Abstract
Objective: To determine if care concordant with 2009 Schizophrenia Patient Outcomes Research Team (PORT) pharmacological recommendations for schizophrenia is associated with decreased mortality. Methods: We conducted a retrospective cohort study of adult Maryland Medicaid beneficiaries with schizophrenia and any antipsychotic use from 1994 to 2004 (N = 2132). We used Medicaid pharmacy data to measure annual and average antipsychotic continuity, to calculate chlorpromazine (CPZ) dosing equivalents, and to examine anti-Parkinson medication use. Cox proportional hazards regression models were used to examine the relationship between antipsychotic continuity, antipsychotic dosing, and anti-Parkinson medication use and mortality. Results: Annual antipsychotic continuity was associated with decreased mortality. Among patients with annual continuity greater than or equal to 90%, the hazard ratio [HR] for mortality was 0.75 (95% confidence interval [CI] 0.57–0.99) compared with patients with annual medication possession ratios (MPRs) of less than 10%. The HRs for mortality associated with continuous annual and average antipsychotic continuity were 0.75 (95% CI 0.58–0.98) and 0.84 (95% CI 0.58–1.21), respectively. Among users of first-generation antipsychotics, doses greater than or equal to 1500 CPZ dosing equivalents were associated with increased risk of mortality (HR 1.88, 95% CI 1.10–3.21), and use of anti-Parkinson medication was associated with decreased risk of mortality (HR 0.72, 95% CI 0.55–0.95). Mental health visits were also associated with decreased mortality (HR 0.96, 95% CI 0.93–0.98). Conclusions: Adherence to PORT pharmacological guidelines is associated with reduced mortality among patients with schizophrenia. Adoption of outcomes monitoring systems and innovative service delivery programs to improve adherence to the PORT guidelines should be considered.
Key words: schizophrenia, antipsychotic continuity, mortality
Introduction
Antipsychotic medication is effective for the treatment of acute psychotic symptoms and maintenance of symptom relief among persons with schizophrenia. Randomized clinical trials have consistently found that both first- and second-generation antipsychotic medications are more effective than placebo at reducing acute positive symptoms among persons with schizophrenia, and antipsychotic medications have been shown to reduce relapse rates in those with schizophrenia.1–3 Recent studies that compare the efficacy of first-generation antipsychotics with the efficacy of second-generation antipsychotics find few differences in the ability of the 2 types of medications to reduce positive symptoms.4,5 In addition, multiple clinical trials have investigated optimal dosing of antipsychotics.6–8 Studies where patients are randomly assigned to receive different doses of first-generation antipsychotics consistently find suboptimal responses below 300 chlorpromazine (CPZ) equivalents and show little evidence of benefit and increased risk for side effects for doses greater than 1000 CPZ equivalents.8
The schizophrenia Patient Outcomes Research Team (PORT) 2009 pharmacological recommendations reflect the results of these and other clinical trials.9 The PORT program was initiated by the Agency for Healthcare Research and Quality (AHRQ) in 1992 to promote the adoption of evidence-based practices supported by strong scientific evidence for various medical conditions.9 The schizophrenia PORT recommendations were originally published in 1998,6 and the most recent updated recommendations were presented in December 2009.9 The 2009 schizophrenia PORT guidelines recommend that all persons with treatment responsive, multiepisode schizophrenia experiencing an acute exacerbation of their illness be prescribed antipsychotic medications as the first line of treatment.9 The 2009 PORT also recommends that persons with schizophrenia continually use antipsychotics in order to maintain symptom relief and reduce the risk of relapse.9 For first-generation antipsychotics, the 2009 PORT recommends a daily dose between 300–1000 CPZ equivalents for acute exacerbations and 300–600 CPZ equivalents for maintenance doses.9 In addition, the 2009 PORT recommends prescription of anti-Parkinson medications for patients taking first-generation antipsychotics on a case-by-case basis.9
Despite its efficacy in controlling positive symptoms of schizophrenia, prior research has hypothesized that antipsychotic therapy contributes to premature mortality among patients with schizophrenia by increasing risk for cardiovascular disease.10 Antipsychotic medications have been shown to cause weight gain and are associated with risk factors for cardiovascular disease such as metabolic syndrome and diabetes mellitus.11,12 Cardiovascular mortality rates among persons with schizophrenia are approximately double the rates in the overall population.13,14 Studies investigating the association between antipsychotic use and mortality have yielded contradictory results.10 A 2004 study found that risk of mortality was 4 times higher among privately insured patients with schizophrenia using antipsychotics compared with their counterparts with no antipsychotic use.15 Similarly, a 2006 study of patients with serious mental illness, including schizophrenia, found that the risk of mortality due to coronary heart disease was 4 times higher among persons using antipsychotics compared with persons not using antipsychotics.16 In contrast, a 2008 study comparing current vs past users of antipsychotics found a decrease in all-cause mortality among current users.17 Studies investigating the association between antipsychotic dosage and mortality also show similarly mixed results. At least one study showed a positive relationship between antipsychotic dosage and mortality among persons with schizophrenia,18 although a 2005 retrospective cohort study found no association between clozapine dosage and mortality.19
