Abstract
Background: Decreased birth weight (BW) is associated with later psychosis, but the sources of decreased BW for those at risk for psychosis remain unclear. Aim: To determine whether fetal exposure to influenza and/or hypoxia accounts for BW decreases among psychotic cases and controls. Method: Subjects were 111 cases diagnosed with schizophrenia or affective psychosis and 333 matched controls from the Collaborative Perinatal Project. Psychiatric diagnoses were ascertained from medical records. Influenza and hypoxia were determined from maternal and cord sera collected at birth. Results: Cases exposed to severe fetal hypoxia or influenza had significantly lower BW compared with unexposed cases and controls, regardless of exposure status. No significant differences in BW were observed among controls based on exposure status. Conclusions: Decreased BW appears to be a risk factor for psychosis only in the presence of other teratogens. Liability to psychosis likely renders fetuses vulnerable to decreased fetal growth in response to hypoxia and influenza.
Key words: obstetric complications, pregnancy, schizophrenia
Introduction
Previous research has shown that low birth weight is more common among individuals who develop schizophrenia in adulthood as compared with unaffected siblings and control subjects. 1 , 2 Other studies, however, have found no association between decreases in birth weight and risk for schizophrenia. 3 Birth weight is a general marker of the viability of the intrauterine environment; therefore, many types of obstetric complications (OCs) can contribute to lower birth weight. Nevertheless, prior studies in schizophrenia research have not examined whether low birth weight is specific to cases with exposures to particular types of OCs. If this is found to be the case, discrepancies between prior birth weight studies in schizophrenia research could be understood in light of differential exposure to OCs among cases.
Although a number of obstetric insults have been associated with both schizophrenia and fetal growth, this study focuses on two OCs: maternal influenza during pregnancy and fetal hypoxia (lack of oxygen to the fetus) that have previously been associated with schizophrenia and premorbid abnormalities in the present cohort. 4 , 5 Although there are some conflicting findings, the link between influenza infection during pregnancy and increased risk of schizophrenia in offspring was first found in ecologic studies of mothers pregnant during influenza epidemics, 6 and has since been replicated in studies using serologically documented influenza exposure during pregnancy. 5 , 7 Similarly, fetal exposure to hypoxia-associated OCs has been linked to increased risk of schizophrenia in a number of studies. 8 , 9 In addition, maternal influenza infection and fetal hypoxia also have been associated with decreased birth weight in both human and animal studies. 10–13 Despite the aforementioned findings, no prior study has investigated whether low birth weight is confined to those schizophrenia cases with histories of prenatal exposure to influenza or hypoxia.
The purpose of this study was to investigate whether prenatal and perinatal factors associated with schizophrenia accounted for the decreases in birth weight found in the histories of individuals who later developed psychosis. Analyses controlled for length of gestation, because this is the most optimal measure of fetal growth, adjusting for birth weight decreases secondary to preterm delivery. 14 Our primary hypothesis was that prenatal exposure to hypoxia or influenza would account for the observed decreases in birth weight among cases who later developed psychosis. We also hypothesized that cases would be preferentially sensitive to prenatal exposure to maternal influenza or hypoxia compared with controls (case status × OC interaction), based on previous studies from this and other cohorts suggesting that cases are especially vulnerable to prenatal exposure to both influenza and hypoxia. 5 , 15 Third, we hypothesized that unexposed cases and controls will not show significant decrements in birth weight, demonstrating selectivity of low birth weight to cases with the additional obstetric risk factors of exposure to maternal influenza or hypoxia. Last, we hypothesized that controls exposed to these OCs would also exhibit decreases in birth weight based on pregnancy studies in the general population; 9 , 16 however, the most pronounced decreases in birth weight would be among exposed cases.
Methods
This study was reviewed and approved by the institutional review boards (IRBs) of the University of Pennsylvania, University of California (Los Angeles), and Temple University.
