Abstract
Certain cognitive measures are heritable and differentiate individuals at risk for schizophrenia from unaffected family members and healthy comparison subjects. These deficits in neurocognitive performance in patients with schizophrenia appear stable in the short-term. However, the duration of most, but not all, longitudinal studies is modest and the majority have relied on traditional average performance measures to examine stability. Using a computerized neurocognitive battery (CNB), we assessed mean performance (accuracy and speed) and intra-individual variability (IIV) in a longitudinal study aimed to examine neurocognitive stability in European-American multiplex families with schizophrenia. Thirty-four patients with schizophrenia, 65 unaffected relatives, and 45 healthy comparison subjects completed the same computerized neurocognitive assessment over approximately 5 years. Measures of mean performance showed that patients had stable accuracy performance but were slower in many neurocognitive domains over time as compared with unaffected family members and healthy subjects. Furthermore, patients and family members showed dissociable patterns of change in IIV for speed across cognitive domains: compared with controls, patients showed higher across-task IIV in performance compared with family members, who showed lower across-task IIV. Patients showed an increase in IIV over time, whereas family members showed a decrease. These findings suggest that measures of mean performance and IIV of speed during a CNB may provide useful information about the genetic susceptibility in schizophrenia.
Key words: intra-individual variability, schizophrenia, cognition, family
Introduction
Schizophrenia is a heritable disorder with persistent neurocognitive1–3deficits in executive functioning, learning and memory, and processing speed.1–4 These deficits, examined as endophenotypic markers5 in unaffected relatives of patients with schizophrenia (eg, Cannon et al.6), are heritable.3,7 Deficits in neurocognitive performance in patients with schizophrenia appear stable in the short-term.8–10 For example, little change in variability was found over short (ie, hours) and intermediate (ie, 1 month) intervals in patients on a brief neuropsychological battery.11 However, the duration of most, but not all,9 longitudinal studies is modest and most have relied on traditional average performance measures to examine stability.
Mean Measures as an Index of Neurocognitive Performance
Neurocognitive deficits in schizophrenia commonly examine mean performance measures. Such comparisons indicate greater between-subject variability in patients relative to healthy individuals or family members. Greater group differences persist even when other demographic and illness-associated factors are considered.4 Reducing group differences by classifying patients based on specific symptoms indicates differentiable neurocognitive performance patterns.12 These group differences in neurocognitive performance are linked to genetic polymorphisms implicated in schizophrenia (eg, catechol-O-methyl transferase; COMT).13 Moreover, unaffected relatives show substantial inter-individual variability in some neurocognitive domains and differ from healthy individuals.14 While, mean performance captures neurocognitive performance, the emphasis on the mean may disregard other important facets of performance,5 which may decrease the likelihood of detecting change over time.
Across-Task Intra-Individual Variability
Intra-individual variability (IIV) reflects within-person fluctuations in neurocognitive performance assessing the stability of cognitive processing.5,15 When within-person variability increases and is systematic, indexing performance based upon a single measurement (eg, mean performance) may result in poor estimates of group differences.16 Thus, IIV has emerged as a useful construct for assessing cognitive performance in disorders such as attention deficit hyperactivity disorder (ADHD)17 and schizophrenia.18–20 Typically, IIV is measured across trials within a given domain21,22 and is limited to measures of performance speed. But IIV can also be calculated across neurocognitive domains, providing a broad index of brain function for accuracy or speed.23 Recently, a large study found greater across-task IIV in patients compared with their unaffected siblings, who showed more IIV than healthy individuals.24 Yet, longitudinal changes in IIV across neurocognitive measures have not been measured in patients with schizophrenia and their family members. Evaluating longitudinal neurocognitive performance using across-task IIV may provide a robust measure of neurocognitive performance and enable detection of change over time that better correlates with genetic liability. The aim of this study was to assess the stability of cognitive performance over time using mean performance and a measure of across-task IIV in patients with schizophrenia and their family members using the computerized neurocognitive battery (CNB), which targets cognitive domains that show characteristic dysfunction in schizophrenia.4
Methods
Participants
The sample of 99 European-Americans from 26 multiplex multigenerational families was recruited at two sites (Philadelphia and Pittsburgh) and completed two computerized neurocognitive assessments. This cohort is a subsample of a previously characterized sample.3 Briefly, patients had an extended multigenerational family, with at least 10 first- and/or second-degree relatives. Consensus best-estimate DSM-IV diagnosis of schizophrenia or schizoaffective disorder was made for each proband via clinical interview or in some cases (patient incarceration or deceased) via evaluation of medical records. Participants were older than 15 years of age at initial contact and provided signed informed consent. The Institutional Review Boards of participating institutions approved the study. For minors < 18 years old, assent was obtained from the child and consent from a parent. These data were collected as part of a larger genomics project examining genetic mechanisms of schizophrenia. To reduce genetic heterogeneity, the sample was restricted to Caucasian individuals.