Importantly, the majority of studies examining the association between antipsychotic use and mortality among persons with schizophrenia do not examine continuity of antipsychotic use or dosage of medications over time. One exception is a 2009 study by Tiihonen et al.20 in which the authors examined the association between antipsychotic drug use and all-cause mortality among persons with schizophrenia using nationwide registry data from Finland. Tiihonen et al.20 found that increased duration of use of antipsychotic drugs was associated with decreased mortality and that long-term treatment with antipsychotic drugs was associated with lower mortality compared with no antipsychotic use.20
To our knowledge, no study to date has examined mortality associated with high vs low continuity over time among antipsychotic users with schizophrenia or evaluated the association between adherence to schizophrenia PORT pharmacological guidelines and mortality. Our study will address these gaps by examining the effects of antipsychotic continuity, antipsychotic dosing, and use of anti-Parkinson medications among a cohort of Maryland Medicaid beneficiaries with schizophrenia who used any antipsychotic medications from 1994 to 2004. As a secondary objective, our study will examine whether continuity of mental health visits is associated with reduced mortality independently of antipsychotic continuity.
Methods
Study Design and Population
We performed a retrospective cohort study of adult Maryland Medicaid beneficiaries living in the Baltimore metropolitan area or the rural eastern shore region of the state. Medicaid beneficiaries were included in the study cohort if they (1) were 21–62 years of age between July 1, 1992 and July 1, 1993; (2) were continuously enrolled in Medicaid between July 1, 1992 and June 30, 1994; (3) were classified as having a diagnosis of schizophrenia (ICD-9 code 295.0–295.9) at baseline; and (4) used any antipsychotic medication during the study period. The sample was restricted to black and white beneficiaries due to the small number of persons of other races. The study was approved by the Johns Hopkins Medical Institutions and Maryland Department of Health and Mental Hygiene Institutional Review Boards.
Data Sources
Baseline information on age, sex, race, area of residence, and schizophrenia diagnoses were obtained from Maryland Medicaid administrative claims files for 1992–1994. Maryland Medicaid hospitalization data for 1994–2004 were used to identify medical comorbidities. Data on antipsychotic and anti-Parkinson medication use were obtained from Medicaid pharmacy claims data from 1994 to 2004, and mortality data, including cause of death, were obtained by linking Medicaid claims data to data from the National Death Index (NDI) using the NDI’s probabilistic scoring technique.21
Measures
Antipsychotic Continuity
Medication Possession Ratio.
We used medication possession ratios (MPRs) or the number of days each study participant used antipsychotic medication divided by the total number of days each participant was eligible for antipsychotic medication (calculation of eligibility is described below), as a measure of antipsychotic continuity. We calculated 2 types of MPRs: annual and average. Annual MPRs were measured in order to assess the association between antipsychotic continuity in the year prior to death and risk of mortality. Average MPRs were measured in order to assess the association between average antipsychotic continuity over the 10-year study period and risk of mortality.
The annual MPR was calculated as the total number of days each cohort member was on any antipsychotic medication in a given year divided by the total number of days each participant was eligible for antipsychotic medication in that year. The total days each cohort member was on any antipsychotic medication in a given year was calculated by summing the days of supply of antipsychotics across consecutive prescription refills in that year. Days on multiple antipsychotics were only counted once, so that the maximum number of days on antipsychotic medication per year was 365. Given that pharmacy claims data were not available from inpatient stays, the total number of days eligible for antipsychotic medications per year was calculated as 365 days − inpatient hospitalization days. In order to establish temporality between antipsychotic continuity and death, the MPRs were lagged by 1 year. The annual MPR was classified as an ordinal variable with the following categories: <10%, 10%–49%, 50%–89%, and >90%. Annual MPR was included as a time-varying exposure (per year) in the statistical models.