Participants were members of the Philadelphia site of the Collaborative Perinatal Project (CPP). The CPP was a large-scale birth cohort study that prospectively followed over 50 000 women during pregnancy, between 1959 and 1966, and offspring until age 7 at multiple sites throughout the United States. The Philadelphia cohort of the CPP was composed of 9236 offspring of 6753 mothers who delivered at two urban hospitals, the Pennsylvania Hospital and the Children’s Hospital of Philadelphia. 4 The Philadelphia site of the CPP was chosen to overrepresent African American individuals of lower socioeconomic status to ensure demographic variability across study sites. 17
Case ascertainment has been described in detail in previous publications, but in brief, cases were identified in two stages. 4 First, in 1996 (when cohort members were between the ages of 30 and 37) the Penn Longitudinal Database was searched to identify CPP members diagnosed with psychotic disorders. This database documents contact with public mental health facilities from 1985 to 1995 in Philadelphia. This search yielded 1197 CPP participants, 339 of whom had a previous diagnosis of a psychotic disorder. Second, a diagnostic validation study was conducted using psychiatric medical records to verify psychotic disorder diagnoses from public claims data. Six raters (two psychiatrists, two clinical psychologists, and two advanced clinical psychology graduate students) conducted chart reviews using a standard coding form based on DSM-IV criteria. Ten charts were used to train diagnosticians and 56 cases were randomly selected to establish inter-rater reliability between examiners (Kappa = 0.85; 93% simple agreement). To confirm chart diagnoses, 14 individuals diagnosed with a psychotic disorder were interviewed with the structured clinical interview based on DSM-IV criteria (SCID). 18 Thirteen of 14 psychosis diagnoses were confirmed. Only cases with chart-review confirmed diagnoses were used in the study, because there was only moderate agreement between the chart-based DSM-IV diagnoses and the register-based diagnoses (Kappa = 0.63). Chart reviews were conducted for the 144 patients with medical records. Of these patients, 72 were diagnosed with schizophrenia or schizoaffective disorder-depressed type (.8% of cohort), 41 were diagnosed with a psychotic form of major depression or bipolar disorder (.4% of cohort), and 31 received other diagnoses (.3% of cohort; for specific diagnoses, see Cannon et al., 1999). 19
Three controls were matched to each case based on the following criteria: (1) from the same hospital as case, (2) same sex and race as case, and (3) being the next 3 CPP births (ie, closest in date of birth) that match on these criteria. The final sample comprised 111 cases (70 schizophrenia/schizoaffective disorder and 41 affective psychoses) and 333 matched controls, all of whom had available maternal sera from birth. Umbilical cord serum samples were also collected for a smaller portion of the sample (n = 257; 38 diagnosed with schizophrenia/schizoaffective disorder, 24 with affective psychoses, and 195 nonpsychiatric controls). Demographic characteristics for the sample are listed in table 1. Subjects were predominantly African American, of relatively low socioeconomic status, and had approximately 10 years of education. Socioeconomic status was determined using a composite index based on yearly family income as well as educational level and occupation of the primary wage earner. 20
Table 1.
Demographic Characteristics of the Sample
| Characteristic | Category | Cases (n = 111) | Controls (n = 333) | Analysis P Value |
|---|---|---|---|---|
| Female | N (%) | 47 (42.34%) | 141 (42.34%) | 1.00 |
| Birth weight (grams) | Mean (SD) | 3074.6 (523.2) | 3130.7 (515.39) | .323 |
| Gestational age (weeks)a | Mean (SD) | 38.77 (4.81) | 39.01 (3.65) | .595 |
| Mother’s agea | Mean (SD) | 24.17 (6.90) | 24.17 (6.14) | .994 |
| Previous pregnanciesa | Mean (SD) | 2.71 (2.66) | 2.61 (2.64) | .717 |
| Maternal social statusa | Mean (SD) | 36.1 (18.6) | 40.7 (15.5) | .01 |
| Mother’s education (years)a | Mean (SD) | 9.97 (2.02) | 10.33 (2.18) | .13 |
| African American | N (%) | 103 (92.79%) | 308 (92.49%) | .917 |
| Influenza B | N (%) | 35 (31.53%) | 80 (24.02%) | .118 |
| Moderate fetal hypoxia (75th percentile) | N (%) | 15 (13.51%) | 48 (14.41%) | .32 |
| Severe fetal hypoxia (90th percentile) | N (%) | 4 (3.60%) | 21 (6.31%) | .95 |
aInformation was incomplete for 10 subjects on gestational age, for 8 subjects on maternal age, for 8 subjects on previous pregnancies, for 18 subjects on maternal social status, and for 11 subjects on maternal education.
