Abstract
Neurocognitive dysfunction is well established in psychosis, but recent work suggests that processing speed deficits might represent a particularly important cognitive deficit. A number of significant confounds, however, such as disease chronicity and antipsychotic medication use, have been shown to affect processing speed, causing debate as to the core cognitive features of psychosis. We adopted a novel strategy of testing neurocognitive performance in the “extended psychosis phenotype,” involving community-based adolescents who are not clinically psychotic but who report psychotic symptoms and who are at increased risk of psychosis in adulthood. This allows investigation of the earliest cognitive factors associated with psychosis risk, while excluding potential confounds such as disease chronicity and antipsychotic use. A population sample of 212 school-going adolescents aged 11–13 years took part in this study. Psychotic symptoms were assessed using the psychosis section of the Schedule for Affective Disorders and Schizophrenia. Neurocognition was assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus neurocognitive battery. Adolescents with psychotic symptoms performed significantly more poorly on 3 processing speed tasks: Trail Making Test-A (F = 3.3, P < .05), Trail Making Test-B (F = 3.1, P < .05), and digit symbol coding task (F = 7.0, P < .001)—as well as on a nonverbal working memory (spatial span) task (F = 3.2, P < .05). Our findings support the idea that neurocognitive impairment, and processing speed impairment in particular, is a core feature of psychosis risk. This group likely demonstrates some of the earliest cognitive impairments associated with psychosis vulnerability.Key words: epidemiology/adolescents/cognition
Key words: epidemiology, adolescents, cognition
Introduction
Neurocognitive impairments are among the most replicated findings in schizophrenia. Deficits have been demonstrated across a wide range of cognitive domains, including in attention, executive function, spatial ability, verbal learning, and memory. 1 Similar deficits have been demonstrated even in young people who are at high risk of psychosis. In ultra high-risk (UHR) samples, eg, deficits have been demonstrated across a number of neurocognitive domains, including in attention, verbal learning, executive function, and in verbal and nonverbal working memory. 2 – 7 Recently, however, debate has emerged about the relative importance of deficits in particular neurocognitive domains. For example, do particular deficits emerge earlier than others or show stronger effects in terms of impairment. Addressing this question is crucial because neurocognitive deficits inform us about the pathophysiology of the underlying disease and, therein, provide important direction for research on treatment. The importance of this has been reflected in the recent galvanization of efforts from government, industry, and academia to produce a consensus cognitive battery for the purposes of research into treatment of psychosis—the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery, developed under the aegis of the US National Institute of Mental Health.8
Recently, some researchers have argued that processing speed, that is, the speed with which cognitive operations can be performed, is the core cognitive deficit in psychosis. Dickinson et al., in a meta-analysis of neuropsychological studies of schizophrenia, demonstrated that performance on a measure of processing speed showed the largest single impairment of any task. 9 Knowles et al. subsequently demonstrated that a number of variables moderate the effect size of processing speed, most notably anti-psychotic medication. 10 This raises questions about how intrinsic processing speed impairment is to schizophrenia itself as opposed to downstream factors associated with the illness.
An alternative approach to this methodological problem is to examine the association between neurocognition and psychosis in a population sample who have not yet had contact with mental health services and who, thus, have not been exposed to treatment effects, including antipsychotic medication. One such approach has been to recruit young people who are not clinically psychotic but who evidence risk for psychosis based on the presence of subclinical psychotic symptoms. 11 – 15 A systematic review of population studies of psychotic symptoms in young people showed a median symptom prevalence of 17% in 9- to 12-year olds and 7.5% in 13- to 17-year olds. 16 Studies have shown that these individuals share a wide range of risk factors with schizophrenia patients 17 and are at increased risk for psychosis in adulthood, 18 , 19 leading researchers to advocate their recruitment for psychosis research as part of an “extended psychosis phenotype” 20 , 21 from whom we can learn about the pathogenesis of psychosis. 22 , 23 More recently, researchers have demonstrated that psychotic symptoms are associated with a number of psychiatric disorders, not limited to psychosis, in particular for severe, multimorbid psychopathology. 24 , 25
In this study, we investigated the neurocognitive profile of a population sample of adolescents who report psychotic symptoms, using the MATRICS consensus battery. 8 This group, while part of a psychosis continuum, are not clinically psychotic and are, thus, free of potential confounds such as disease chronicity and medication, allowing investigation of the earliest cognitive risk factors underlying an extended psychosis phenotype.
