Abstract
The major excitatory and inhibitory neurotransmitters, glutamate (Glu) and gamma-aminobutyric acid (GABA), respectively, are implicated in the pathophysiology of schizophrenia. N-acetyl-aspartyl-glutamate (NAAG), a neuropeptide that modulates the Glu system, may also be altered in schizophrenia. This study investigated GABA, Glu + glutamine (Glx), and NAAG levels in younger and older subjects with schizophrenia. Forty-one subjects, 21 with chronic schizophrenia and 20 healthy controls, participated in this study. Proton magnetic resonance spectroscopy (1H-MRS) was used to measure GABA, Glx, and NAAG levels in the anterior cingulate (AC) and centrum semiovale (CSO) regions. NAAG in the CSO was higher in younger schizophrenia subjects compared with younger control subjects. The opposite pattern was observed in the older groups. Glx was reduced in the schizophrenia group irrespective of age group and brain region. There was a trend for reduced AC GABA in older schizophrenia subjects compared with older control subjects. Poor attention performance was correlated to lower AC GABA levels in both groups. Higher levels of CSO NAAG were associated with greater negative symptom severity in schizophrenia. These results provide support for altered glutamatergic and GABAergic function associated with illness course and cognitive and negative symptoms in schizophrenia. The study also highlights the importance of studies that combine MRS measurements of NAAG, GABA, and Glu for a more comprehensive neurochemical characterization of schizophrenia.
Key words: schizophrenia, MRS, GABA, NAAG, glutamate, neuroimaging
Introduction
Effective brain and behavior function depends on a carefully orchestrated balance of inhibition and excitation in the central nervous system. That dynamic is dependent on the actions of glutamate (Glu), the principal excitatory neurotransmitter, and gamma-aminobutyric acid (GABA), the principal inhibitory transmitter. A disturbance in the actions of these transmitters is implicated in the pathophysiology of schizophrenia.1,2 A wide range of pathophysiological and therapeutic studies has implicated glutamatergic alterations in schizophrenia,3,4 while evidence for altered GABAergic function in schizophrenia comes primarily from postmortem studies.2,5 An endogenous neuropeptide, N-acetyl-aspartyl-glutamate (NAAG), a precursor of Glu, which modulates the Glu system as an agonist of the metabotropic type-3 Glu receptor (mGluR3) and as a weak antagonist of the N-Methyl- d -aspartate receptor (NMDAR), may be altered in schizophrenia based on postmortem and rodent studies.6–8
Proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive technique capable of measuring in vivo Glu, GABA, and NAAG levels in the human brain. Several studies have reported altered Glu, glutamine (Gln), or combined Glu + glutamine (Glx) levels in schizophrenia with conventional spectroscopic methods.9,10 GABA and NAAG are difficult to measure with conventional spectroscopic methods at 3 T due to their relatively low concentrations and overlap with other compounds that have more intense signals. However, they can be measured by spectral-editing methods, in particular, the “MEGA-PRESS” editing sequence.11 To date, there are only a few reports of GABA levels measured with edited MRS in patients with schizophrenia and the results are inconsistent. One study reported increased medial frontal and parietooccipital GABA/creatine (Cr) in chronic schizophrenia compared with controls, but these results may have been influenced by the adjunctive medication used in the patient group.12 Another reported increased medial prefrontal GABA in unmedicated patients.13 Other studies reported lower GABA:Cr ratios in the occipital lobe14 and basal ganglia15 in schizophrenia. Finally, one study found no significant differences between groups but did find correlations between GABA levels and dosage/type of antipsychotic medication.16 To date, there is just a single study of NAAG measured in vivo with MRS in patients with schizophrenia,17 which found a trend for increased NAAG in the anterior cingulate (AC) and an inverse correlation between frontal lobe NAAG levels and negative symptoms of schizophrenia.
The purpose of this study was to measure GABA, Glx, and NAAG in a medial frontal region that included the rostral AC and the centrum semiovale (CSO) brain region in subjects with and without schizophrenia. These regions were chosen as representative gray and white matter regions, respectively; pathology in the AC region has been implicated in schizophrenia in many studies.18 The CSO was chosen because it is a representative white matter region and white matter involvement has also been linked to schizophrenia.19 Because of clinical20 and neurochemical21 descriptions suggestive of differential effects in the first 10 years of the illness, we examined younger and older subjects. Based on prior postmortem and MRS studies of schizophrenia, we hypothesized that GABA, Glx, and NAAG would be reduced in the schizophrenia group and more pronounced in the older schizophrenia subjects when compared with controls. AC GABA was hypothesized to be positively correlated to performance on attention tasks (ie, higher AC GABA, better attention performance). NAAG was hypothesized to be negatively correlated to severity of positive and negative symptoms (ie, higher NAAG, lower psychiatric symptom severity).