Schizophrenia participants (n = 34) were competent to provide informed consent, capable of participate and not exhibiting acute positive symptomatology that required medication adjustment or hospitalization within the past year. Thirty-one patients were medicated with antipsychotic medication at baseline, one was not medicated and medication information was not available for two patients. Family members (n = 65) were excluded if they had mental retardation (IQ < 70), a central nervous system disorder that hindered performance, or were not proficient in English. Patients and family members were diagnostically reevaluated at follow-up. Diagnosis was unchanged in all patients and in 53 family members. Twelve family members had minor diagnostic changes that were deemed clinically insignificant (eg, past substance abuse/dependence, currently in full remission). Global functioning and clinical symptomatology were assessed at baseline in most patients and family members. Global functioning was measured using the Global Assessment of Functioning (GAF)25 with higher scores indicating better functioning. The Scale for the Assessment of Negative Symptoms (SANS)26 and the Scale for the Assessment of Positive Symptoms (SAPS)27 were used to rate the presence and severity of negative and positive symptoms. The Wide Range Achievement Test, version 3 (WRAT3),28 a brief estimate of IQ, was measured in most subjects at the initial testing. Of those individuals who had two neurocognitive assessments, 18 families (78 individuals) consisted of at least 1 patient with schizophrenia and at least 1 family member, 3 families (4 individuals) consisted of only patients, and 5 families (17 individuals) consisted of only family members.
The comparison group included 45 psychiatrically, medically, and neurologically healthy European-Americans with no axis I and axis II cluster A disorders, no history of psychosis or mood disorder in their first-degree relatives and stable over time. Healthy comparison subjects were recruited from the same communities as patients and families underwent urine drug testing to screen for current substance abuse. At project initiation, the research teams at the two test sites participated in a 3-day workshop that covered all procedures related to ascertainment, screening, assessment, consensus diagnosis, reliability, and neurocognitive testing to ensure cross-site consistency. We found no difference is performance across sites for the comparison subjects in this sample. Demographic information for each group is provided in table 1.
Table 1.
Demographic Information for Healthy Comparison Subjects, Schizophrenia Patients, and Their Unaffected Relatives
| Healthy Comparison (n = 45) | Schizophrenia (n = 34) | Unaffected Family (n = 65) | |
|---|---|---|---|
| Age (Time 1), years | 46.82 (16.77) | 44.53 (8.48) | 40.45 (16.20) |
| Age (Time 2), years | 50.69 (17.49) | 51.00 (8.87) | 46.18 (16.78) |
| Age (range), years | 23–80 | 20–56 | 16–79 |
| Retest interval, years | 3.93 (2.22) | 6.43 (1.59)** | 5.67 (1.62)** |
| Retest interval range, months | 1–155 | 47–107 | 42–107 |
| Education, years | 15.31 (2.20) | 12.50 (2.03)** | 13.58 (2.87)** |
| Parental EDU, years | 13.24 (2.70) | 12.44 (2.78) | 11.57 (2.90)** |
| Sex, % M | 42.22% | 55.88% | 46.15% |
| Handedness, % R | 86.67% | 91.18% | 84.62% |
| WRAT (standard) | 107.11 (9.80) | 90.16 (16.25)** | 98.84 (14.90)* |
| GAF | n/a | 50.51 (12.67) # | 79.44 (13.19) |
| SANS | n/a | 17.33 (14.21)# | 5.88 (9.79) |
| SAPS | n/a | 18.96 (17.38)# | 1.97 (5.54) |
| Medication Atypicals Typicals None/Unknown |
— | 24 7 3 |
— |
Note: GAF, Global Assessment of Functioning Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; WRAT, Wide Range Achievement Test.
** P < .01, * P < .05 as compared with healthy comparison subjects.
# P < .05 as compared with unaffected family members.
Computerized Neurocognitive Battery
The CNB evaluates several domains including abstraction and mental flexibility, attention, language, memory—verbal, facial, visuo-spatial—as well as sensorimotor and emotion processing. Details regarding tests and administration have been published.3,29,30
Statistical Methods and Analyses
Test-Retest Reliability of the CNB.
Reliability of neurocognitive measures was calculated with intraclass correlation coefficients (ICC) for degree of consistency for both raw accuracy and speed in those with complete data at baseline and follow-up. The magnitudes of the ICCs for patients and comparison subjects were compared using a Fisher r-to-z transformation for ICC.31
Mean Performance Comparisons.
Group differences in neurocognition were analyzed using z-transformed scores for each domain. General linear models with mixed effects were used to compare overall CNB performance. The between-subject factor was diagnostic group and the within-subject factor was CNB domain. Age, parental education, and interval between CNB test administrations were included as covariates. Analyses were performed for each test administration (Time 1 and Time 2). Overall effects were followed-up by comparing performance between group pairs (eg, patients versus healthy comparison subjects) using mixed model analyses taking into account family relationships through incorporation of a kinship coefficient. Raw performance score are presented in table 3.
Table 3.