Birth Weight Assessment
Birth weight was assessed in the delivery room, or taken as the weight on nursery admission if the delivery room weight was missing. 21 Gestational age was also assessed and controlled for in analyses, as this is the most optimal measure of fetal growth and accounts for decreased birth weight that is secondary to preterm delivery. 14
Processing of Serum and Immunoglobulin Measurements
A maternal blood sample was collected at the time of birth for each study participant. Serum samples were stored at the National Institutes of Health repository at −70ºC. All assays of serum samples were carried out in the Stanley Laboratory of Developmental Neurovirology at Johns Hopkins University Medical Center under the supervision of Dr Robert Yolken.
Measurement of Infection
Solid-phase enzyme immunoassays were used to measure levels of specific immunoglobin G (IgG) class antibodies to influenza B viruses in human serum and plasma (ELISA, kits RE56541 & RE56511, IBL, Hamburg, Germany). This procedure has previously been described in detail. 5 This study focused on Influenza B because no previous associations with Influenza A and risk for schizophrenia or premorbid cognitive problems were found in this sample. 5
Most people have been exposed to influenza B and therefore are seropositive (carry antibodies) to this virus. If an individual has been previously exposed to influenza B, an increase in IgG antibodies occurs in response to influenza B re-exposure. This increase in IgG antibodies to influenza persists for approximately several months in healthy adults, 22 and previous studies suggest that the IgG response to infection in pregnant women does not differ from nonpregnant women. 23 This study used IgG optical density values greater than the 75th percentile to indicate the likelihood of recent exposure to influenza B; specifically, women with IgG antibodies greater than the 75th percentile likely had an influenza infection at some point during the third trimester, given that the samples were collected at birth. 5 All samples were analyzed under code, with the laboratory performing the studies blind to clinical statuses of participants.
Measurement of Fetal Hypoxia
Fetal hypoxia was assessed through measurement of erythropoietin (EPO) ascertained from umbilical cord serum. EPO is a glycoprotein hormone produced in response to anemia and hypoxia in human fetuses and adults. 24 Maternal EPO does not appear to cross the placenta, meaning that umbilical cord EPO is entirely from fetal origins. 25 Last, EPO is a fairly good measure of the severity of a hypoxic event, because EPO levels rise as oxygen demands increase. 24
Erythropoietin levels were determined using solid- phased enzyme immunoassay (ELISA Kit RE56011, IBL, Hamburg, Germany). Wells were filled with 150 μl of phosphate buffer, protein, 0.02% NaN3, pH 7.4. Fifty microliters of each standard (contains EPO, buffer/protein solution, NaN3), control (contains NaN3), and study participant sample were pipetted into respective wells. Plates were covered with adhesive foil and incubated for 3–4h at room temperature (18–25°C) on an orbital shaker (500rpm). Adhesive foil was removed and incubation solution was discarded. Plates were washed 3 times with 250 μl of diluted assay buffer (phosphate buffer, Tween, 0.2% NaN3). Excess solution was removed by tapping the inverted plate on a paper towel. One hundred microliters of freshly prepared anti-EPO antiserum (rabbit), conjugated to biotin, buffer, 0.02% NaN3 was placed into each well. Plates then were covered with new adhesive foil and incubated overnight (16–20h at 2–8°C).