Methods
Subjects
Twenty-one outpatients with chronic schizophrenia treated with antipsychotic medication and 20 healthy volunteers participated in this study. The inclusion/exclusion criteria for subjects with schizophrenia were as follows: (1) diagnosis of schizophrenia as determined with the Structured Clinical Interview for DSM-IV-TR, patient version, (2) age within 18–55 years old, (3) no current or past neurological condition, (4) no DSM-IV-TR substance abuse or dependence in the last 6 months, (5) clinically stable as determined by their treatment psychiatrist, (6) same type and dose of antipsychotic for at least 3 months, and (7) not currently treated with benzodiazepines or mood stabilizers. All but 2 patients were taking second-generation antipsychotic medication. The inclusion/exclusion criteria for healthy volunteers were as follows: (1) no past or present psychiatric disorder as determined with the Structured Clinical Interview for DSM-IV-TR, non-patient version, (2) age within 18–55 years old, (3) no first-degree relatives with a diagnosis of a psychotic disorder, (4) no current or past neurological condition, and (5) no DSM-IV-TR substance abuse in the last 6 months or dependence in lifetime. Subjects with schizophrenia were evaluated for their ability to provide informed consent before signing consent documents. All subjects gave written informed consent prior to participation in the study. This study was approved by the University of Maryland and Johns Hopkins University Internal Review Boards.
Patients were evaluated for psychopathology with the Brief Psychiatric Rating Scale (BPRS) and the Scale for the Assessment of Negative Symptoms (SANS). All subjects completed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) to provide indices of attention (digit span and coding tests), immediate and delayed memory (story and list recall tests), language (fluency), visual spatial construction, and combined score as a measure of general cognitive function. Subjects were monetarily compensated for their time.
MR Acquisition and Analyses
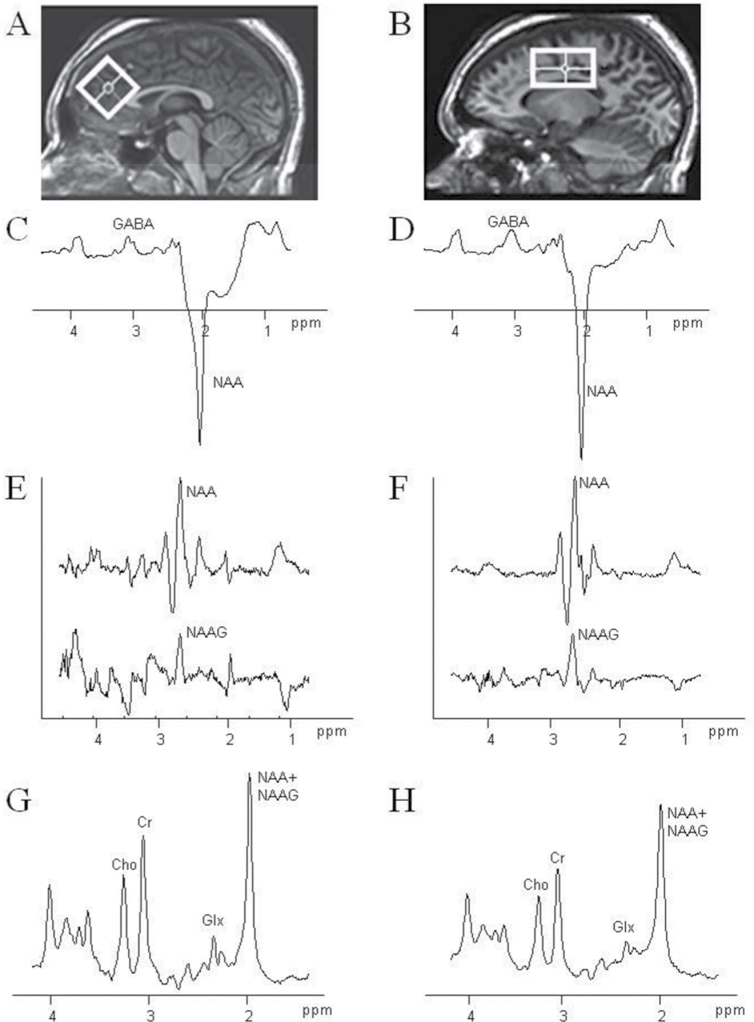
Magnetic resonance imaging (MRI) and MRS were conducted on a 3T “Achieva” scanner (Philips Healthcare, Best, the Netherlands) equipped with an 8-channel “SENSE” head coil. Anatomical T1-weighted images were acquired for spectroscopic voxel placement with a Magnetization Prepared RApid Gradient Echo (MP-RAGE) sequence (SENSE factor 2, 1-mm isotropic voxels, 256×256mm FOV, TR/TE/TI = 8/3.8/842.5ms, flip angle = 8°). Head position was fixed with foam padding to minimize movement. Spectroscopic voxel sizes were 3.5×3.5×3.5cm for a medial frontal region including the AC and 5.0×3.0×3.0cm for the CSO. The AC voxel was prescribed on the midsagittal slice and positioned parallel to the genu of the corpus callosum and scalp with the midline of the voxel placed directly above the most anterior tip of the genu of the corpus callosum. The CSO voxel was prescribed on the axial slice superior to the corpus callosum and positioned parallel and midline to the body of the corpus callosum. Voxels were placed to minimize cerebrospinal fluid (CSF) content. Based on the MP-RAGE images, spectroscopic voxels were segmented into gray, white, and CSF tissues using MATLAB code,22 and metabolite concentrations were corrected for the proportion of CSF. Figure 1 illustrates voxel position and representative spectra.
Fig. 1.
