 11: 1142–1146 [DOI] [PubMed] [Google Scholar]
- 15.Shellock FG. MR imaging of metallic implants and materials: a compilation of the literature. AJR Am J Roentgenol 1988; 151: 811–814 10.2214/ajr.151.4.811 [DOI] [PubMed] [Google Scholar]
- 16.Costa AL, Appenzeller S, Yasuda CL, Pereira FR, Zanardi VA, Cendes F. Artifacts in brain magnetic resonance imaging due to metallic dental objects. Med Oral Patol Oral Cir Bucal 2009; 1: E278–E282 [PubMed] [Google Scholar]
- 17.Klocke A, Kahl-Nieke B, Adam G, Kemper J. Magnetic forces on orthodontic wires in high field magnetic resonance imaging (MRI) at 3 Tesla. J Orofac Orthop 2006; 67: 424–429 10.1007/s00056-006-0621-x [DOI] [PubMed] [Google Scholar]
- 18.Gill A, Shellock FG. Assessment of MRI issues at 3-Tesla for metallic surgical implants: findings applied to 61 additional skin closure staples and vessel ligation clips. J Cardiovasc Magn Reson 2012; 14: 3 10.1186/1532-429X-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann W, Von Ammon K, Fink U, Weik T, Yasargil GM. Aneurysm clips made of titanium: magnetic characteristics and artifacts in MR. AJNR Am J Neuroradiol 1997; 18: 939–944 [PMC free article] [PubMed] [Google Scholar]
- 20.Anusavice KJ. Phillips’ science of dental materials. 11th edn Philadelphia, PA: Saunders; 2003 [Google Scholar]
- 21.Sekhar KR, Vasa AAK, Sahana S, Vijaya Prasad KE. Comparative evaluation of marginal microleakage in amalgam restorations of permanent and primary teeth: a stereomicroscopic study. Ann Essence Dent 2010; 2: 6–9 [Google Scholar]
- 22.Bharti R, Wadhwani KK, Tikku AP, Chandra A. Dental amalgam: an update. J Conserv Dent 2010; 13: 204–208 10.4103/0972-0707.73380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans MC. 12-year survival of composite vs. amalgam restorations. J Dent Res 2010; 89: 1063–1067 10.1177/0022034510376071 [DOI] [PubMed] [Google Scholar]
- 24.Toledano M, Osorio E, Osorio R, García-Godoy F. Microleakage and SEM interfacial micromorphology of amalgam restorations using three adhesive systems. J Dent 2000; 28: 423–428 [DOI] [PubMed] [Google Scholar]
- 25.Cenci MS, Piva E, Potrich F, Formolo E, Demarco FF, Powers JM. Microleakage in bonded amalgam restorations using different adhesive materials. Braz Dent J 2004; 15: 13–18 10.1590/S0103-64402004000100003 [DOI] [PubMed] [Google Scholar]
- 26.Bates LF, Ireland AW. The magnetic properties of silver amalgams. Proc Phys Soc 1937; 49: 642–645 Available from: http://iopscience.iop.org/0959-5309/49/6/302 [Google Scholar]
- 27.Bates LF, Tai LC. The magnetic properties of amalgams. Proc Phys Soc 1936; 48: 795–809 Available from: http://iopscience.iop.org/0959-5309/48/5/312 [Google Scholar]
- 28.Uçar Y, Brantley WA. Biocompatibility of dental amalgams. Int J Dent 2011; 2011: 981595 10.1155/2011/981595 [DOI] [PMC free article] [PubMed] [Google Scholar]