On the second day, adhesive foil was removed and incubation solution was discarded. Plates were washed 3 times with 250 μl of diluted assay buffer and excess solution was removed by tapping the inverted plate on a paper towel. Each well was filled with 100 μl of freshly prepared anti-biotin antiserum (goat), conjugated to alkaline phosphatase, buffer, 0.01% NaN3 and then covered with new adhesive foil. Plates were incubated for 120min at room temperature (18–25°C) on an orbital shaker (500rpm). Adhesive foil was removed, incubation solution was discarded, and plates were washed 3 times with 250 μl of diluted assay buffer (excess solution removed by tapping the inverted plate on a paper towel). In each well, 200 μl of freshly prepared p-nitrophenyl phosphate (pNPP) solution was pipetted and then wells were covered with new adhesive foil and incubated for 120min at room temperature (18–25°C) on an orbital shaker (500rpm). The substrate reaction was stopped by adding 50 μl of pNPP stop solution (1M NaOH, 0.25M EDTA) into each well and then the contents were briefly mixed by gently shaking the plate. Optical densities were measured with a photometer at 405nm (reference wavelength: 600–650nm) within 60min after pipetting of the stop solution. The obtained optical densities of the standards (y-axis, linear) were plotted against their concentration (x-axis, logarithmic). The concentration of the samples was read from the standard curve and values were expressed as international units of activity (mIU/ml). Univariate analyses were conducted to determine the 75th and 90th percentile values for EPO (mIU/ml). EPO values greater than the 75th percentile cut-off (12.50 mIU/ml) were considered moderate levels of fetal hypoxia and values greater than the 90th percentile (28.30 mIU/ml) were considered severe fetal hypoxia. Our a priori decision to distinguish between moderate and severe fetal hypoxia was based on previous research suggesting that the effects of hypoxia on offspring development differ depending on the severity and duration of the hypoxic event, as well as the period of gestation. 26
Statistical Analyses
The primary goal of data analysis was to examine the relationships between birth weight and prenatal exposure to influenza B or fetal hypoxia among those who later developed psychosis in adulthood and controls. Analyses were conducted collapsing across schizophrenia, schizoaffective disorder, and affective psychoses to create a single group of persons with psychosis, herein referred to as cases, to maximize power to detect significant results, given the limited sample size. Exploratory analyses were conducted on all significant results to determine if results differed by diagnostic category. All analyses were conducted in SAS 9.2 (SAS, Inc, Cary, NC). Associations between the main dependent variables (birth weight and case status) and potential covariates, including maternal age, race, and offspring sex, were examined. Because these variables did not significantly predict the main dependent variables, they were not included as covariates in the analysis. Analyses of covariance (ANCOVAs) were conducted controlling for socioeconomic status as a proxy of postnatal adversity. 14 In addition, gestational age was controlled for in all analyses related to birth weight to disentangle the contribution of gestational age from other factors that might influence fetal growth. Three post hoc analyses were conducted: (1) unexposed cases were compared with exposed cases to determine whether exposure to influenza B or fetal hypoxia led to more pronounced decreases in birth weight among those at risk for psychosis; (2) unexposed cases were compared with unexposed control subjects to determine whether there were significant differences in birth weight in the absence of exposure to influenza B or fetal hypoxia; (3) unexposed control subjects were compared with exposed control subjects to determine whether prenatal exposure to influenza B or fetal hypoxia led to decreases in birth weight among infants at presumed low genetic risk for psychosis. Effect sizes (Cohen’s d) were computed for post hoc comparisons. Effect sizes of .0 to .2 are considered small, .3 to .5 are considered medium, and greater than .5 are considered large. 27
Results
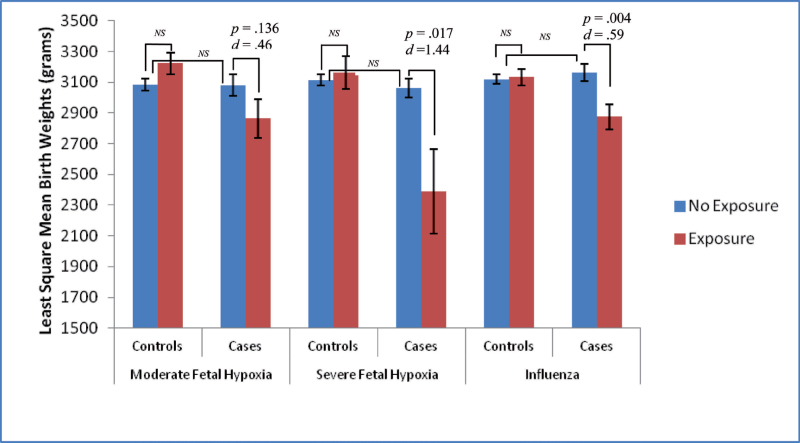
Results from ANCOVA analyses are presented in figure 1 and table 2. There were significant interactions between case status and exposure to influenza B (F = 6.39, df = 1, P = .012), exposure to moderate fetal hypoxia (F = 4.57, df = 1, P = .034), and exposure to severe fetal hypoxia (F = 5.78, df = 1, P = .017) on birth weight, after controlling for socioeconomic status and gestational age. These findings are particularly interesting given similar levels of fetal hypoxia among cases and controls (for moderate fetal hypoxia: χ2 = 1.00, df = 1, P = .32; for severe fetal hypoxia: χ2 = 0.004, df = 1, P = .95).
Fig. 1.