Methods
Recruitment
The study was carried out in Dublin, Ireland, and neighboring counties, with testing conducted over 3 consecutive years during school summer breaks. The study methodology has been previously reported. 26 However, briefly, a total of 1131 pupils from 16 schools in fifth and sixth class (ie, the two most senior years in the Irish national/primary school system), aged 11–13 years, participated in a survey of psychiatric symptoms, using the Strengths and Difficulties Questionnaire (SDQ), 27 which is a validated self-report instrument that assesses emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behavior. This sample was also assessed for psychotic symptoms, using the Adolescent Psychotic Symptom Screener (APSS), which is a validated self-report instrument that assesses hallucinations and delusions. 28 These instruments were completed in school, with a member of the research team present in the classroom. Data from these instruments were not used as part of a selection process; rather, these instruments provided baseline data on psychopathology and psychotic symptoms in the total school-based population. Written informed consent was obtained from the parent or guardian of participants. Of the total 1131 adolescents, 656 (58%) indicated an interest in taking part in a more in-depth study and a sample of 212 attended for clinical interview and neurocognitive testing. Because a relationship has been shown between low socioeconomic status (SES) and psychosis, 29 , 30 SES of each study participant was determined using parental occupation, assessed according to the Irish Social Class Scale from the national Central Statistics Office. We divided the sample into 2 major groups according to social class: the first group contained SES groups 1 and 2 (professional/managerial) and the second group contained SES groups 3–7: (nonmanual skilled, skilled manual, semi-skilled manual, unskilled manual, and unemployed).
Assessment of Psychotic Symptoms
Psychotic symptoms were assessed using the psychosis section of the Schedule for Affective Disorders and Schizophrenia for School-aged Children (K-SADS) (available online: http://www.moodresearch.com/resources/scales/children/KSADS-PL.pdf). The K-SADS is a well- validated semi-structured research diagnostic interview for the assessment of Axis-1 psychiatric disorders in children and adolescents. The psychosis section contains questions designed to assess a range of hallucinations and delusional thinking. Children and parents were interviewed separately, both answering the same questions about the child. Interviews were conducted by 2 psychiatrists and 4 psychologists with extensive training on the assessment of psychotic symptoms. All interviewers recorded detailed notes of potential psychotic phenomena in this section of the interview. A clinical consensus meeting was held following all of the interviews in order to classify potential symptoms as psychotic or not, blind to all other information on the participants.
Neurocognitive Assessment
The neurocognitive assessment was carried out in years 2 and 3 of the study, which included approximately 80% of the total sample (n = 165). The battery was administered by psychologists with training in standardized neurocognitive testing. The MATRICS neurocognitive battery was used to assess neurocognition. 8 The Wide Range Achievement Test 4 (WRAT4) 31 was used as a brief assessment of general scholastic ability/IQ. The MATRICS covers 7 putative cognitive domains in 10 tests. Because of time constraints, the social cognition task was excluded from our panel of tests.
In relation to the assessment of differential deficits in the extended psychosis phenotype, it is important to ensure that deficits are due to a differential ability rather than simply being due to good discriminant ability of some tasks and poor discriminant ability of others. Two factors that have been recognized to be important in recognizing true differential deficits in neurocognitive ability, as opposed to differences in discriminant properties of tasks, are test-retest reliability and spread of scores. 32 The test-retest reliability of MATRICS tasks has been shown to be excellent and the number of scores reaching floor or ceiling levels to be low. 8 , 33 What is more, clear deficits have been demonstrated for each of the MATRICS tasks in schizophrenia patients. 8 , 33 In addition, specifically in relation to childhood populations, Holmen and colleagues have previously reported on administering the MATRICS in children as young as age 12 with early onset schizophrenia and showed it to be a sensitive marker of cognitive dysfunction in this age group. 34 They found that patients demonstrated deficits on all MATRICS tasks used in this study. Therefore, any differential deficits in the extended psychosis phenotype would be unlikely to be due to a problem with the discriminant ability of the MATRICS tasks and should reflect true differential deficits in ability. The following nine MATRICS tasks were used, with one additional task: the Trail Making Test-Part B (TMT-B).
Tasks
TMT—Parts A and B: Pencil and paper task that requires the participant to draw a line connecting, in consecutive order, numbers arranged randomly on a page (Part A), followed by both numbers and letters arranged randomly on a page (Part B); outcome: total time for completion. Putative cognitive domain: processing speed.
Brief assessment of cognition in schizophrenia-symbol coding (BACS-SC): A pencil and paper task that requires participants to write numbers that correspond to nonsense symbols as rapidly as possible in 90 s; outcome: number of symbols coded correctly. Putative cognitive domain: processing speed.
Category fluency (Fluency): verbal fluency for animals in 60 s; outcome: number of correct words spoken. Putative cognitive domain: processing speed.