The average MPR across all years of study participation (1999–2004) was calculated as the total days each cohort member was on any antipsychotic medication in the study period divided by the total number of days each participant was eligible for antipsychotic medication in the study period. Days on antipsychotic medication and days of eligibility were defined as described above for the annual MPR. The average MPR was classified as an ordinal variable with the same categories as the annual MPR. Average MPR was included as a time-fixed exposure in the statistical models.
Antipsychotic Dosage.
For the subset of patients who ever used first-generation antipsychotics (N = 2027), we examined dose appropriateness. An average dose of first-generation antipsychotics using CPZ equivalents was calculated per year for each cohort member ever using first-generation antipsychotics. CPZ equivalents were calculated using the therapeutically equivalent oral doses defined by the American Psychiatric Association.22 To define CPZ equivalent dose per year, we averaged the CPZ equivalent dose of all first-generation antipsychotics taken during a given year. Dosing was measured as an ordinal variable using the following categories of CPZ equivalents: 0–299, 300–599, 600–1500, and >1500. First-generation antipsychotic dosage was included as a time-varying exposure (per year) in the statistical models. We did not measure dose appropriateness for second-generation antipsychotics, for 2 reasons. First, CPZ dosing equivalents are only relevant to first-generation antipsychotics.9 Second, the 2009 PORT guidelines note that upper effective dose limits for the majority of second-generation antipsychotics have not been established.9
Anti-Parkinson Medication Use.
For the subset of patients who ever used first-generation antipsychotics (N = 2027), information on whether or not each patient used any anti-Parkinson medication in a given year was measured. Anti-Parkinson medication use was included as a time-varying exposure (per year) in the statistical models.
Covariates.
We applied the AHRQ’s Clinical Classification System to primary ICD-9 diagnoses and their comorbidity software (version 3.3) to secondary ICD-9 diagnoses to identify medical comorbidities. The Clinical Classification System clusters patient diagnoses into 289 clinically meaningful categories. The comorbidity software, developed based on Elixhauser’s23 work on comorbidity classification, groups secondary diagnoses into 29 categories of comorbidities. We combined conditions identified using these 2 tools and measured presence of cardiovascular disease, renal failure, cancer, diabetes, HIV infection, alcohol abuse, and drug abuse. These comorbidities were included as time-varying covariates (per year) in the statistical models. Number of outpatient mental health visits per year and age were also measured and included in statistical models as time-varying covariates. Measures of sex, race (white/black), and area of residence (urban/suburban/rural) were obtained from the Medicaid administrative claims data and included in the statistical models as time-fixed covariates.
Statistical Analyses
We used Cox proportional hazards models to calculate the risk of mortality associated with antipsychotic continuity, CPZ dosing, and anti-Parkinson medication use. We used 3 models with different exposures of interest. First, we examined risk of death associated with annual MPRs as time-varying exposures among all study participants. Second, we examined risk of death associated with average MPR as a time-fixed exposure among all study participants. Third, a prespecified subgroup analysis was performed among patients who ever used first-generation antipsychotics, in which we examined risk of death associated with CPZ dosing and anti-Parkinson medication use as time-varying exposures, controlling for annual MPR as a time-varying covariate. Mental health visits were included as a time-varying covariate in all models in order to assess the association of mental health visits on mortality independent of antipsychotic continuity, dosing, and anti-Parkinson medication use. All models controlled for the time-fixed covariates of sex, area of residence, and race and the time-varying covariates of age, cardiovascular disease, renal failure, cancer, diabetes, HIV infection, alcohol abuse, and drug abuse.
Results
The study cohort was comprised of 2132 adult Maryland Medicaid beneficiaries with schizophrenia who received any antipsychotic medication between 1994 and 2004. The average age of the study cohort was 42 years. Forty-seven percent of cohort members were female, 57% were African American, and 87% lived in an urban area (table 1). The median number of mental health outpatient visits during the study period was 16. A total of 337 (16%) cohort members died during follow-up. Of the 2132 cohort members, 2027 were prescribed first-generation antipsychotics during the study period. The demographic profile of cohort members prescribed first-generation antipsychotics was similar to the profile in the overall group (table 1). Among study participants prescribed first-generation antipsychotics, 83.9% were prescribed anti-Parkinson medication during the study period.
Table 1.