Least square mean birth weights by exposure and case status. ANCOVA analyses for birth weights by hypoxia, influenza B, and case status. Figure displays least square mean values for birth weight by moderate fetal hypoxia, severe fetal hypoxia, and influenza B. All analyses controlled for gestational age and socioeconomic status (NS = nonsignificant at P < .05).
Table 2.
Post Hoc Comparisons From Birth Weight ANCOVA Analyses of Exposure by Case Status
| Exposed Control Subjects vs Unexposed Control Subjects | Unexposed Cases vs Unexposed Control Subjects | Exposed Cases vs Unexposed Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | t | df | P | Cohen’s d | t | df | P | Cohen’s d | t | df | P | Cohen’s d |
| Influenza B | .198 | 1 | .8435 | .030 | .671 | 1 | .503 | .090 | 2.865 | 1 | .004 | .590 |
| Moderate fetal hypoxia | 1.691 | 1 | .092 | .28 | –.067 | 1 | .946 | .01 | 1.495 | 1 | .136 | .46 |
| Severe fetal hypoxia | .454 | 1 | .650 | .11 | –.694 | 1 | .489 | .10 | 2.410 | 1 | .017 | 1.44 |
Note: All analyses controlled for gestational age and socioeconomic status.
Planned post hoc analyses indicated significant differences in birth weight between cases exposed to severe fetal hypoxia compared with unexposed cases (t = −2.410, df = 1, P = .017, Cohen’s d = 1.44) and between cases exposed to influenza B compared with unexposed cases (t = –2.865, df = 1, P = .004, Cohen’s d = .59), after controlling for socioeconomic status and gestational age. Cases exposed to moderate fetal hypoxia also had lower birth weights compared with unexposed cases, but this difference did not reach statistical significance (t = −1.495, df = 1, P = .136, Cohen’s d = 0.46). There were no significant differences in birth weight between unexposed control subjects and control subjects exposed to influenza B or hypoxia (see table 2). There also were no significant differences in birth weight between unexposed cases and unexposed controls (see table 2).
Diagnostic Specificity
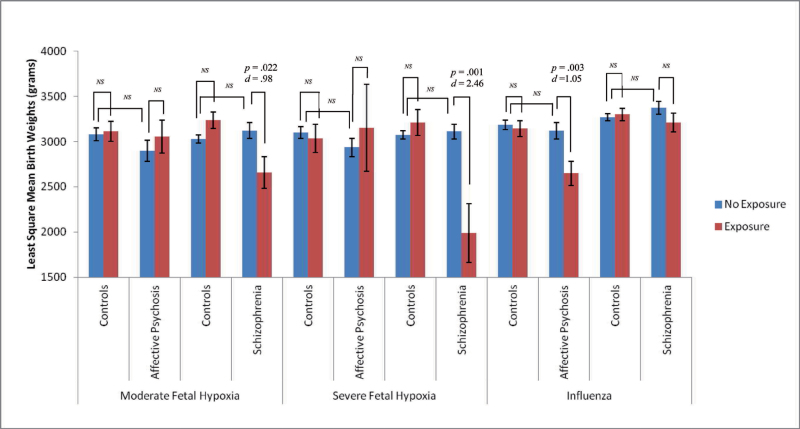
Exploratory analyses (see figure 2) were conducted to determine if significant results differed by diagnostic category (affective psychosis or schizophrenia/schizoaffective disorder). Fetal exposure to influenza B was associated with significant decreases in birth weight among individuals diagnosed with affective psychosis in adulthood compared with unexposed affective psychosis cases (t = −3.036, df = 1, P = .003, Cohen’s d = 1.05). In contrast, fetal hypoxia was associated with decreased birth weight only among exposed schizophrenia cases compared with unexposed schizophrenia cases (moderate hypoxia: t = −2.318, df = 1, P = .022, Cohen’s d = .98; severe hypoxia: t = −3.357, df = 1, P = .001, Cohen’s d = 2.46). There were no significant birth weight differences among individuals diagnosed with affective psychoses who were exposed to moderate or severe fetal hypoxia in utero compared with unexposed affective psychoses cases (moderate hypoxia: t = .508, df = 1, P = .612, Cohen’s d = .24; severe hypoxia: t = .268, df = 1, P = .789, Cohen’s d = .29). There also were no significant birth weight differences among schizophrenia cases exposed to influenza B compared with unexposed schizophrenia cases (t = 1.412, df = 1, P = .159, Cohen’s d = .37).