Regions of interest used for magnetic resonance spectroscopy (MRS) centered on the anterior cingulate (AC) gyrus (A) and centrum semiovale (CSO) white matter (B). The AC voxel size was 3.5 × 3.5 × 3.5 cm3, whereas the CSO voxel was 5.0 × 3.0 × 3.0cm3. Example MEGA-PRESS spectra for gamma-aminobutyric acid (GABA) (C, D) and N-acetyl-aspartyl-glutamate (NAAG) (E, F), as well as the conventional short TE PRESS spectrum (G, H), corresponding to each region are shown.
Conventional MRS.
Spectra were acquired with a point resolved pulse sequence (“PRESS”, TR = 2000ms, TE = 35ms, 2048 points, 2000-Hz spectral width, 64 averages with water suppression and 16 averages without water suppression). Water suppression was achieved using variable pulse powers and optimized relaxation delays (VAPOR) presaturation pulses. Spectra were analyzed using fully automated curve fitting software, “LCModel.”23 The basis set was created from individual metabolites in solution with the same sequence parameters and included aspartate, alanine, GABA, NAAG, Glu, Gln, lactate, myo-inositol (mI), scyllo-inositol, taurine, N-acetylaspartate (NAA), glutathione, glucose, glycerophosphorylcholine, phosphorylcholine, and Cr. Spectra were normalized to the unsuppressed water signal, yielding quantification of the “total NAA” signal (NAA + NAAG), Glx, mI, choline (Cho)-containing compounds, and total Cr in “institutional units.” Metabolite concentration uncertainties that exceeded a Cramer-Rao Lower Bound (CRLB), as provided by LCModel, of 20% were considered poor quality and were excluded from further analyses. Spectra were also considered poor quality and rejected from further analyses if the LCModel FWHM (full-width half maximum) exceeded 0.1 ppm. One spectrum from a young control subject was excluded because the CRLB for Glx exceeded 20. Reported results for Glx were derived from the PRESS sequence due to improved conspicuity of Glu + Gln at shorter TEs,24–26 although several recent studies have reported Glx data from GABA-selective MEGA-PRESS.13,27,28
Spectral-Edited MRS.
The measurement of the relative amounts of NAA and NAAG spectra were acquired with a MEGA-PRESS sequence as described previously11,29 (TR = 2000ms; TE = 140ms; 40ms editing pulses applied at 4.61 and 4.15 ppm for selecting NAAG, and 4.84 and 4.38 ppm for selecting NAA; 256 averages). For quantification, the ratio of signal intensity of NAA to NAAG was calculated (using software developed in-house (MATLAB code and “csx3”)30 and individual NAA and NAAG levels were then calculated by multiplying the relative proportions of NAA and NAAG by the total NAA + NAAG concentration obtained from the LCModel analysis of the conventional PRESS spectrum. For the detection of GABA, spectra were acquired with a MEGA-PRESS sequence (TR = 2.0 s; TE = 68ms; 14ms editing pulse applied at 1.9 ppm and 7.5 ppm, 256 averages). For quantification, the integral of the GABA peak as measured in the program “csx3” was referenced to the integral of the unsuppressed water peak. One GABA spectrum from a young schizophrenia subject was not available for analysis.
Statistical Analysis
Demographic variables were analyzed with chi-square tests for categorical data and ANOVAs for continuous variables. Two (diagnostic group) × 2 (age group) × 2 (region) ANOVAs with repeated measures on region were conducted to investigate the 3 metabolites of interest, GABA, NAAG, and Glx. Subject assignment to age group was determined by median split of age (43 years old). Significant effects were followed up with analysis of simple effects and Tukey’s B post hoc tests when appropriate.
The relationships between NAAG levels and psychiatric symptom severity and GABA levels and attention scores (ie, digit span and coding tests) were computed with partial correlations controlling for age with Bonferroni-corrected significance level set to 0.0125 and 0.025, respectively. Analyses were conducted with the Statistical Package for Social Sciences (SPSS) version 12.0 software package.
Results
Subject demographic and clinical characteristics by diagnostic and age group are provided in table 1. The duration of illness was significantly shorter for the younger (7.7 years) compared with the older (25.5 years) subjects with schizophrenia (t(1,19) = 7.5, P < .001). There were no significant differences in psychiatric symptom severity between the younger and older schizophrenia groups. As commonly observed, subjects with schizophrenia had significantly fewer years of education as revealed by a main effect of group (F(1,37) = 6.2, P = .018) and significantly lower RBANS total scores as revealed by a main effect of group (F(1,37) = 13.3, P = .001) compared with the control group. There were no significant differences in age (F(1,37) = 0.2, P = .7) or gender (chi square = 1.2, P = .3) between the groups.
Table 1.