Mean, Standard Deviation, and Effect Size of the Difference Over Time for Accuracy and Speed Scores on the Computerized Neurocognitive Battery (cnb) for Healthy Comparison Subjects, Schizophrenia Patients, and Unaffected Family Members with Test at Both Time Points29
| Domain (test) | Healthy | Schizophrenia | Unaffected Family | ||||||||||
| n b | Time 1 | Time 2 | d d | n b | Time 1 | Time 2 | d d | n b | Time 1 | Time 2 | d d | ||
| Accuracya | |||||||||||||
| ABF | 40 | 2.40 (0.83) | 2.75 (0.77) | 0.17 | 32 | 0.85 (0.85) | 1.09 (0.96) | 0.26 | 62 | 2.20 (1.04) | 2.54* (0.87) | 0.35 | |
| ATT | 27 | 116.48 (5.55) | 117.37 (3.03) | 0.19 | 28 | 103.86 (13.04) | 102.61 (14.65) | −0.09 | 61 | 112.45 (9.42) | 115.20* (6.77) | 0.33 | |
| VMEM | 38 | 35.42 (2.77) | 35.04 (3.02) | −0.13 | 34 | 32.67 (3.52) | 31.69 (4.24) | −0.25 | 64 | 34.65 (3.69) | 33.86 (3.68) | −0.21 | |
| FMEM | 44 | 32.19 (4.11) | 34.25* (3.38) | 0.54 | 34 | 28.81 (3.51) | 29.50 (3.79) | 0.18 | 64 | 30.92 (4.19) | 32.64* (4.48) | 0.39 | |
| SMEM | 41 | 15.43 (1.81) | 15.85 (2.21) | 0.21 | 31 | 13.19 (2.39) | 12.88 (2.13) | −0.14 | 63 | 15.08 (2.05) | 15.27 (2.18) | 0.09 | |
| LAN | 34 | 69.37 (15.27) | 69.27 (13.59) | −0.01 | 14 | 45.57 (16.46) | 45.07 (16.59) | −0.03 | 42 | 63.13 (19.01) | 62.80 (19.05) | −0.02 | |
| SMc | — | — | — | — | — | — | — | — | — | — | — | — | |
| EMO | 40 | 33.25 (2.96) | 33.30 (3.08) | 0.02 | 32 | 29.31 (5.16) | 28.47 (4.61) | −0.17 | 65 | 32.55 (4.30) | 31.96 (4.15) | −0.14 | |
| Speed (ms) | |||||||||||||
| AMF | 40 | 2906 (1474) | 2522 (1404) | 0.27 | 32 | 4146 (1898) | 3941 (1846) | 0.11 | 62 | 2729 (955) | 2598 (1004) | 0.13 | |
| ATT | 27 | 511 (51) | 495 (61) | 0.28 | 28 | 570 (76) | 560 (89) | 0.12 | 61 | 503 (68) | 490 (56) | 0.21 | |
| VMEM | 38 | 1310 (270) | 1696* (697) | −0.19 | 34 | 1700 (460) | 3095* (1719) | −1.10 | 64 | 1440 (493) | 1870* (667) | −0.73 | |
| FMEM | 44 | 1892 (541) | 1940 (752) | −0.07 | 34 | 2062 (681) | 2831* (1295) | −0.74 | 64 | 2003 (738) | 2190* (826) | −0.23 | |
| SMEM | 41 | 1584 (381) | 1648 (407) | −0.16 | 31 | 1793 (680) | 2106 (671) | −0.46 | 63 | 1732 (625) | 1801 (608) | −0.11 | |
| LAN | 34 | 8420 (4034) | 7577 (1973) | 0.26 | 14 | 9455 (5107) | 8573 (2750) | 0.21 | 42 | 9578 (3781) | 8742 (3518) | 0.22 | |
| SM | 43 | 915 (445) | 867 (272) | 0.13 | 31 | 1509 (646) | 1760* (687) | −0.37 | 63 | 951 (316) | 1023 (362) | −0.21 | |
| EMO | 40 | 2300 (704) | 2272 (590) | 0.04 | 32 | 3488 (1248) | 4047 (1344) | −0.48 | 65 | 2854 (1165) | 2723 (870) | 0.12 | |
Note: Negative effect size indicates poorer performance at Time 2.
ABF, Abstraction and Mental Flexibility; ATT, Attention; VMEM, Verbal Memory; FMEM, Face Memory (FMEM); SMEM, Spatial Memory; LAN, Language Reasoning; SM, Sensorimotor Processing Speed; EMO, Emotion Processing (EMO).
aAccuracy measures include number of items correct (Attention, VMEM, FMEM, SMEM, EMO); % correct (LAN) and (number correct/number of categories attained) + 1 (ABF)-see text for detailed description.
b This number reflects only those individuals who had completed the CNB at both Time 1 and Time 2.
cNot amenable for calculation.
dCohen’s d.
* P < .05.
Across-Task Intra-Individual Variability.
Within-person across-test variability was calculated as in a previous study (see “Appendix”).23 Variability scores were computed for accuracy and speed at Time 1 and Time 2 and were compared as described for the CNB tasks. For this analysis, diagnostic group was considered a between-subjects factor.
Stability of Neurocognitive Performance.
Difference in performance was calculated for each CNB measure as accuracy, speed, or IIV at Time 2 minus accuracy, speed, or IIV at Time 1, respectively. A variance component model was used to test whether the mean of difference in performance was different from zero in the subgroups of healthy controls, individuals with schizophrenia, and family members, while accounting for the nonindependence between patients and their family members via incorporation of a kinship matrix. The likelihood of a model estimating the mean difference in trait values between testing sessions was compared with the likelihood of a model in which the mean difference was fixed to zero, resulting in a one degree of freedom chi-square test. Age, parental education, and interval between CNB test administrations were included as covariates. The significance threshold was set at P < .05.