Hopkins verbal learning -revised (HVLT-R): Participants heard a list of 12 words and were asked to repeat these in a series of 3 trials; outcome: number of correct responses summed over 3 trials. Putative cognitive domain: verbal learning/memory.
Wechsler memory scale-spatial span (WMS-SS) (nonverbal memory): Requires participants to remember and repeat which of a series of blocks the test administrator points to, first forward then backward; outcome: sum of raw scores for both conditions. Putative cognitive domain: working memory (nonverbal).
Letter number span (LNS) (verbal memory): Participants heard sets of letters and numbers, which they were required to repeat after mentally reordering numerically and alphabetically; outcome: total number correctly spoken. Putative cognitive domain: working memory (verbal).
Mazes (neuropsychological assessment battery): Pencil and paper test where participants attempted 7 mazes of increasing difficulty; outcome: scores were based on the speed with which participants completed the 7 mazes. Putative cognitive domain: reasoning and problem solving.
Brief visuospatial memory test-revised (BVMT-R): Participants were shown a page displaying six geometric figures for 10 s over 3 trials and asked to draw these figures on a sheet of paper after each trial; outcome: points were awarded for the accuracy of the drawings over the 3 trials. Putative cognitive domain: visual learning.
Continuous performance test-identical pairs (CPT-IP): Participants were required to monitor numbers as they flashed on screen and press a button whenever 2 numbers in a row were identical; outcome: summed mean d’value, which is an index of signal/noise discrimination, across 2-, 3-, and 4-digit conditions. Putative cognitive domain: attention/vigilance.
Statistical Analyses
Statistical analyses were conducted using STATA version 11.0 for Windows. Chi-square and t tests were used to measure differences in participants who took part in the interview study compared with the larger surveyed population sample. We report means and standard deviations for adolescents with and without psychotic symptoms. Standardized z scores were calculated for each participant across each of the tasks, using the formula Z = (data point – mean)/standard deviation. Analysis of covariance was used to examine neurocognitive performance on the MATRICS in adolescents with psychotic symptoms compared with the rest of the population sample, using z scores as the dependent variables and controlling for sex and number of years of education (see table 1). Significant findings were also analyzed for interactions between group and sex. Cohen’s f statistic was used as a measure of effect size for differences between adolescents with psychotic symptoms compared with adolescents with no psychotic symptoms using the Stata command effectsize (see table 1). By convention, a Cohen’s f of about 0.10 is considered small, about 0.25 is considered medium, and about 0.40 is considered large.
Table 1.
Neurocognitive Performance on the MATRICS Battery, Adjusted for Sex and Years of Education
| Test (Putative Neurocognitive Domain) | Males (n = 85)Mean (SD) | Females (n = 80) Mean (SD) | F (P value) | Psychotic Symptoms Group (n = 42) Mean (SD) | Comparison Group (n = 123)Mean (SD) | F (P value) | Cohen’s f |
|---|---|---|---|---|---|---|---|
| TMT-Aa (processing speed) | 44.9 (13.3) | 41.8 (12.1) | 2.47 (0.12) | 45.5 (14.0) | 42.6 (12.3) | 3.33 (<0.05) | 0.10 |
| TMT-Ba (processing speed) | 74.3 (29.0) | 68.3 (28.8) | 1.46 (0.23) | 83.4 (32.3) | 67.4 (26.8) | 3.06 (<0.05) | 0.25 |
| BACS-SC (processing speed) | 45.5 (9.4) | 50.3 (8.6) | 11.66 (<0.001) | 44.4 (10.2) | 49.1 (8.7) | 7.03 (<0.001) | 0.22 |
| Category fluency (processing speed) | 20.2 (5.4) | 20.1 (4.9) | 0.01 (0.91) | 19.8 (5.2) | 20.3 (5.2) | 1.20 (0.31) | 0.04 |
| HVLT-R (verbal learning/memory) | 24.6 (4.2) | 26.0 (4.5) | 3.71 (0.06) | 24.3 (5.3) | 25.6 (4.0) | 2.59 (0.05) | 0.13 |
| WMS-SS(working memory: nonverbal) | 14.7 (3.1) | 15.9 (3.1) | 6.89 (<0.01) | 14.9 (3.7) | 15.4 (2.9) | 3.17 (<0.05) | 0.07 |
| LNS (working memory: verbal) | 13.1 (2.8) | 13.5 (2.9) | 0.84 (0.36) | 13.4 (2.9) | 13.3 (2.8) | 0.37 (0.78) | 0.02 |
| NAB: Mazes (reasoning and problem solving) | 16.5 (5.1) | 14.9 (5.0) | 3.97 (<0.05) | 15.2 (5.1) | 15.9 (5.1) | 2.38 (0.07) | 0.06 |
| BVMT-R (visual learning) | 25.1 (7.9) | 27.5 (6.6) | 4.56 (<0.05) | 26.9 (7.0) | 26.1 (7.5) | 2.24 (0.09) | 0.05 |
| CPT-IP (attention/vigilance) | 5.0 (4.8) | 5.4 (1.7) | 0.24 (0.63) | 4.5 (2.8) | 5.3 (4.0) | 0.27 (0.85) | 0.09 |
Note: SD, standard deviation; TMT, Trail Making Test (Part A and Part B); BACS-SC, brief assessment of cognition in schizophrenia-symbol coding; HVLT-R, Hopkins verbal learning test-revised; WMS, Wechsler Memory Scale; LNS, letter number span; NAB, Neuropsychological Assessment Battery; BVMT-R, brief visuospatial memory test-revised; CPT, continuous performance test-identical pairs.