Characteristics of Maryland Medicaid Cohort With Schizophrenia Using Any Antipsychotic and First-Generation Antipsychotics
| All Using an Antipsychotic Medication (n = 2132) | Participants Using FGA (n = 2027) | |
|---|---|---|
| Mean age in years (SD) | 41.6 (10.1) | 41.6 (10.1) |
| Sex—female (%) | 1035 (46.5) | 994 (49) |
| Race—white (%) | 924 (43.3) | 857 (4.3) |
| Area of residence (%) | ||
| Urban | 1850 (86.8) | 1756 (86.6) |
| Suburban | 170 (8) | 161 (7.9) |
| Rural | 111 (5.2) | 109 (5.4) |
| Comorbidity at baseline (%) | ||
| Cardiovascular disease | 38 (1.8) | 35 (1.7) |
| Renal failure | 3 (0.1) | 2 (0.1) |
| Cancer | 10 (0.5) | 10 (0.5) |
| Diabetes | 27 (1.3) | 26 (1.3) |
| HIV | 6 (0.3) | 6 (0.3) |
| Alcohol abuse | 101 (4.7) | 100 (4.9) |
| Drug abuse | 104 (4.9) | 100 (4.9) |
| Mean number mental health visits per year | 4 (10.9) | 4 (11.0) |
| Average CPZ of FGA during study period (range) | — | 330 (140–715) |
| Number using FGA ever on anti-Parkinson medication (%) | — | 1701 (83.9) |
| Mortality (%) | 337 (15.8) | 329 (15.4) |
Notes: CPZ, chlorpromazine; FGA, First-Generation Antipsychotics; HIV, Human Immunodeficiency Virus.
Of the total deaths experienced by the study cohort, the 5 most common causes of death were cardiovascular disease (28%), neoplasms (17%), infectious and parasitic diseases (10%), respiratory disease (9%), and undetermined harm (which may include suicide; 8%). Remaining deaths were accounted for by cerebrovascular disease (5%), diabetes (4%), unintentional injuries (4%), gastrointestinal disease (2%), kidney disease (2%), intentional harm against self (1%), intentional harm by others (1%), neural disease (1%), pregnancy or perinatal condition (1%), and other disease (7%). Among study participants with a 90% or higher average MPR across the study period (n = 610), 0.5% died due to suicide or undetermined harm, compared with 3% of those with a 10% or lower MPR during the same period (n = 197). About 4.1% of persons with an average MPR of less than 10% died due to cardiovascular disease, compared with 4.2%, 3.8%, and 4.3% with average MPRs of 10%–50%, 50%–90%, and greater than 90%, respectively. Among study participants who used first-generation antipsychotics in the year that they died (n = 151), 53% of those taking 1500 or more CPZ equivalents (n = 21) died from cardiovascular disease, compared with 31%, 33%, and 27% of those taking 0–299 (n = 33), 200–599 (n = 64), or 600–1499 (n = 33) CPZ equivalents, respectively.
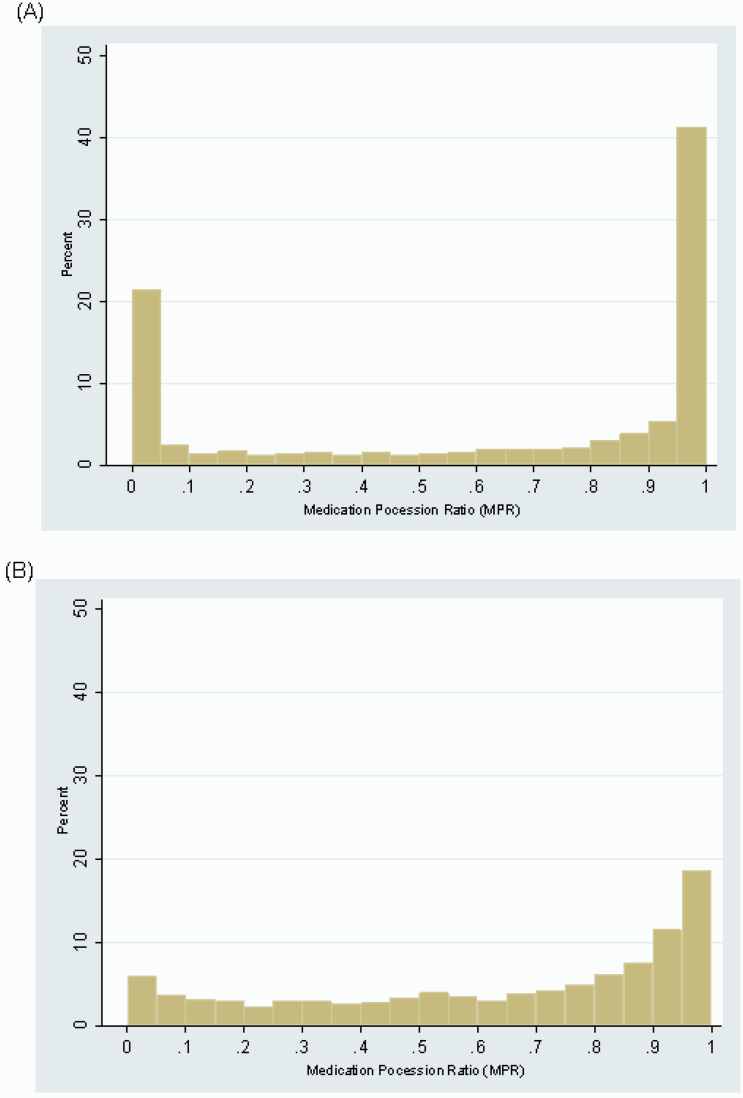
Figure 1 shows the distribution of annual MPRs per person-year (figure 1A) and average MPRs across the study period per person (figure 1B). Forty-seven percent of annual antipsychotic MPRs were greater than 90% and 25% were less than 10% (figure 1A), suggesting that in a given year, the majority of study participants had either very high or very low adherence. In contrast, 29% and 10% of average antipsychotic MPRs across the 10-year study period were greater than 90% and less than 10%, respectively, suggesting that participants’ adherence fluctuated over time rather than remaining very high or very low for the entire time studied (figure 1B).