Results
Test-Retest Reliability of the cnb Over an Extended Period
Test-retest reliability across the two versions of the CNB (table 2) is acceptable to be high across most domains in comparison subjects, although accuracy for spatial memory and attention, and speed of verbal reasoning showed lower ICCs. A similar pattern of ICCs was observed in patients and family members. Comparison of CNB ICCs between groups, using Fisher r-to-z transformations, indicated no group difference in the test-retest reliability.
Table 2.
Test-Retest Reliability of the Accuracy and Speed on the Computerized Neuropsychological Battery for Each Domain in Comparison Subjects, Patients with Schizophrenia, and Unaffected Relatives with Tests at Both Time Points29
| Domain (Test) | Healthy Subjects | Schizophrenia | Unaffected Family | HC versus Schizophrenia | HC versus Unaffected Family | ||
|---|---|---|---|---|---|---|---|
| z | P | z | P | ||||
| Accuracy | |||||||
| ABF | 0.70 | 0.34# | 0.70 | 1.45 | 0.15 | −0.01 | 0.99 |
| ATT | 0.45# | 0.70 | 0.64 | −0.90 | 0.37 | −0.78 | 0.44 |
| VMEM | 0.76 | 0.64 | 0.83 | 0.54 | 0.59 | −0.28 | 0.78 |
| FMEM | 0.87 | 0.84 | 0.86 | 0.11 | 0.91 | 0.03 | 0.98 |
| SMEM | 0.32# | 0.22# | 0.56 | 0.40 | 0.69 | −1.13 | 0.26 |
| LAN | 0.77 | 0.89 | 0.87 | −0.34 | 0.73 | −0.44 | 0.66 |
| SMa | — | — | — | — | — | — | — |
| EMO | 0.75 | 0.63 | 0.76 | 0.46 | 0.65 | −0.03 | 0.98 |
| Speed | |||||||
| ABF | 0.85 | 0.55 | 0.80 | 1.20 | 0.23 | 0.23 | 0.82 |
| ATT | 0.76 | 0.86 | 0.67 | −0.29 | 0.77 | 0.40 | 0.69 |
| VMEM | 0.62 | 0.39# | 0.63 | 0.90 | 0.37 | −0.03 | 0.98 |
| FMEM | 0.83 | 0.56 | 0.87 | 1.12 | 0.26 | −0.19 | 0.85 |
| SMEM | 0.66 | 0.66 | 0.78 | 0.00 | 0.99 | −0.58 | 0.56 |
| LAN | 0.32# | 0.29# | 0.62 | 0.07 | 0.94 | −1.23 | 0.22 |
| SM | 0.84 | 0.85 | 0.54 | −0.05 | 0.96 | 1.47 | 0.14 |
| EMO | 0.87 | 0.41# | 0.72 | 1.84 | 0.06 | 0.70 | 0.48 |
Note: Test-retest reliability values are intraclass correlation coefficients (ICC) for degree of consistency between measurements.30 The z and P values reflect the comparison of ICC magnitude between groups. The magnitude of the ICC was compared by using a Fisher r-to-z transformation for ICC.
Note: ABF, Abstraction and Mental Flexibility; ATT, Attention; VMEM, Verbal Memory; FMEM, Face Memory; SMEM, Spatial Memory; LAN, Language Reasoning; SM, Sensorimotor Processing Speed; EMO, Emotion Processing; HC, Healthy comparison subjects.
a Speed is the only dependent measure used for this task.
#Reliability testing did NOT reach significance (P > .05).
Neurocognitive Performance
The neurocognitive profiles for each group are presented in table 3. Groups differed in accuracy (Time 1: [F(2,74) = 16.62, P < .001]; Time 2: [F(2,122) = 32.77, P < .001]) and speed (Time 1: [F(2,74) = 5.11, P = .008]; Time 2: [F(2,122) = 27.38, P < .001]) at each time point. There was a main effect of domain for accuracy [F(6,444) = 3.54, P = .002] but not speed [P = .07] at Time 1. The main effect of domain was significant for accuracy [F(6,732) = 3.94, P < .001] and speed [F(7,854) = 2.09, P = .04] at Time 2. The group-by-domain interaction was significant for accuracy (Time 1: [F(12,444) = 2.64, P = .002]; Time 2: [F(12,732) = 2.27, P = .008]) and speed (Time 1: [F(14,518) = 3.03, P < .001]; Time 2: [F(14,854) = 5.87, P = .001]) at both time points.