aHigher score = poorer performance. By convention, a Cohen’s f of about 0.10 is considered small, about 0.25 is considered medium, and about 0.40 is considered large.
Ethical approval for the study was granted by the Beaumont Hospital Medical Ethics Committee.
Results
Adolescents who attended for interview and neurocognitive testing were no more likely to have an abnormal or borderline-abnormal score on the SDQ (x 2 = 1.22 [df = 1] P = .27) and did not differ significantly in their scores on the APSS (interviewed group mean = 1.8 [SE = 0.12], non-interviewed group mean = 1.9 [SE = 0.19]; t = 0.26, df = 1130, P = 0.79). The nationalities of adolescents taking part in the study approximated the representative figures from the 2006 national census, including 88.9% Irish-born participants (compared with 90.3% of 0–14-year olds nationally). A total of 34.6% of participants were categorized as SES groups 1 and 2 (compared with 32.1% of the general population nationally) and 65.4% were categorized as SES groups 3–7. There was no relationship between SES and psychotic symptoms or performance on neurocognitive tasks; therefore, SES was not included as a covariate in our analyses. However, females performed significantly better on the symbol coding task, the spatial span task, and the visual learning task, while males performed significantly better on the Mazes problem-solving task; thus, sex was controlled for in the statistical analyses (see table 1).
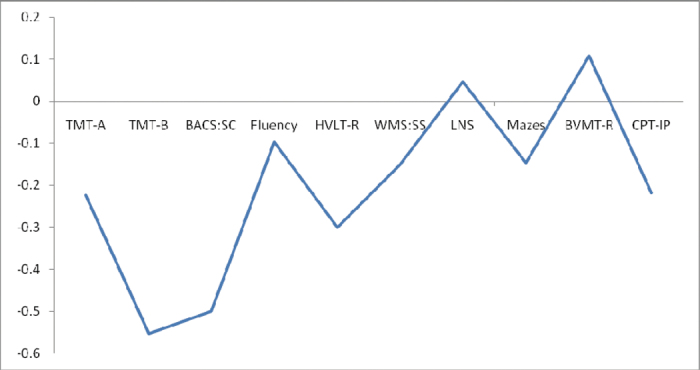
A total of 22.6% of the sample reported psychotic symptoms, principally auditory hallucinations. Psychotic symptoms were more common among fifth class participants compared with sixth class participants (χ2 = 5.95, P < .05) and among males compared with females (χ2 = 7.03, P < .01) and so number of years of education and sex were used as covariates in the neurocognitive analyses. Adolescents with psychotic symptoms did not differ in general scholastic ability/IQ as measured with the WRAT4 (F = 0.02, df = 1, P = .89). In tests of neurocognitive function, adolescents with psychotic symptoms performed significantly more poorly on the TMT-A, TMT-B, BACS-SC, and WMS-SS tasks (see table 1 and figure 1). The effect sizes were in the small range for differences in performance on TMT-A and WMS-SC and in the medium range for differences in performance on TMT-B and BACS-SC (see table 1). Differences on the HVLT-R were just outside statistical significance at the level of P = .05. Significant interactions were detected between group and sex on two tasks: BACS-SC (F = 5.77, P < .05) and WMS-SS (F = 3.90, P < .01), though controlling for these did not affect the overall results. In contrast to the findings on psychotic symptoms, depressive symptoms, as assessed using the SDQ, and depressive disorder, as assessed using the K-SADS, showed no relationship with performance on any of these tasks (results available upon request).
Fig. 1.
Mean differences in z scores between adolescents with and without psychotic symptoms on the MATRICS neurocognitive battery.