Subject Characteristics. Mean (SD)
| Schizophrenia Younger (n = 11) | Control Younger (n = 10) | Schizophrenia Older (n = 10) | Control Older (n = 10) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 9 | 5 | 7 | 7 |
| Female | 2 | 5 | 3 | 3 |
| Age (y) | 30.2 (6.6) | 33.4 (6.5) | 51.1 (4.0) | 49.4 (3.9) |
| Education (y)a | 12.8 (2.4) | 14.7 (2.8) | 12.4(1.3) | 13.8 (1.7) |
| RBANS totala | 88.4 (14.0) | 97.4 (11.0) | 83.8 (13.2) | 106.2 (16.3) |
| Duration of illness (y) | 7.7 (4.1) | 25.5 (6.5) | ||
| Psychiatric ratings | ||||
| SANS | 24.3 (12.1) | 19.5 (9.2) | ||
| BPRS (total) | 35.5 (8.5) | 32.2 (6.1) | ||
| BPRS (+ subscale) | 10.2 (4.8) | 10.5 (5.5) | ||
| BPRS (− subscale) | 8.9 (2.8) | 7.4 (2.8) | ||
| Antipsychotic medications | ||||
| Olanzapine | 1 | 5 | ||
| Risperidone | 4 | 1 | ||
| Aripiprazole | 2 | 2 | ||
| Ziprasidone | 1 | 0 | ||
| Seroquel | 3 | 0 | ||
| Fluphenazine | 0 | 2 | ||
Note: SANS, Scale for the Assessment of Negative Symptoms; BPRS, Brief Psychiatric Rating Scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
aMain effect of group (schizophrenia <control; P < .05).
Metabolite Levels
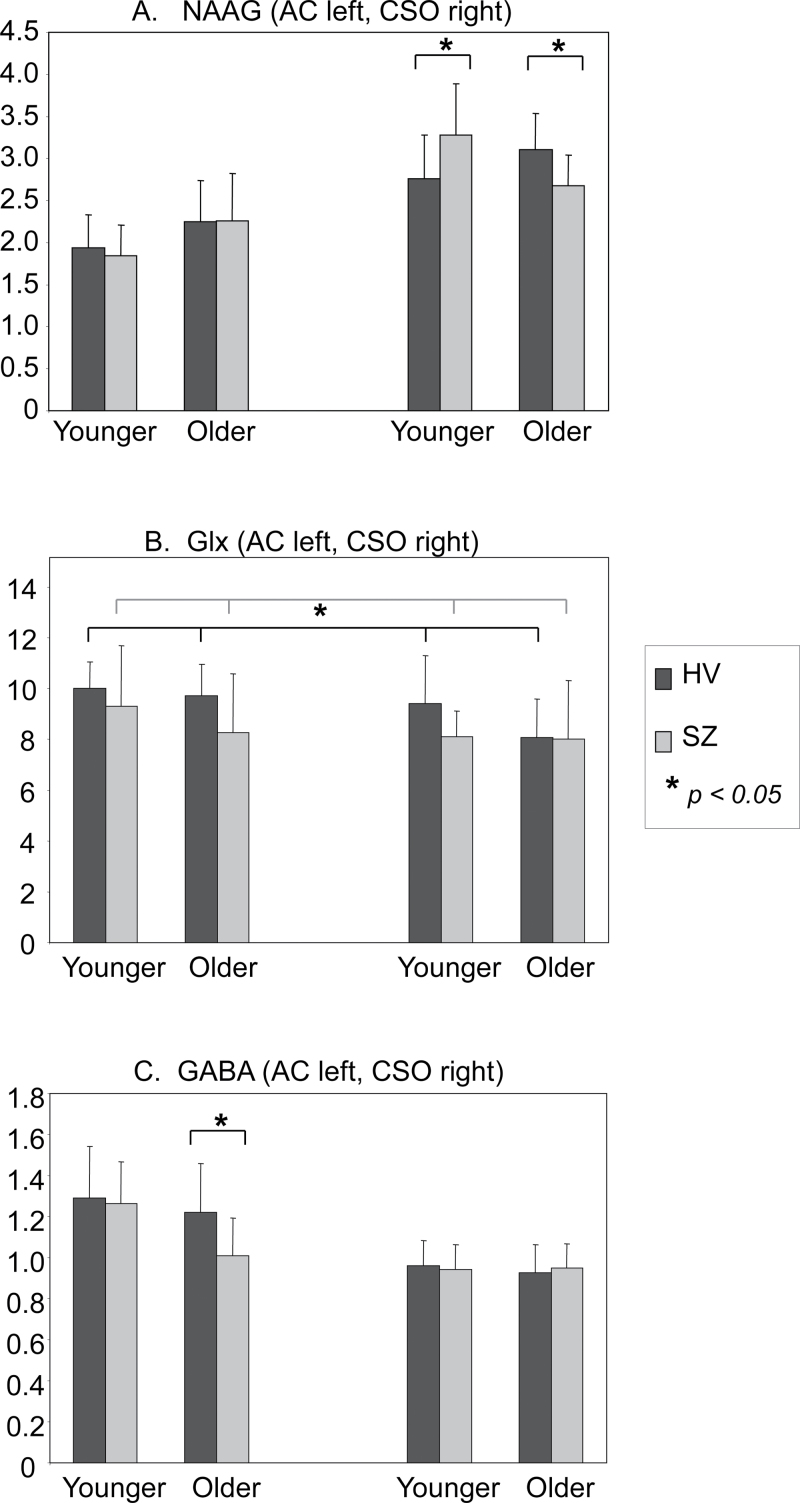
N-Acetyl-Aspartyl-Glutamate. Results revealed a significant 2 (diagnostic group) × 2 (age group) × 2 (region) interaction (F(1,37) = 5.8, P = .02). Analysis of simple interaction effects revealed a significant 2 (diagnostic group) × 2 (age group) interaction for the CSO region only (F(1,37) = 11.4, P = .002). For the schizophrenia group, NAAG levels decreased across age group, whereas for the control group, NAAG levels increased across age group. Post hoc tests revealed that the younger schizophrenia group had higher NAAG levels than the younger control group (P = .012) and the older control group had higher NAAG levels than the older schizophrenia group (P = .04). Means (SD) by diagnostic and age groups are displayed in figure 2A.