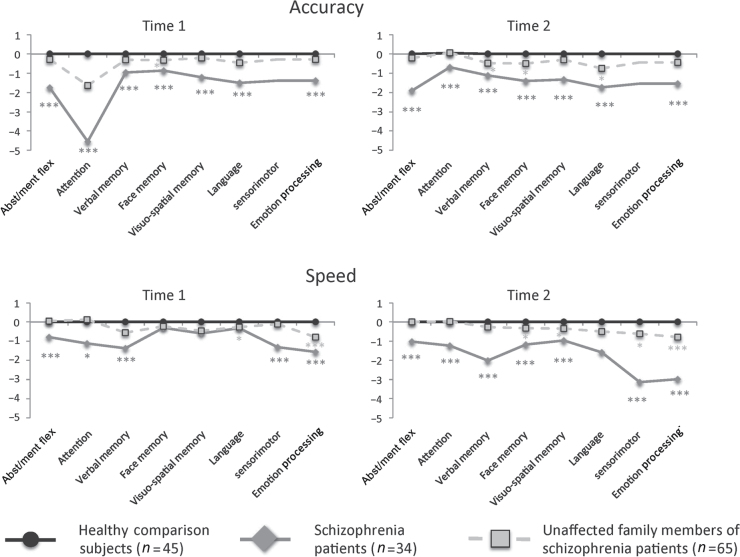
Patients with schizophrenia performed most poorly across domains and measures, while relatives performed at an intermediate level (figure 1). At Time 1, patients were significantly less accurate than comparison subjects (all P < 0.001) and family (all P < 0.05) members for all CNB tasks. Family members were less accurate for face memory (P < .05) relative to comparison subjects but equivalent on all other CNB measures. Patients’ speed was significantly slower than comparison subjects (all P < .05) for all tasks except language, face, and visuo-spatial memory. Patients’ speed was slower compared with family members for all CNB tasks except language, emotion processing, face, and visuo-spatial memory. Family members were slower than comparison subjects for language (P < .05) and emotion processing (P < .01). At Time 2, patients were significantly less accurate than comparison subjects (all P < 0.001) and family (all P < 0.05) members for all CNB tasks. Family members were less accurate for language and face memory (P < .05) relative to comparison subjects but equivalent on the remaining CNB task. Patients’ speed was significantly slower than comparison subjects (all P < .01) for all CNB tasks except language. Patients’ speed was significantly slower than family members (P < .05) for all CNB tasks except for visuo-spatial memory. Family members were slower than comparison subjects for sensorimotor and emotion processing and face and visuo-spatial memory (P < .05).
Fig. 1.
z-transformed neurocognitive performance in healthy comparison subjects, patients with schizophrenia, and unaffected family members at two time points. Asterisks indicate significant differences as compared with healthy comparison subjects and are coded according to group. * P < .05; ** P < .01; *** P < .001. Abst/ment Flex = Abstraction/mental flexibility.
Across-Task Intra-Individual Differences in cnb Performance
Healthy subjects showed less variability in accuracy [t(108) = 3.57, P < .001] and speed [t(108) = 3.05, P < .01] compared with family members who showed less variability in both accuracy [t(97) = 4.58, P < .001] and speed [t(97) = 4.13, P < .001] than patients. Patients had greater variability than controls in accuracy [t(77) = 7.18, P < .001] and speed [t(77) = 6.29, P < .001].
At both time points, the groups significantly differed in variability for both accuracy (Time 1: [F(2,137) = 7.63, P < .001]; Time 2: [F(2,137) = 22.17, P < .001]) and speed (Time 1: [F(2,137) = 10.85, P < .001]; Time 2: [F(2,137) = 12.85, P < .001]). Follow-up tests revealed that comparison subjects’ accuracy was less variable than patients at both time points (Time 1: P < 001; Time 2: P < 001) and family members (Time 1: P = .002; Time 2: P = .01). Family members were less variable than patients at both time points (Time 1: P = .002; Time 2: P = .001). Healthy subjects’ speed was less variable than family members at Time 1 (P < .01) and Time 2 (P < .05) and patients at Time 1 (P < .001) and Time 2 (P < .001). Family members’ speed variability was not different from patients at Time 1 (P < .10), but they were less variable at Time 2 [t(97) = 4.56, P = .001].
Stability of Performance Over Time in Multiplex Families of Schizophrenia
Mean Performance.
CNB performance stability and effect sizes of the change (Cohen’s d) in performance are presented in table 3. Comparison subjects’ performance accuracy remained stable in most domains but improved in face memory. Patients showed stable CNB accuracy in all domains while family members’ accuracy increased in attention, abstraction/mental flexibility, and face memory but remained stable in the other domains. Comparison subjects’ reaction time on the CNB remained stable in most domains but became slower during the verbal memory. Patients had stable reaction time in five domains: attention, verbal reasoning, abstraction/mental flexibility, visuo-spatial memory, and emotion processing but became slower for face memory, word memory, and sensorimotor processing. Family members’ reaction time was stable across most domains but slowed for face and verbal memory.
Across-Task Intra-Individual Variability.
IIV stability and effect sizes of its change are presented in table 4. Across-task variability for accuracy was stable in patients but decreased in comparison subjects and family members over time. Comparison subjects and patients also showed stable variability in speed, while family members’ speed variability decreased over time.
Table 4.