TMT-A, Trail Making Test-Part A; TMT—B, Trail Making Test-Part B; BACS-SC, brief assessment of cognition in schizophrenia-symbol coding; Fluency, verbal fluency test (animal naming); HVLT-R, Hopkins verbal learning test-revised; WMS-SS, Wechsler memory scale-spatial span; LNS, letter number span; Mazes, Neuropsychological Assessment Battery: Mazes; BVMT-R, brief visuospatial memory test-revised; CPT, continuous performance test-identical pairs.
Discussion
In this study, we found processing speed deficits in adolescents with psychotic symptoms, using the TMT-A, the TMT-B, and the BACS digit symbol coding tasks, as well as (nonverbal) working memory deficits on the WMS-SS task. Effect sizes were small for TMT-A and WMS-SS tasks but medium for both the symbol coding and TMT-B tasks (see table 1). There were significant interactions between group and sex in the BACS-SC and WMS-SS tasks, indicating that males with psychotic symptoms performed more poorly than females with psychotic symptoms on these tasks. Interaction results must be interpreted with caution, however, considering that there were fewer females (n = 20) than males (n = 33) in the psychotic symptom group and, thus, more limited power to test interactions.
This is the first study to report on symbol coding in a population sample with psychotic symptoms, with clear findings of impairment demonstrated on this task. This is in keeping with findings from Dickinson et al., 9 who have argued that symbol coding deficits reflect slowed information processing that is the central feature of cognitive dysfunction in psychosis. Interestingly, in a longitudinal study, Niendam et al. showed a number of neurocognitive deficits at age 7 in both individuals, who would go on to develop schizophrenia, and in their siblings; 35 however, only symbol coding scores differentiated those who would later develop schizophrenia from their unaffected siblings. We also found deficits in TMT-A and TMT-B performance in adolescents with psychotic symptoms in this study. This is in line with a previous cohort study, which demonstrated that childhood performance on processing speed tasks, uniquely among tests of neurocognition, predicted adulthood schizophrenia. 36 This finding is also in keeping with a previous report on a small sample of adolescents with psychotic symptoms (n = 17), which showed poorer performance on the TMT-B. 37
Waber et al., in the National Institutes of Health study of normal childhood brain development, demonstrated a general pattern of rapid improvement in cognitive domains, such as executive function and verbal fluency from age 6 up to ages 10–12, followed by a slow or minimal increase in scores up to age 16. 38 Interestingly, however, symbol coding scores demonstrated little change in trajectory at ages 10–12; rather, scores continued to increase linearly right throughout adolescence, reflecting continued rapid development in this domain. For example, between ages 11 and 13 (the ages of this study participants), scores in executive function did not increase, while scores in verbal learning/memory increased by approximately 2%. This contrasts with an increase of approximately 20% in symbol coding scores over the same period. Symbol coding scores, then, given that they develop rapidly during this time, may be a particularly sensitive marker of perturbations in normal brain development during the adolescent period. Abnormalities in neurodevelopment during this adolescent period that stunt cognitive development would take a much longer period of time to become readily apparent in other cognitive domains due to the slower rate of change in cognitive ability in these domains. For example, with regard to development in verbal learning/memory, even a 50% disruption to development in this domain between the ages of 11 and 13 would result in only an approximately 1% difference in scores compared with normally developing young people. The same degree of disruption to the development of symbol coding ability, however, would result in an approximately 10% difference in scores compared with healthy controls. Therefore, symbol coding ability might be particularly sensitive to disordered neurodevelopment in adolescence compared with many other cognitive domains. This might explain why deficits on all of the MATRICS tasks are evident in psychotic disorder (which tends to come on in late adolescence or early adulthood), when abnormalities in adolescent brain development have been occurring over a sufficient number of years to result in identifiable deficits in a wider range of cognitive domains. This may also explain why research on early stages of the psychosis prodrome has found that deficits are limited to processing speed, while in later stages of the prodrome deficits are evident across a range of neurocognitive tasks. 39
Spatial working memory deficits were also found in the current sample, though these deficits were less pronounced than those of processing speed (see figure 1). Interestingly, Waber et al. also found that spatial working memory was a relative exception to the general rule of slow change from age 10–12 (though to a lesser degree than that of symbol coding). 38 In fact, they found that errors in nonverbal working memory decreased most rapidly at ages 10–14 (rather than at ages 6–10 as with most other tasks). Spatial working memory, then, might also be a relatively sensitive marker of neurodevelopmental dysfunction occurring in early adolescence.