Fig. 1.
(A) Annual and (B) average antipsychotic medication possession ratio per patient with schizophrenia, 1994–2004. *Lagged by 1 year.
High antipsychotic continuity was associated with decreased risk of mortality (table 2), controlling for medical comorbidities and mental health visits. Annual antipsychotic continuity greater than 90% was associated with significantly reduced risk of mortality (HR 0.75, 95% CI 0.57–0.99). The HR comparing high average antipsychotic continuity across the entire study period compared with low average antipsychotic continuity was less than 1 but not statistically significant at the P < .05 level (HR 0.81, 95% CI 0.55–1.19). We estimated the same models presented in table 2 with annual (lagged by 1 year) and average antipsychotic continuity defined as continuous rather than categorical exposures. High annual antipsychotic continuity was associated with reduced mortality at the P < .05 level (HR 0.75, 95% CI 0.58–0.98), and average antipsychotic continuity across the 10-year study period appeared to have a protective effect on mortality but was not significant at the P < .05 level (HR 0.84, 95% CI 0.58–1.21).
Table 2.
Association Between Annual* and Average Antipsychotic Continuity and Risk of Mortality Among Maryland Medicaid Beneficiaries With Schizophrenia (N = 2132), 1994–2004
| Model 1: Annual* MPR HR (95% CI) |
Model 2: Average MPR HR (95% CI) |
|
|---|---|---|
| MPR | ||
| <10% | Reference | Reference |
| 10%–49% | 0.87 (0.61–1.23) | 0.78 (0.54–1.13) |
| 50%–89% | 0.88 (0.64–1.20) | 0.71 (0.50–1.01) |
| >90% | 0.75 (0.57–0.99) | 0.81 (0.55–1.19) |
| Age | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) |
| Sex | ||
| Female | Reference | Reference |
| Male | 0.89 (0.81–1.12) | 0.89 (0.70–1.12) |
| Area of residence | ||
| Urban | Reference | Reference |
| Suburban | 1.05 (0.66–1.67) | 1.04 (0.65–1.67) |
| Rural | 0.89 (0.51–1.57) | 0.88 (0.50–1.55) |
| Race | ||
| White | Reference | Reference |
| Black | 0.94 (0.74–1.20) | 0.97 (0.76–1.23) |
| Comorbidity | ||
| Cardiovascular disease | 2.25 (1.72–2.93) | 2.26 (1.73–2.95) |
| Renal failure | 2.07 (1.35–3.18) | 2.18 (1.43–3.30) |
| Cancer | 6.01 (4.37–8.27) | 6.02 (4.37–8.31) |
| Diabetes | 1.19 (0.86–1.65) | 1.22 (0.89–1.68) |
| Human immunodeficiency virus (HIV) | 5.33 (3.25–8.75) | 5.33 (3.26–8.71) |
| Alcohol abuse | 1.10 (0.80–1.50) | 1.11 (0.81–1.51) |
| Drug abuse | 1.51 (1.04–2.18) | 1.50 (1.04–2.17) |
| Number of mental health visits | 0.95 (0.93–0.98) | 0.95 (0.93–0.98) |
Notes: *Lagged by 1 year HR, Hazard Ratio; MPR, Medication Possession Ratio.