Mean, Standard Deviation, and Effect Size of the Difference Over Time for across-Task iiv (iiv) Scores on the Computerized Neurocognitive Battery for in Healthy Comparison Subjects, Schizophrenia Patients, and Unaffected Family Members with Test at Both Time Points. Higher iiv Score Indicates Poorer Performance
| IIV scorea | Healthy | Schizophrenia | Unaffected Family | |||||||
| Measure | Time 1 | Time 2 | d b | Time 1 | Time 2 | d b | Time 1 | Time 2 | d b | |
| Accuracy | 0.86 (0.30) | 0.79 (0.24)* | 0.26 | 1.98 (1.33) | 1.97 (1.29) | 0.01 | 1.36 (1.00) | 1.01 (0.54)* | 0.43 | |
| Speed | 0.69 (0.32) | 0.65 (0.39) | 0.11 | 1.17 (0.49) | 1.51 (1.05) | 0.41 | 0.97 (0.60) | 0.81 (0.47)* | 0.30 | |
Discussion
In a multiplex multigenerational cohort, patients with schizophrenia showed stable accuracy performance, less stable speed performance, and greater across-task IIV compared with their relatives and healthy subjects on a CNB. Longitudinal measures revealed subtle, domain-specific decreases in performance in affected individuals but improved performance in unaffected family members. The use of an across-task measure of performance variability confirmed previous results of more IIV for neurocognitive measures in patients with schizophrenia compared with family members and healthy controls. Moreover, patients’ IIV for speed increased, nominally, over time relative to family members or comparison subjects. In contrast, family members’ accuracy and speed IIV decreased significantly over time. These changes in performance were small to modest in effect size. In addition, traditional measures of mean performance, particularly speed measures, reliably differentiated the affected from the unaffected family members and healthy subjects. Our findings suggest that both mean performance measures and across-tasks variability measurements of speed on the CNB appear to differentiate affected and unaffected individuals and that using the CNB to measure stability of neurocognitive performance over time may help future genetic association studies of schizophrenia.
Neurocognitive Performance: Across-Task Intra-Individual Variability
Our results confirm and extend prior findings on the neuropsychological deficits in schizophrenia. Like other studies, IIV not only differentiated patients from healthy subjects but also distinguished unaffected family members. Comparison subjects and family members showed reductions in accuracy IIV over time, but only family members showed reductions in speed IIV over time. Conversely, patients’ accuracy IIV was unchanged, but their speed IIV increased by approximately 30%, although this increase did not reach statistical threshold. Reduction in accuracy and speed IIV in comparison subjects and family members over time may reflect the ability to maintain consistency across tasks that involve rapid complex cognitive processing. Other studies16,24 suggest consistency, or lack thereof, may be a sensitive index of performance over time that is related, in part, to dopaminergic function.21,32 Thus, IIV may be a sensitive marker of disease given the relationship between schizophrenia and dopaminergic functioning.33 Importantly, patients take longer to perform at the same level of accuracy as family members and healthy subjects suggesting an increase in monitoring while responding during the CNB. Future studies should consider measuring the relationship between response slowing and brain function (ie, functional magnetic resonance imaging) in schizophrenia. Directly measuring within-person variability in neurocognitive performance provides a general view of neurocognitive ability. However, Cole et al.24 argue that using a composite index of neurocognitive domains provides a better index of performance coherence across neurocognitive domains. Furthermore, IIV can be advantageous in elucidating common underlying mechanisms of information processing that result in increased variability.34 This approach may be more sensitive to detecting change over time by taking advantage of the variability within an individual to aid in determining individuals at risk for schizophrenia. In fact, individuals with high variability on IQ measurements have been shown to be three times more likely to develop schizophrenia than individuals with low variability.35 Yet, the specific mechanism underlying reduced speed and accuracy IIV over time in family members remains unclear. A reduction may reflect familiarity with the tasks or learning over time. Notably, even after this improvement family members remain intermediate to healthy subjects suggesting some genetic influence on IIV. Other factors such as age36 or task complexity21 may explain part of the IIV differences, yet these associations tend to be task-specific37 and our longitudinal comparison considered factors such as age. Nonetheless, domain-specific measures of IIV in large multiplex families with schizophrenia may prove useful in further differentiating genetic susceptibility.
A single measure of variability over a battery of computerized tasks cannot fully replace a through neuropsychological evaluation, as variability is common even in healthy adults.38 However, assessment of within-person variability as a complementary measure to standard outcomes provides a generalizable metric of neurocognitive performance that is informative and potentially useful in genetic association studies. The neural substrate of across-task IIV has not been investigated in patients with schizophrenia. However, our findings in patients are similar to other studies demonstrating that focal lesions of the frontal lobes21 and other brain disorders,17 including schizophrenia20,24 are associated with increased IIV. Increased across-trial IIV is linked to neural disconnectivity of the frontal cortex in aging39 and ADHD.17 ADHD and schizophrenia have similar neurodevelopmental mechanisms40 and may share a common neural pathway that results in information processing instability due to a reduction in prefrontal dopamine.20 Furthermore, cortico-cortical disconnectivity of the frontal cortex is a potential neurobiological mechanism underlying poor neurocognitive performance in schizophrenia.41,42 This disconnection hypothesis is further supported by findings indicating that alterations in brain white matter in schizophrenia are associated with deficits in neurocognitive performance (eg, Kubicki et al.43, Szeszk et al.44, Perez-Iglesias et al.45), including impairments in prefrontal cortex-mediated task switching43 and abstraction/mental flexibility.45 While these studies note specific brain-behavior relationships in schizophrenia, it is possible that more comprehensive performance metric, such as across-task (IIV), may be more closely associated with microstructural alterations in the brain. However, symptomatology and illness course may lead to disparate patterns of variability, either in the timing of the deficits or in the specific neuropsychological domains responsible for increased IIV. Future studies could examine patterns of neurodevelopment and their relationship to across-task neurocognitive IIV. In addition, attempts to differentiate the neuropsychological domains that contribute to increases in IIV in specific neurological disorders should be considered.