There has been a great deal of recent interest in processing speed in schizophrenia. Processing speed deficits have not only been shown in patients with chronic schizophrenia 9 but have also been demonstrated in first-episode psychosis 40 and in the psychosis prodrome.6 Some researchers, in fact, have argued that impairments in processing speed represent the most important or the core neurocognitive deficit of psychosis. 5 , 9 , 41 , 42 A number of converging lines of evidence support this hypothesis. First, longitudinal research has shown that childhood deficits in processing speed, but not deficits in a range of other neurocognitive tasks, are associated with increased risk for psychotic disorder in adulthood. 36 Second, processing speed scores, uniquely in a panel of neurocognitive tests, were shown to predict social and role functioning in patients at UHR for psychosis 5 and to predict real-world functioning in schizophrenia patients across all domains. 43 Third, meta-analytic research has demonstrated that deficits in processing speed are more pronounced in schizophrenia than deficits in other neurocognitive domains. 9 In a meta-analysis of 40 studies, Dickinson et al. found that the effect size of the impairment on symbol coding tasks, a classic measure of processing speed, significantly exceeded the effect sizes of tasks commonly used to measure other cognitive domains, including episodic memory, executive function, and working memory. Furthermore, in a sample of first-episode psychosis patients, Rodriguez-Sanchez et al. reported that, while patients demonstrated deficits across a wide range of neurocognitive domains, performance on processing speed, on multivariate analysis, accounted for deficits across all other domains. 41 These research findings, taken together, point to the importance of processing speed in the etiology of schizophrenia. The results of this study add further support to the importance of processing speed deficits in psychosis by showing that these deficits are present even in young adolescents in the community with psychotic symptoms (the extended psychosis phenotype).
The significance of processing speed deficits to “real world” measures of function has recently been highlighted by Bowie et al., 43 using the Specific Level of Function Scale, an observer-rated assessment of a patient’s behavior and functioning. In a sample of more than 200 schizophrenia patients, they found that processing speed tasks, uniquely in a battery of neurocognitive tests, predicted functioning in all 3 domains of functioning, including interpersonal relationships, community involvement, and work skills. More recently, among a sample of UHR patients, Carrion et al. showed that both social and role functioning related specifically to processing speed (a combined symbol coding and TMT score) 5 and argued that processing speed represents a rate-limiting step in the formation of good social and role functioning. Our own community findings suggest that deficits in processing speed may represent an early neurocognitive marker of psychosis vulnerability, present not only in patients but even in community-based young adolescents with psychotic symptoms.
In contrast to many neurocognitive tasks that might be attributed to specific neural networks or specific anatomical regions, processing speed tasks have been argued to measure a “systems”-based process, reflecting speeded integration and coordination between distributed brain networks. 42 Empirical support for this has come from digital tractography imaging of white matter microstructural organization in both healthy and brain injured individuals. Turken et al., eg, showed that processing speed is closely related to the structural integrity of major white matter tracts that run along the anterior-posterior axis of the brain, allowing fronto-posterior network interactions, including the superior longitudinal fasciculus, occipitofrontal fasciculus, and inferior longitudinal fasciculus. 44 The findings of this study, then, are in line with the dysconnection hypothesis of schizophrenia, which asserts that impaired communication within the brains of schizophrenia patients occurs when there is focal disruption that adversely affects the entire network. 45 This is in keeping with neuroimaging findings that showed impaired connectivity in a community sample of adolescents with psychotic symptoms. 46 Processing speed deficits, then, may point to aberrant functional connectivity within and between whole-brain neural systems, rather than indexing impairment in discrete neural networks.
Strengths and Limitations
This is the largest population-based neurocognitive assessment of young people with psychotic symptom to date. In addition, this is, to our knowledge, the first community-based study in children to use the MATRICS battery. The use of a standardized neurocognitive battery in this study will facilitate comparison with other studies. None of the adolescents in this study had a diagnosis of a psychotic disorder and none had ever used antipsychotic medication, outruling disease chronicity or treatment effects in the relationship between psychotic symptoms and neurocognitive performance. While we surveyed a relatively large number of adolescents, a relatively small proportion was brought to interview, introducing the risk of ascertainment bias, whereby individuals with a personal or family history of disorder may be more likely to agree to participate, thus self-selecting for increased rates of the disorder under study (in this case psychotic disorders). However, we do not believe this to be the case in this study for a number of reasons: (1) adolescents who attended the full interview study did not differ from the larger surveyed school sample from which they were drawn in terms of symptoms of general psychopathology, as measured by the SDQ, or in terms of psychotic symptoms, as measured by the APSS; (2) only 1.3% of participants had a first degree relative with a history of psychotic illness, suggesting that families with psychosis were not more likely to participate; (3) the prevalence of mental disorders was very similar to previous epidemiological work both nationally and internationally. 47 , 48 Participants were also representative of the general population in terms of ethnicity and socioeconomic status. It is important to note that, while young people with psychotic symptoms are considered to form part of an “extended psychosis continuum,” they do not represent a straightforward phenotype in terms of psychosis risk, in that they have been shown to be at risk for a variety of diagnoses not limited to psychosis. 24 , 49
An interesting methodological finding of this study was that, while adolescents with psychotic symptoms demonstrated deficits on 3 processing speed tasks, there was no evidence of impairment on a fourth test of processing speed (verbal fluency). In addition, while the impairment effect size for one of the processing speed tasks was in the small range (TMT-A), 2 were in the medium range (TMT-B and BACS-SC). This illustrates a problem inherent in reporting “domain” scores, which is frequently the case in the literature, rather than reporting performance on individual tasks. By reporting only a domain score, tasks which, at face value, may involve a number of different processes are reduced to the mean when, in fact, there might be substantial differences in effect size across the tasks. The findings of this study, then, highlight the fact that putative “domain scores” should be used with caution in the MATRICS and other neurocognitive batteries, with individual task scores always also being reported.