Fig. 2.
Least square mean birth weights by exposure and specific diagnoses. ANCOVA analyses for birth weights by hypoxia, influenza B, and diagnostic status. Figure displays least square mean values for birth weight by moderate fetal hypoxia, severe fetal hypoxia, and influenza B. All analyses controlled for gestational age and socioeconomic status (NS = nonsignificant at P < .05).
Discussion
This is the first study to investigate whether prenatal exposure to influenza or hypoxia is associated with decreases in birth weight among infants who developed psychosis in adulthood. These findings indicate that fetal exposure to influenza or hypoxia is associated with significantly decreased weight at birth among individuals who later developed a psychotic disorder but not among control subjects, suggesting that factors associated with liability to psychosis rendered the fetuses particularly vulnerable to these OCs, leading to decreases in fetal growth. Further, birth weights of unexposed cases did not significantly differ from controls, suggesting that exposure to a prenatal insult was necessary for cases to exhibit decreases in birth weight. Among controls, hypoxia and influenza B had no significant influence on birth weight, despite similar levels of exposure to these OCs among cases and controls, which suggests that liability for schizophrenia and/or reduced fetal growth was a necessary condition for hypoxia- and influenza B-associated decreases in birth weight. The results from this study also may account for the discrepancy in birth weight findings among previous studies in schizophrenia research, given that previous studies did not account for the prenatal environment when examining birth weight. Fetal hypoxia and influenza B were especially good first candidates to explore, given that the association between the OCs in this study and risk for psychosis are well-replicated in schizophrenia research, 1 , 3 , 28 including in previous studies of the CPP cohort, 4 , 5 and have been associated with decreases in birth weight in both animal and human studies. 10 – 13 Further, our measures of the prenatal environment were a major strength of this study, as it is the first study to use serological indicators of exposure to influenza B and hypoxia, rather than confirmation through self-report or medical records.
The main findings of this study appeared to vary by type of psychosis; however, these are tentative findings given limited sample sizes. Specifically, fetal hypoxia only lead to decreases in birth weight among individuals diagnosed with schizophrenia, while exposure to influenza B only lead to decreases in birth weight among individuals diagnosed with affective psychosis. Our results suggest that the vulnerability for affective psychosis versus schizophrenia may differentially influence how the fetus responds to different teratogens. Given that these were exploratory analyses, future studies are necessary to determine whether certain OCs may be differentially related to specific psychosis diagnoses.
Although the exact mechanisms through which exposure to influenza and hypoxia impacts the fetus is unknown, there are a number of plausible explanations. Influenza is believed to not cross the placenta; thus, damaging effects may result from the maternal immune response to infection. 29 Proinflammatory cytokine polymorphisms (eg, Interleukin IL-1 complex [IL-1α (-889) allele 2, IL-1β (-511) allele 1, and IL-1RA allele 1] and tumor necrosis factor-alpha (TNF-α) have been found to be associated with both schizophrenia outcome and particular OCs, such as preterm delivery, and may at least partially account for the findings. 30 – 32 Similarly, reduced fetal growth has been associated with increased risk of hypoxia-associated complications during labor, suggesting that there may be a shared liability between reduced birth weight and risk of these OCs. 9
This study had limitations that should be addressed by future studies. First, our study included predominantly African American participants. African Americans have been found to be at increased risk for schizophrenia, 33 OCs, and low birth weight, even when controlling for socioeconomic status and other sociodemographic variables. 34 , 35 The present results may have reduced generalizability to other ethnic/racial groups but may provide clues about the contributors to racial disparities in schizophrenia that should be examined by future investigations. Second, other maternal conditions during pregnancy, such as diabetes, malnutrition, and maternal psychosocial stress have been associated with the OCs examined in this study and could have contributed to our findings. 36 Because this is the first study to examine prenatal contributors to decreased birth weight among those who develop psychosis, two OCs that have been associated with schizophrenia in the present cohort and in other similar cohorts were focused on in this study. 10 – 13 Nevertheless, future studies should investigate whether additional maternal conditions of pregnancy contribute to decreased birth weight among those who later develop schizophrenia. Third, the exact onset of hypoxia cannot be discerned from umbilical EPO; however, umbilical EPO has a longer half-life than other biomarkers of hypoxia, therefore the hypoxic events assessed by EPO were more likely to include events that occurred prior to delivery compared with other biologic measures of hypoxia, such as umbilical cord pH which assesses acute exposure. 24 One limitation of discerning hypoxia from umbilical EPO is that we cannot rule out the possibility that the hypoxic event occurred at birth. Because there is some evidence that growth restricted fetuses are at increased risk of hypoxia around birth, 9 , 37 future studies should examine the direction of the association between fetal growth restriction and hypoxia.