Neurocognitive Performance: Mean Performance and Stability
We confirmed previous findings3,4 that patients are globally impaired across a range of neurocognitive domains and that relatives without schizophrenia are also impaired in specific domains compared with healthy subjects without a family history of psychosis. Our study extends findings that neurocognitive deficits in performance accuracy in outpatients with schizophrenia are stable over a 5-year interval, suggesting that these deficits are a feature of the disease. However, this stable pattern is noted in outpatients, whereas chronically institutionalized patients over the age of 65 decline in neurocognitive performance as compared with age-matched controls.46 We also found an intermediate stability pattern for family members, with accuracy improving in many, but not all domains. The stability profile of family members was similar to that of the comparison subjects. Although we increased detection power in family members, this finding could reflect less genetic vulnerability or resilience in unaffected relatives.
We found less stability in performance speed for specific domains in patients. Comparison subjects and family members showed minimal changes in performance speed. Indeed, these groups showed the traditional speed-accuracy trade-off noted in previous studies3 but not in verbal memory. However, this pattern was not seen in patients who showed robust slowing with no complementary increase in accuracy, similar to previous studies.18,47 Patients were slower at baseline and slowing continued over time. Given these dissociable patterns in affected and unaffected family members, parsing performance into accuracy and speed components results in increased detection of genetic vulnerabilities associated with cognitive performance.
A distinct advantage of mean performance measures is the ability to inspect differences in performance in specific neurocognitive domains. This specificity is useful for probing particular neurocognitive deficits in patients with schizophrenia or their family members. Future studies that focus on domain-specific changes in performance would be noteworthy, particularly if the trajectories of change were compared within and across multiplex families with schizophrenia. Family-specific deficits should be further probed, but larger family units are needed to provide sufficient power.
Reliability of the CNB
We show that the test-retest reliability of the CNB is high over an extended period in most cognitive domains. This is similar to previous studies using standard measurements.9 Importantly, the test-retest reliability was similar across the diagnostic groups. Regardless of outcome measure used, it is apparent that the CNB offers precise, reliable measurement of neurocognitive performance that allows for sensitive monitoring over time, which may be critical for determining trajectories in individuals at clinical risk for psychosis or those with significant genetic susceptibility.
Limitations
Our use of multiplex family members provides a unique perspective on cognitive performance over time, but this data may not translate to simplex families as mulitplex families may have higher incidences of other axis I or axis II disorders. In addition, we cannot discern the importance of the degree of biological relationship in this limited sample. The older age and inclusion of only Caucasian individuals makes generalizations more difficult. However, the pattern of results with the CNB is similar to that found in sporadic schizophrenia4 and other studies of stability8,9,11,48 and variability.24 In addition to controlling for age and the duration between testing, we clinically reevaluated all participants at their second visit. Given the duration of time between tests, it is possible that other significant changes in functioning may have gone undetected or that alterations of medication regiments may have affected neurocognitive performance over time. It is also possible that the duration of illness or illness severity may also contribute to changes in performance over time or willingness to participate in a longitudinal assessment. It should also be noted that not all tests showed high reliability during our test-retest evaluation (eg, SMEM), yet in these cases, the reliability was similar across groups. Such differences in reliability may be the result of using a computerized method for acquiring neuropsychological test scores. This method has many advantages, yet computers and software evolve. Thus, there are small changes to the administration or display of the tasks over 5 years, which could result in performance changes, affecting test-retest reliability.
Funding
National Institute of Mental Health [R01MH042191 and T32 MH019112 to R.E.G., R01MH063480 to V.L.N., R01MH061622 to L.A.A., and R01MH084856 to R.C.G.].
Acknowledgments
The authors thank Robert Witalec, Sue Clifton, and Amy Cassidy for data gathering and processing. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix
Intra-individual variability (IIV) was calculated in the following manner. Raw scores for each computerized neurocognitive battery (CNB) test were z-transformed based on the sample as a whole. These transformed scores were then used to calculate variability using the following equation:
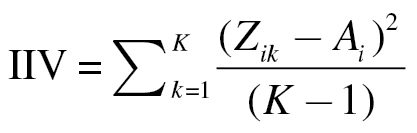 |
where Zik is the kth CNB test score for the ith individual and
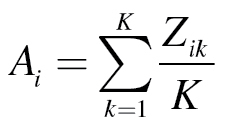 |
is the individual’s mean z-transformed score based on all of the CNB tasks performed. 23
References
- 1. Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131 [DOI] [PubMed] [Google Scholar]
- 2. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445 [DOI] [PubMed] [Google Scholar]
- 3. Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819 [DOI] [PubMed] [Google Scholar]
- 4. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–788 [DOI] [PubMed] [Google Scholar]
- 5. Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannon TD, Zorrilla LE, Shtasel D, et al. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry. 1994;51:651–661 [DOI] [PubMed] [Google Scholar]