Conclusion
In a sample of adolescents with psychotic symptoms tested with the MATRICS consensus battery, we found impairment on processing speed tasks and in nonverbal working memory. These individuals, while part of an extended psychosis phenotype, are not clinically psychotic and are free of complicating medication/treatment effects, which may affect neurocognitive findings in schizophrenia samples. Our findings support hypotheses on the importance of processing speed deficits to psychosis. This adds to the evidence that a systems-based dysfunction may be at the heart of cognitive impairment in psychosis, as opposed to cognitive features that suggest that the pathology emerges from localized or region-specific deficits. These findings also support existing evidence which suggests that processing speed should be a key target for research aimed at ameliorating cognitive impairment in schizophrenia. Further neurocognitive and neuroimaging research in the extended psychosis phenotype will add to our understanding of the core underlying deficits and provide guidance on the best approaches to developing treatments targeted at improving cognition in psychosis.
Funding
European Community’s Seventh Framework Programme (HEALTH-F2-2010-241909 [Project EU-GEI]). EU-GEI is the acronym of the project European network of National Schizophrenia Networks Studying Gene-Environment Interactions. Essel National Alliance for Research on Schizophrenia and Depression/The Brain and Behavior Research Foundation Independent Investigator award (to M.C.). Clinician Scientist Award (CSA/2004/1) from the Health Research Board (Ireland) (to M.C.). C.R. was supported by an Irish Research Council for the Humanities and Social Sciences Postgraduate Scholarship.
Acknowledgments
We thank the Clinical Research Centre at Beaumont Hospital for use of research rooms. None of the study sponsors had any role in the data collection, analysis, or write up of this article. The authors report no conflicts of interest.
References
- 1. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445 [DOI] [PubMed] [Google Scholar]
- 2. Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871 [DOI] [PubMed] [Google Scholar]
- 3. Wood SJ, Pantelis C, Proffitt T, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247 [DOI] [PubMed] [Google Scholar]
- 4. Hawkins KA, Addington J, Keefe RS, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67:115–122 [DOI] [PubMed] [Google Scholar]
- 5. Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168:806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niendam TA, Bearden CE, Zinberg J, Johnson JK, O’Brien M, Cannon TD. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophr Bull. 2007;33:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213 [DOI] [PubMed] [Google Scholar]
- 9. Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542 [DOI] [PubMed] [Google Scholar]
- 10. Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–835 [DOI] [PubMed] [Google Scholar]
- 11. Scott J, Martin G, Bor W, Sawyer M, Clark J, McGrath J. The prevalence and correlates of hallucinations in Australian adolescents: results from a national survey. Schizophr Res. 2009;107:179–185 [DOI] [PubMed] [Google Scholar]
- 12. Yung AR, Nelson B, Baker K, Buckby JA, Baksheev G, Cosgrave EM. Psychotic-like experiences in a community sample of adolescents: implications for the continuum model of psychosis and prediction of schizophrenia. Aust N Z J Psychiatry. 2009;43:118–128 [DOI] [PubMed] [Google Scholar]
- 13. Bartels-Velthuis AA, Jenner JA, van de Willige G, van Os J, Wiersma D. Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196:41–46 [DOI] [PubMed] [Google Scholar]
- 14. Varghese D, Scott J, Welham J, et al. Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull. 2011;37:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laurens KR, Hobbs MJ, Sunderland M, Green MJ, Mould GL. Psychotic-like experiences in a community sample of 8000 children aged 9 to 11 years: an item response theory analysis. Psychol Med. 2012;42:1495–1506 [DOI] [PubMed] [Google Scholar]
- 16. Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42:1857–1864 [DOI] [PubMed] [Google Scholar]
- 17. Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41:1–6 [DOI] [PubMed] [Google Scholar]
- 18. Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058 [DOI] [PubMed] [Google Scholar]
- 19. Welham J, Scott J, Williams G, et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med. 2009;39:625–634 [DOI] [PubMed] [Google Scholar]