Given the small sample size and this study being the first of its kind, multiple tests were conducted without adjusting P values for multiple comparisons. Therefore, there is a possibility of type 1 errors; however, this possibility is somewhat mitigated by the findings being completely restricted to cases, which is unlikely to occur due to chance. Future studies with larger sample sizes are needed to test whether our results are resilient under more conservative P values and whether fetal exposure to OCs act in combination with genetic risk factors and/or other obstetric risk factors within the neurodevelopmental course of psychosis. Although we did not examine genetic factors in this study, our findings are consistent with previous research suggesting that environmental and constitutional factors coalesce to influence risk of schizophrenia. 38
Potential limitations associated with ascertainment methods include the possibility of undetected psychiatric morbidity among control subjects. However, given the pattern of findings, such errors would likely lead to an underestimation of the association between fetal exposure to hypoxia or influenza and decreased birth weight among cases and controls. Further, this study did not examine the whole spectrum of psychosis, as non-schizophrenia psychosis (eg, delusional disorder and brief psychotic disorder) cases were not ascertained. Future studies are necessary to determine whether our results are generalizable to other disorders along the schizophrenia spectrum.
Another caveat is that serum samples used in this study have been frozen for more than 40 years, which could raise concerns about the long-term stability of IgG antibodies. Sera samples were carefully maintained to remain frozen, undisturbed, and protected against the breakdown of proteins. Samples were handled uniformly for cases and controls; therefore, differences among these groups should not be attributable to decay over time. Additionally, multiple antibodies have been assayed from maternal sera from the CPP and have shown significant relationships with offspring psychosis, suggesting that the sera stored under the conditions employed for the CPP study are suitable for these measurements (eg, Ellman et al., 2009). 5
Despite relatively small sample sizes, the calculated effect sizes were large, suggesting that these OCs, particularly severe hypoxia, are especially damaging to those who later develop psychosis as compared with controls. Among individuals exposed to severe fetal hypoxia, infants fell into the clinical ranges of very low birth weight, suggesting that infants exposed to OCs that exhibit severe fetal growth restriction may be particularly vulnerable to developing psychosis in adulthood.
In conclusion, the findings of this study suggest that low birth weight may be a risk factor for psychosis only among individuals exposed to other obstetric risks, including influenza and hypoxia. The results further suggest that an underlying liability to psychosis might moderate the effects of these OCs on fetal growth. Future studies are needed to examine specific liabilities that could underlie this association, including polymorphisms in genes that confer risk for psychotic disorders.
Funding
This work was supported by the Stanley Medical Research Institute and a gift from Garen and Shari Staglin to T.D.C., a NIMH fellowship to L.M.E. as part of the UCLA Health Psychology Doctoral Program (MH15750), and a postdoctoral NIMH fellowship to L.M.E. as part of Columbia University’s schizophrenia research fellowship (5 T32 MH018870-20).