Fig. 2.
(A) N-acetyl-aspartyl-glutamate (NAAG) means (SD) (institutional units, IU) for diagnostic group, age group and region (anterior cingulate [AC] left-handed side, centrum semiovale [CSO] right-handed side). There was a 2 (diagnostic group) × 2 (age group) interaction for the CSO region (F(1,37) = 11.4, P = .002). Schizophrenia vs control (P = .012 [younger], P = .04 [older]). (B) Glu + glutamine [Glx] means (SD) (institutional units, IU) for diagnostic group, age group and region (AC left-handed side, CSO right-handed side). There was a significant main effect of diagnostic group (F(1,36) = 4.2, P = .048) with lower Glx in schizophrenia compared with control group averaged across age group and region. (C) gamma-aminobutyric acid (GABA) means (SD) (institutional units, IU) for diagnostic group, age group and region (AC left-handed side, CSO right-handed side). AC GABA levels were lower in the older schizophrenia compared with the older group (t(18) = 2.3, P = .037).
Glutamate + Glutamine.
Results revealed no significant interactions. However, there was a main effect of diagnostic group (F(1,36) = 4.2, P = .048), such that the schizophrenia group had lower levels of Glx compared with the control group averaged across region and age group (average difference = 0.88 + 0.43). Analysis of simple main effects revealed no statistically significant differences between the schizophrenia and control group for AC (P = .082) or CSO (P = .36). There was also a significant main effect of region (F(1,36) = 5.8, P = .02) such that Glx levels were higher in the AC compared with CSO average across diagnostic and age groups. Glx means (SD) by diagnostic group and age group are displayed in figure 2B.
Gamma-Aminobutyric Acid.
Results revealed no significant interactions or main effect of diagnostic group. There was a main effect of age group (F(1,36) = 4.7, P = .037), such that the younger age group had higher levels of GABA compared with the older age group averaged across diagnostic group and region. Means (SD) by diagnostic and age groups are displayed in figure 2C. Visual inspection of means suggested that there may be a significant difference in AC GABA levels between the older schizophrenia and control groups only. Therefore, a t test was conducted. Results revealed a significantly lower AC GABA level in the older schizophrenia group compared with the older control group (t(1,18) = 2.25, P = .037).
Mean (SD) for all metabolite levels and tissue segmentation for each diagnostic and age group per region are presented in online supplementary table S1.
Relationship to Symptoms and Attention
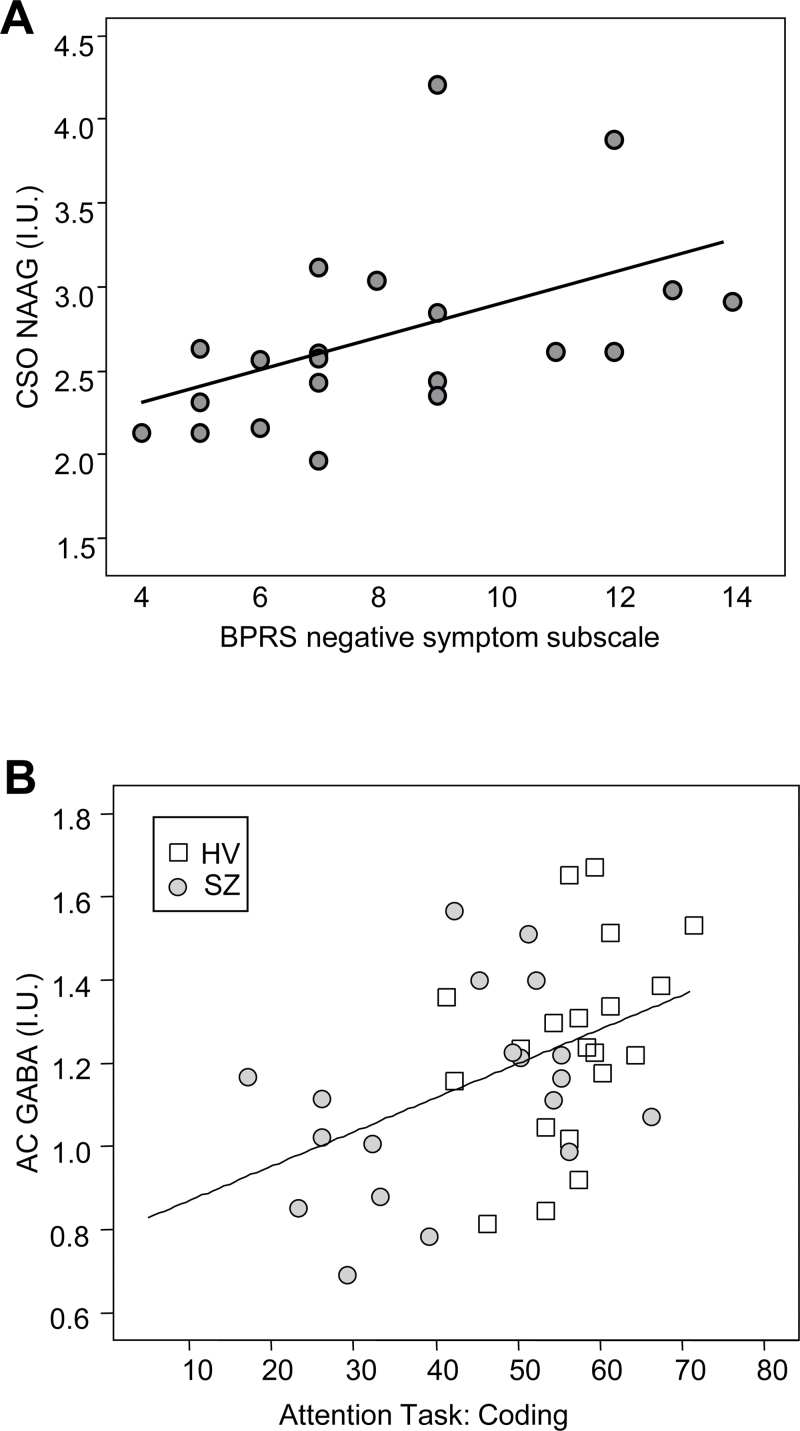
BPRS negative symptom subscale scores were positively correlated to CSO NAAG (r(18) = 0.59, P = .006; see figure 3A). Greater negative symptom severity was associated with a higher NAAG level in the CSO. Negative symptom severity was not significantly correlated to AC NAAG. BPRS positive symptom subscale scores also were not significantly correlated to CSO or AC NAAG.