- 7. Calkins ME, Tepper P, Gur RC, et al. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): evidence for impairment and heritability of neurocognitive functioning in families of schizophrenia patients. Am J Psychiatry. 2010;167:459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nayak Savla G, Moore DJ, Roesch SC, et al. An evaluation of longitudinal neurocognitive performance among middle-aged and older schizophrenia patients: use of mixed-model analyses. Schizophr Res. 2006;83:215–223 [DOI] [PubMed] [Google Scholar]
- 9. Heaton RK, Gladsjo JA, Palmer BW, et al. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32 [DOI] [PubMed] [Google Scholar]
- 10. Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. J Am Acad Child Adolesc Psychiatry. 2007;46:867–878 [DOI] [PubMed] [Google Scholar]
- 11. Pietrzak RH, Snyder PJ, Jackson CE, et al. Stability of cognitive impairment in chronic schizophrenia over brief and intermediate re-test intervals. Hum Psychopharmacol. 2009;24:113–121 [DOI] [PubMed] [Google Scholar]
- 12. Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res. 2008;99:218–224 [DOI] [PubMed] [Google Scholar]
- 13. Stefanis NC, van Os J, Avramopoulos D, et al. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psychiatry. 2005;162:1752–1754 [DOI] [PubMed] [Google Scholar]
- 14. Heydebrand G. Cognitive deficits in the families of patients with schizophrenia. Curr Opin Psychiatry. 2006;19:277–281 [DOI] [PubMed] [Google Scholar]
- 15. MacDonald SW, Li SC, Backman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging. 2009;24:792–808 [DOI] [PubMed] [Google Scholar]
- 16. Hultsch DF, Strauss E, Hunter MA, MacDonald SWS. Intraindividual variability, cognition, and aging. In: Craik FIM, Salthouse TA, eds. The Handbook of Aging and Cognition. 3rd ed New York, NY: Psychology Press; 2008:491–556 [Google Scholar]
- 17. Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst). 2000;104:167–190 [DOI] [PubMed] [Google Scholar]
- 18. Kaiser S, Roth A, Rentrop M, et al. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008;66:73–82 [DOI] [PubMed] [Google Scholar]
- 19. Carroll CA, O'Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009;70:181–190 [DOI] [PubMed] [Google Scholar]
- 20. Rentrop M, Rodewald K, Roth A, et al. Intra-individual variability in high-functioning patients with schizophrenia. Psychiatry Res. 2010;178:27–32 [DOI] [PubMed] [Google Scholar]
- 21. Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126(Pt 11):2363–2380 [DOI] [PubMed] [Google Scholar]
- 22. Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097 [DOI] [PubMed] [Google Scholar]
- 23. Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. J Am Med Assoc. 2008;300:823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole VT, Weinberger DR, Dickinson D. Intra-individual variability across neuropsychological tasks in schizophrenia: a comparison of patients, their siblings, and healthy controls. Schizophr Res. 2011;129:91–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771 [DOI] [PubMed] [Google Scholar]
- 26. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa: Unversity of Iowa; 1984 [Google Scholar]
- 27. Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa: Unversity of Iowa; 1984 [Google Scholar]
- 28. Wilkinson GS. Wide Range Achievement Test—Revision 3. Wilmington, DE: Jastak Association; 1993 [Google Scholar]
- 29. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776 [DOI] [PubMed] [Google Scholar]
- 30. Gur RC, Richard J, Hughett P, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psych Methods. 1996;1:30–46 [Google Scholar]
- 32. Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690 [DOI] [PubMed] [Google Scholar]
- 33. Remington G, Agid O, Foussias G. Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev Neurother. 2011;11:589–607 [DOI] [PubMed] [Google Scholar]
- 34. Li S, Aggen SH, Nesselroade JR, Baltes PB. Short-term fluctuations in elderly people’s sensorimotor functioning predict text and spatial memory performance: The Macarthur Successful Aging Studies. Gerontology. 2001;47:100–116 [DOI] [PubMed] [Google Scholar]
- 35. Reichenberg A, Weiser M, Rapp MA, et al. Premorbid intra-individual variability in intellectual performance and risk for schizophrenia: a population-based study. Schizophr Res. 2006;85:49–57 [DOI] [PubMed] [Google Scholar]
- 36. Myerson J, Robertson S, Hale S. Aging and intraindividual variability in performance: analyses of response time distributions. J Exp Anal Behav. 2007;88:319–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–432 [DOI] [PubMed] [Google Scholar]
- 38. Binder LM, Iverson GL, Brooks BL. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol. 2009;24:31–46 [DOI] [PubMed] [Google Scholar]
- 39. Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P101–P115 [DOI] [PubMed] [Google Scholar]
- 40. Oie M, Rund BR. Neuropsychological deficits in adolescent-onset schizophrenia compared with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1216–1222 [DOI] [PubMed] [Google Scholar]
- 41. Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156 [DOI] [PubMed] [Google Scholar]
- 42. Gold JM, Weinberger DR. Cognitive deficits and the neurobiology of schizophrenia. Curr Opin Neurobiol. 1995;5:225–230 [DOI] [PubMed] [Google Scholar]
- 43. Kubicki M, Westin CF, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984 [DOI] [PubMed] [Google Scholar]
- 45. Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–458 [DOI] [PubMed] [Google Scholar]
- 46. Friedman JI, Harvey PD, Coleman T, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer's disease and normal aging. Am J Psychiatry. 2001;158:1441–1448 [DOI] [PubMed] [Google Scholar]
- 47. Vinogradov S, Poole JH, Willis-Shore J, Ober BA, Shenaut GK. Slower and more variable reaction times in schizophrenia: what do they signify? Schizophr Res. 1998;32:183–190 [DOI] [PubMed] [Google Scholar]
- 48. Harvey PD, Silverman JM, Mohs RC, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry. 1999;45:32–40 [DOI] [PubMed] [Google Scholar]