Acknowledgments
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kunugi H, Nanko S, Murray RM. Obstetric complications and schizophrenia: prenatal underdevelopment and subsequent neurodevelopmental impairment. Br J Psychiatry Suppl. 2001;40:s25––s29 [DOI] [PubMed] [Google Scholar]
- 2. Lane EA, Albee GW. Comparative birth weights of schizophrenics and their siblings. J Psychol. 1966;64:227––231 [Google Scholar]
- 3. Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923––930 [DOI] [PubMed] [Google Scholar]
- 4. Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351––366 [DOI] [PubMed] [Google Scholar]
- 5. Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65:1040––1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261––280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774––780 [DOI] [PubMed] [Google Scholar]
- 8. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080––1092 [DOI] [PubMed] [Google Scholar]
- 9. Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490––496 [DOI] [PubMed] [Google Scholar]
- 10. Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523––559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz JE, Kovach A, Meyer J, McConnell C, Iwamoto HS. Brief, intermittent hypoxia restricts fetal growth in Sprague-Dawley rats. Biol Neonate. 1998;73:313––319 [DOI] [PubMed] [Google Scholar]
- 12. Siegel M, Fuerst HT. Low birth weight and maternal virus diseases. A prospective study of rubella, measles, mumps, chickenpox, and hepatitis. JAMA. 1966;197:680––684 [PubMed] [Google Scholar]
- 13. Tanaka M, Natori M, Ishimoto H, Miyazaki T, Kobayashi T, Nozawa S. Experimental growth retardation produced by transient period of uteroplacental ischemia in pregnant Sprague-Dawley rats. Am J Obstet Gynecol. 1994;171:1231––1234 [DOI] [PubMed] [Google Scholar]
- 14. Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23 905––916 [DOI] [PubMed] [Google Scholar]
- 15. Cannon TD, Yolken R, Buka S, Torrey EF. Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64:797––802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neerhof MG. Causes of intrauterine growth restriction. Clin Perinatol. 1995;22:375––385 [PubMed] [Google Scholar]
- 17. Niswader KR, Gordon M. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and their Pregnancies. Philadelphia: W. B. Saunders; 1972 [Google Scholar]
- 18. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders–—Patient Edition (SCID-I/P, Version 2.0). Institute NYSP, ed. New York: Biometric Research; 1995 [Google Scholar]
- 19. Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467––485 [DOI] [PubMed] [Google Scholar]
- 20. Saha S, Barnett AG, Buka SL, McGrath JJ. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res. 2009;115:130––135 [DOI] [PubMed] [Google Scholar]
- 21. Klebanoff MA, Zemel BS, Buka S, Zierler S. Long-term follow-up of participants in the Collaborative Perinatal Project: tracking the next generation. Paediatr Perinat Epidemiol. 1998;12:334––346 [DOI] [PubMed] [Google Scholar]
- 22. Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. New York: Garland Science Publishing; 2005 [Google Scholar]
- 23. Gyamfi C, Fernandez-Sesma A, Marcell N, Steren C, Sperling R, Moran T. Evaluation of the maternal IgG response to influenza vaccine during pregnancy. Am J Obstet Gynecol. 2005;193:S84 [Google Scholar]
- 24. Vora M, Gruslin A. Erythropoietin in obstetrics. Obstet Gynecol Surv. 1998;53:500––508 [DOI] [PubMed] [Google Scholar]
- 25. Reviewed in Cunningham GF, Gant NF, Leveno KJ, Gilstrap LC, III, Hauth JC, Wenstrom KD. William’s Obstetrics. 21st ed. International: McGraw-Hill Companies; 2001 [Google Scholar]
- 26. Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia: W. B. Saunders; 2001 [Google Scholar]
- 27. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988 [Google Scholar]
- 28. Larsen JK, Bendsen BB, Foldager L, Munk-Jorgensen P. Prematurity and low birth weight as risk factors for the development of affective disorder, especially depression and schizophrenia: a register study. Acta Neuropsychiatr. 2010;22(6):284––291 [DOI] [PubMed] [Google Scholar]
- 29. Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299––305 [DOI] [PubMed] [Google Scholar]
- 30. Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M. Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry. 2001;6:79––82 [DOI] [PubMed] [Google Scholar]
- 31. Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005;7:593––604 [DOI] [PubMed] [Google Scholar]
- 32. Katila H, Hänninen K, Hurme M. Polymorphisms of the interleukin-1 gene complex in schizophrenia. Mol Psychiatry. 1999;4:179––181 [DOI] [PubMed] [Google Scholar]
- 33. Bresnahan M, Begg MD, Brown A, et al. Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36:751––758 [DOI] [PubMed] [Google Scholar]
- 34. Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004;191:691––699 [DOI] [PubMed] [Google Scholar]
- 35. Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13––30 [DOI] [PubMed] [Google Scholar]
- 36. Maier RF, Böhme K, Dudenhausen JW, Obladen M. Cord blood erythropoietin in relation to different markers of fetal hypoxia. Obstet Gynecol. 1993;81:575––580 [PubMed] [Google Scholar]
- 37. Marsál K. Intrauterine growth restriction. Curr Opin Obstet Gynecol. 2002;14:127––135 [DOI] [PubMed] [Google Scholar]
- 38. Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083––1094 [DOI] [PMC free article] [PubMed] [Google Scholar]