Fig. 3.
(A) Centrum semiovale (CSO) N-acetyl-aspartyl-glutamate (NAAG) values (institutional units, IU) show a positive correlation (r(18) = 0.59, P = .006) with the Brief Psychiatric Rating Scale (BPRS) negative symptom subscale in patients with schizophrenia covaried for age. (B) Anterior cingulate (AC) gamma-aminobutyric acid (GABA) values (institutional units, IU) show a positive correlation with performance on an attention task in healthy controls and subjects with schizophrenia (r(36) = 0.37, P = .024) covaried for age.
AC GABA was positively correlated to the coding test (r(36) = 0.37, P = .024; see figure 3B) but not significantly correlated to digit span (P > .025). Better coding test performance was correlated to higher AC GABA levels.
Discussion
To our knowledge, this is the first study to report in vivo GABA, Glx, and NAAG measurements from the same brain regions in subjects with schizophrenia. Subjects with schizophrenia had reduced levels of Glx irrespective of age group and region. This result supports previous studies of subjects with schizophrenia who, like the subjects in this study, are clinically stabilized with antipsychotic medications.31,32 Elevated medial prefrontal Gln, Gln:Glu ratio, Glu, or Glx levels have been reported in the first episode with no or minimal exposure to antipsychotic medication,13,33,34 during the prodromal state,35 during psychotic state exacerbations,36 and in those at high risk for developing schizophrenia.37 This may reflect increased Glu release early in the illness (ie, within the first year of psychotic symptom onset), possibly a downstream effect of NMDAR hypofunction.38 Increased Glu may play a role in the mechanisms underlying brain volume loss observed early in the illness39 and reduced neuropil in postmortem tissue.40 In contrast, medial prefrontal glutamatergic metabolites are reduced when patients are stabilized with antipsychotic medication and during the chronic phase of the illness, as with this study.31,32,41 Low dose, chronic phencyclidine (PCP) exposure results in blunted extracellular Glu release42 and reduced Glu tissue levels43 in medial prefrontal cortex. This is consistent with reduced medial prefrontal glutamatergic measurements observed in chronic schizophrenia. A recent meta-analysis on MRS studies of Glu and Gln in schizophrenia provide evidence for elevated medial frontal Glu/Gln values early in the illness and lower in the chronic stage.10
The omnibus statistical test for GABA was not significant. However, a post hoc comparison test revealed reduced AC GABA in older schizophrenia subjects compared with older control subjects with a large effect size (Cohen’s d = 1.02). Due to the a posteriori nature of this statistical test, and the nonsignificant test for age × group interaction, this result should be considered cautiously. Few studies have measured in vivo GABA levels in schizophrenia. One study reported reduced GABA/Cr concentrations in the left basal ganglia but not frontal or occipital-parietal regions in subjects with early-stage schizophrenia compared with healthy subjects,15 and another reported lower GABA/Cr levels in the occipital region.14 No significant GABA differences between schizophrenia and control subjects in the medial prefrontal and left basal ganglia regions16 and increased GABA/Cr in medial frontal and parieto-occipital regions in schizophrenia compared with control subjects12 have also been reported. In the latter study, concomitant medication use in the patient group may have influenced the results because group differences were not significant when patients taking anticonvulsants were removed from the analysis.12 This study is unique in that it controlled for concomitant anticonvulsants and benzodiazepines. A recent study reported elevated medial prefrontal cortex GABA in unmedicated schizophrenics vs healthy controls.13 It is likely that GABA levels are affected by antipsychotic medication. However, additional studies focused on within-subject designs, before and after antipsychotic treatment, are needed to substantiate these findings because the majority of off medication patient GABA values fell within the range of control and medicated subjects. Our results suggest that illness course may affect GABA levels because they were higher in younger subjects with schizophrenia and were lower in older subjects with schizophrenia. GABA levels could be reduced because of lower GABA synthesis, greater GABA metabolism, or reduced GABAergic interneuron density. Postmortem studies reveal reduced expression of the enzyme glutamic acid decarboxylase67 (GAD67) in schizophrenia.44 GAD67 is responsible for the majority of GABA synthesis and GAD67 levels correlate strongly with GABA tissue levels. To our knowledge, there is no published postmortem evidence suggesting that GABA metabolism is greater in schizophrenia compared with controls, but postmortem evidence suggests reduced GABAergic neuron number and altered GABA-A receptor number in schizophrenia subjects compared with controls.5 Hence, our results of reduced GABA levels in older schizophrenia subjects likely reflect an interaction between reduced GABA synthesis, compromised GABA interneuron soma size, and diminished dendritic inhibitory neuron arborization in schizophrenia.