- 20. Wigman JT, Vollebergh WA, Raaijmakers QA, et al. The structure of the extended psychosis phenotype in early adolescence—a cross-sample replication. Schizophr Bull. 2011;37:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195 [DOI] [PubMed] [Google Scholar]
- 22. Polanczyk G, Moffitt TE, Arseneault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67:328–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cullen AE, Dickson H, West SA, et al. Neurocognitive performance in children aged 9–12 years who present putative antecedents of schizophrenia. Schizophr Res. 2010;121:15–23 [DOI] [PubMed] [Google Scholar]
- 24. Kelleher I, Keeley H, Corcoran P, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: Evidence from 4 population studies. Br J Psychiatry. 2012;201:26–32 [DOI] [PubMed] [Google Scholar]
- 25. Werbeloff N, Drukker M, Dohrenwend BP, et al. Self-reported attenuated psychotic symptoms as forerunners of severe mental disorders later in life. Arch Gen Psychiatry. 2012;69:467–475 [DOI] [PubMed] [Google Scholar]
- 26. Kelleher I, Murtagh A, Molloy C, et al. Identification and characterization of prodromal risk syndromes in young adolescents in the community: a population-based clinical interview study. Schizophr Bull. 2012;38:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodman R, Ford T, Simmons H, Gatward R, Meltzer H. Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. 2000;177:534–539 [DOI] [PubMed] [Google Scholar]
- 28. Kelleher I, Harley M, Murtagh A, Cannon M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37:362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am J Psychiatry. 2005;162:1652–1657 [DOI] [PubMed] [Google Scholar]
- 30. Scott J, Chant D, Andrews G, McGrath J. Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychol Med. 2006;36:231–238 [DOI] [PubMed] [Google Scholar]
- 31. Wilkinson G, Robertson G. Wide Range Achievement Test. 4th ed. Lutz, FL: Psychological Assessment Resources Inc; 2005 [Google Scholar]
- 32. Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14:303–311 [DOI] [PubMed] [Google Scholar]
- 33. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220 [DOI] [PubMed] [Google Scholar]
- 34. Holmén A, Juuhl-Langseth M, Thormodsen R, Melle I, Rund BR. Neuropsychological profile in early-onset schizophrenia-spectrum disorders: measured with the MATRICS battery. Schizophr Bull. 2010;36:852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062 [DOI] [PubMed] [Google Scholar]
- 36. Cannon M, Moffitt TE, Caspi A, Murray RM, Harrington H, Poulton R. Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. Br J Psychiatry. 2006;189:463–464 [DOI] [PubMed] [Google Scholar]
- 37. Blanchard MM, Jacobson S, Clarke MC, et al. Language, motor and speed of processing deficits in adolescents with subclinical psychotic symptoms. Schizophr Res. 2010;123:71–76 [DOI] [PubMed] [Google Scholar]
- 38. Waber DP, De Moor C, Forbes PW, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746 [DOI] [PubMed] [Google Scholar]
- 39. Frommann I, Pukrop R, Brinkmeyer J, et al. Neuropsychological profiles in different at-risk states of psychosis: executive control impairment in the early—and additional memory dysfunction in the late—prodromal state. Schizophr Bull. 2011;37:861–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336 [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Sanchez JM, Crespo-Facorro B, Gonzalez-Blanch C, Perez-Iglesias R, Vazquez-Barquero JL. Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. Br J Psychiatry. 2007(Suppl);51:s107–110 [DOI] [PubMed] [Google Scholar]
- 42. Dickinson D. Digit symbol coding and general cognitive ability in schizophrenia: worth another look? Br J Psychiatry. 2008;193:354–356 [DOI] [PubMed] [Google Scholar]
- 43. Bowie CR, Leung WW, Reichenberg A, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032– 1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friston K, Frith C. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89– 97 [PubMed] [Google Scholar]
- 46. Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11 13 year old schoolchildren. Neuroimage. 2010;49:1875–1885 [DOI] [PubMed] [Google Scholar]
- 47. Lynch F, Mills C, Daly I, Fitzpatrick C. Challenging times: prevalence of psychiatric disorders and suicidal behaviours in Irish adolescents. J Adolesc. 2006;29:555–573 [DOI] [PubMed] [Google Scholar]
- 48. Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001 2004 NHANES. Pediatrics. 2010;125:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rössler W, Hengartner MP, Ajdacic-Gross V, Haker H, Gamma A, Angst J. Sub-clinical psychosis symptoms in young adults are risk factors for subsequent common mental disorders. Schizophr Res. 2011;131:18–23 [DOI] [PubMed] [Google Scholar]