Reduced GABA levels correlated with poorer performance on attention tests in schizophrenia subjects. This complements research showing that parvalbumin-expressing GABAergic interneurons generate high-frequency electrical activity (gamma band >30 Hz) that mediate attention processes; gamma band activity is also altered in schizophrenia with likely elevated gamma activity at baseline/resting states and reduced gamma activity during evoked/cognitive states.2,45,46 Furthermore, GABA levels measured with MRS are directly correlated to gamma oscillations in healthy subjects.47,48 Preliminary data suggest a similar relationship in schizophrenia with reduced GABA correlating with reduced evoked gamma activity.49 Affected GABA levels may contribute to the attention processing impairments observed in schizophrenia.
There were two findings with respect to NAAG, which are as follows: (1) higher CSO NAAG was correlated to greater negative symptom severity when covarying for age and (2) CSO NAAG was higher in younger subjects with schizophrenia and declined with age; the opposite pattern was observed in control subjects. A recent study found trend-level elevations in NAAG in the left AC in chronic, medicated subjects with schizophrenia.17 Lower levels of left frontal lobe NAAG correlated with greater negative symptom severity, which is in contrast to this study. However, it is difficult to compare these studies due to differences in spectral acquisition, analysis methods, and the brain regions assessed. This study is unique in that it employed a spectral-editing technique specifically optimized to detect NAAG, whereas Jessen et al.17 did not. Our results suggest that illness course impacts CSO NAAG levels because higher levels of CSO NAAG were present in the younger schizophrenia subjects and declined in the older schizophrenia subjects. It is possible that increased CSO NAAG levels in the early phases of the illness serve as a compensatory response to excessive Glu release. As an agonist of the mGluR3 site, NAAG may ameliorate some of the toxic effects of Glu. Increased CSO NAAG levels with normal aging as observed in this study may also reflect this protective mechanism because there is increased vulnerability to Glu-related white matter damage with age.50
The current results suggest that pharmacological enhancement of NAAG through NAAG peptidase inhibitors may be therapeutic for schizophrenia, especially in older subjects who have low levels of CSO NAAG. These inhibitors51 and metabotropic Glu receptor (mGluR)2/3 agonists52–54 prevent the behavioral and physiological effects of NMDAR antagonism in humans and animals by inhibiting Glu release through mGluR2/3 stimulation. Recent clinical trial studies indicate LY2140023, a selective agonist for the mGluR2/3, is as effective as olanzapine in treating positive and negative symptoms in schizophrenia.55 Because higher levels of CSO NAAG were associated with greater negative symptom severity, it is not clear if this treatment approach will be therapeutic. Nevertheless, it is possible that this approach may be beneficial for patients later in the illness course when levels are lower than normal, or for a select group of patients with low NAAG levels.
Several study limitations deserve discussion. First, the spectroscopic voxel sizes were large and, particularly for the voxel centered on the AC gyrus, did not contain specific, homogenous brain regions. This limits the interpretation of the results’ anatomical specificity. It is plausible that the findings would be stronger if the voxel size was reduced and constrained to a specific brain region. Future studies, perhaps performed at higher field strengths and with more sensitive receiver coil arrays, may be able to employ smaller voxel sizes prescribed in specific brain regions. Second, macromolecules (MM) contribute to the GABA peak. While methods are available to account for MM contamination, such as by collecting metabolite-nulled macromolecular spectra, in this study, it was not feasible to collect metabolite-nulled spectra from subjects due to time constraints. Recent advances in MRS sequence development likely to reduce MM contamination could be used in future studies. Third, we did not control for cigarette smoking, and it is possible that nicotine could affect MRS measures. Finally, we could not eliminate the potential confound of antipsychotic medication, which is inherent to the majority of schizophrenia studies.
In conclusion, the results of this study provide support for the concept of altered glutamatergic and GABAergic function in schizophrenia and suggest that MRS measurements of GABA, Glx, and NAAG may be used as biomarkers for schizophrenia. Further studies using MRS combined measurements of NAAG, GABA, and Glu are needed to assess drug effects, disease course spanning from “high-risk” to geriatric samples, and psychiatric state in schizophrenia. Moreover, these results also highlight the importance of further investigations into the relationship between these potential biomarkers and cognition in schizophrenia.
Funding
National Institute of Mental Health (1R21MH082322 to Dr Barker) and (K01MH077230 to Dr Rowland).
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We thank the volunteers, especially the patients, for participating in the study. We thank Terri Brawner, Kathleen Kahl, and Ivana Kusevic for conducting the MR scans. We thank Dr Juan Bustillo for valuable comments regarding this project. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514 [DOI] [PubMed] [Google Scholar]
- 2. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324 [DOI] [PubMed] [Google Scholar]
- 3. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holcomb HH, Rowland LM, Tagamets MA. Cognitive dysfunction in schizophrenia: glutamatergic hypoactivity and dopaminergic failure. Drug Discov Today: Dis Mech. 2004;1:435–439 [Google Scholar]
- 5. Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27 [DOI] [PubMed] [Google Scholar]
- 6. Nudmamud S, Reynolds LM, Reynolds GP. N-acetylaspartate and N-acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: a postmortem study. Biol Psychiatry. 2003;53:1138–1141 [DOI] [PubMed] [Google Scholar]
- 7. Reynolds LM, Cochran SM, Morris BJ, Pratt JA, Reynolds GP. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr Res. 2005;73:147–152 [DOI] [PubMed] [Google Scholar]
- 8. Tsai G, Passani LA, Slusher BS, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–836 [DOI] [PubMed] [Google Scholar]
- 9. Stone JM. Imaging the glutamate system in humans: relevance to drug discovery for schizophrenia. Curr Pharm Des. 2009;15:2594–2602 [DOI] [PubMed] [Google Scholar]
- 10. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies [published online ahead of print July 11, 2011]. Schizophr Bull. 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272 [DOI] [PubMed] [Google Scholar]
- 12. Ongür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459 [DOI] [PubMed] [Google Scholar]
- 14. Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goto N, Yoshimura R, Moriya J, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193 [DOI] [PubMed] [Google Scholar]
- 16. Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91 [DOI] [PubMed] [Google Scholar]
- 17. Jessen F, Fingerhut N, Sprinkart AM, et al. N-acety laspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia [published online ahead of print September 12, 2011]. Schizophr Bull. 10.1093/schbul/sbr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rowland LM, Spieker EA, Holcomb HH. A review of diffusion tensor imaging in schizophrenia. Clinl Schizophr Relat Psychos. 2009;3:142–154 [Google Scholar]
- 20. Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739 [DOI] [PubMed] [Google Scholar]
- 21. Bustillo JR, Chen H, Gasparovic C, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry. 2011;69:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montelius M, Ljungbreg A, Carlsson G, Starck E. Foressell-Aronsson. Matlab tool for segmentation and re-creation of MRS volumes of interst in MRI image stacks. Proc Eur Soc Mag Resonan Med Biol 2009 Congr. 2009;320–321 [Google Scholar]
- 23. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264 [DOI] [PubMed] [Google Scholar]
- 24. Zhong K, Ernst T. Localized in vivo human 1H MRS at very short echo times. Magn Reson Med. 2004;52:898–901 [DOI] [PubMed] [Google Scholar]
- 25. Tkác I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456 [DOI] [PubMed] [Google Scholar]
- 26. Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969 [DOI] [PubMed] [Google Scholar]
- 27. O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 2011;33:1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200 [DOI] [PubMed] [Google Scholar]
- 29. Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–982 [DOI] [PubMed] [Google Scholar]
- 30. Soher BJ, Hurd RE, Sailasuta N, Barker PB. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magn Reson Med. 1996;36:335–339 [DOI] [PubMed] [Google Scholar]
- 31. Tayoshi S, Sumitani S, Taniguchi K, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS). Schizophr Res. 2009;108:69–77 [DOI] [PubMed] [Google Scholar]
- 32. Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233 [DOI] [PubMed] [Google Scholar]
- 33. Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946 [DOI] [PubMed] [Google Scholar]
- 35. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ongür D, Jensen JE, Prescot AP, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118 [DOI] [PubMed] [Google Scholar]
- 38. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007 [DOI] [PubMed] [Google Scholar]
- 39. Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73 [DOI] [PubMed] [Google Scholar]
- 41. Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318 [DOI] [PubMed] [Google Scholar]
- 42. Amitai N, Kuczenski R, Behrens MM, Markou A. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology. 2012;62:1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bustillo J, Perrone-Bizzozero N, Paz R, Griffith G, Galloway M. (eds.). Hypometabolic Medial-Frontal Effects of Chronic Intermitent PCP Exposure in Rats Assessed with High Resolution Magic Angle Spin 11.7T Proton Magnetic Resonance Spectroscopy. Waikoloa, Hawaii: American College of Neuropsychopharmacology 44th Annual meeting; 2005 [Google Scholar]
- 44. Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113 [DOI] [PubMed] [Google Scholar]
- 47. Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106:8356–8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kegeles LS, Chen C, Mao C, Stanford A, Shungu DC, Lisanby SH. Frontal cortex GABA, oscillations, and working memory in schizophrenia. Schizophr Bull. 2011;37–125 [Google Scholar]
- 50. Baltan S. Ischemic injury to white matter: an age-dependent process. Neuroscientist. 2009;15:126–133 [DOI] [PubMed] [Google Scholar]
- 51. Olszewski RT, Bukhari N, Zhou J, et al. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–885 [DOI] [PubMed] [Google Scholar]
- 52. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179:303–309 [DOI] [PubMed] [Google Scholar]
- 53. Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus D-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170 [PubMed] [Google Scholar]
- 54. Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005;93:1989–2001 [DOI] [PubMed] [Google Scholar]
- 55. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.