Among study participants who used first-generation antipsychotics (table 3), heightened risk of death was significantly associated with CPZ dosing equivalents of greater than or equal to 1500mg (HR 1.88, 95% CI 1.10–3.21). In our study sample, polypharmacy increased over time. In 1994, 36% of the study sample (n = 2132) took more than 1 antipsychotic (17%, <1%, and 19% of participants took multiple first-generation, second-generation, or mix of first- and second-generation antipsychotics, respectively), whereas in 2004, 55% of the living study sample (n = 1835) took more than 1 antipsychotic medication (3% took multiple first-generation antipsychotics, 10% took multiple second-generation antipsychotics, and 42% took a mix of first- and second-generation antipsychotics). Use of anti-Parkinson medication was associated with significantly reduced mortality among study participants using first-generation antipsychotics, with a HR of 0.72 (95% CI 0.55–0.95). Mental health visits were protective even when antipsychotic continuity, dosing, and anti-Parkinson use were accounted for (HR 0.96, 95% CI 0.93–0.98).
Table 3.
Association Between Annual* Antipsychotic Continuity, Chlorpromazine (CPZ) Equivalent Dosing, and Anti-Parkinson Medication Use and Risk of Mortality Among Maryland Medicaid Beneficiaries With Schizophrenia (N = 2027), 1994–2004
| Hazard Ratio, HR (95% CI) |
|
|---|---|
| Medication possession ratio (MPR), per year* | |
| <10% | Reference |
| 10%–49% | 0.90 (0.62–1.30) |
| 50%–89% | 0.93 (0.66–1.31) |
| >90% | 0.79 (0.59–1.07) |
| CPZ dosing | |
| 0–299 | Reference |
| 300–599 | 1.17 (0.76–1.81) |
| 600–1500 | 1.07 (0.67–1.72) |
| >1500 | 1.88 (1.10–3.21) |
| Anti-Parkinson medication use | 0.72 (0.55–0.95) |
| Age | 1.04 (1.03–1.05) |
| Sex | |
| Female | Reference |
| Male | 0.89 (0.70–1.12) |
| Area of residence | |
| Urban | Reference |
| Suburban | 1.03 (0.64–1.66) |
| Rural | 0.85 (0.48–1.51) |
| Race | |
| White | Reference |
| Black | 0.94 (0.74–1.20) |
| Comorbidity | |
| Cardiovascular disease | 2.25 (1.72–2.95) |
| Renal failure | 2.05 (1.31–3.20) |
| Cancer | 5.69 (4.12–7.87) |
| Diabetes | 1.17 (0.85–1.62) |
| Human immunodeficiency virus (HIV) | 5.27 (3.18–8.75) |
| Alcohol abuse | 1.07 (0.78–1.47) |
| Drug abuse | 1.55 (1.07–2.23) |
| Number of mental health visits | 0.96 (0.93–0.98) |
Note: *Lagged by 1 y.
Discussion
The results of our study show that among this Maryland Medicaid population with schizophrenia, antipsychotic continuity is associated with reduced risk of mortality. An MPR of 90% or higher in the year prior to death was associated with a 25% lower risk of mortality compared with cohort members with an MPR of 10% or lower, controlling for medical comorbidities and mental health visits. High average MPRs across the study period of 1994–2004 were not associated with a statistically significant decrease in risk of mortality, although coefficients were less than 1. Among the 2027 cohort members who ever used first-generation antipsychotics, high CPZ equivalents were associated with increased risk of mortality, and use of anti-Parkinson medications was associated with decreased risk of mortality. These findings suggest that adherence to the 2009 PORT recommendations is associated with reduced risk of mortality. To our knowledge, this retrospective cohort study is one of the first studies to demonstrate that adherence to PORT pharmacological recommendations is associated with reduced mortality among persons with schizophrenia. In addition, number of mental health visits was associated with decreased risk of mortality even when antipsychotic continuity was controlled.
Our study examined the association between antipsychotic continuity and mortality among a cohort of antipsychotic users with schizophrenia. Previous studies have compared mortality among antipsychotic users with schizophrenia to mortality among non-antipsychotic users with schizophrenia, with mixed results. Unlike the results of prior studies suggesting that long-term antipsychotic use contributes to the excess mortality among persons with schizophrenia by increasing the risk for cardiovascular disease,16 our study showed that high average antipsychotic continuity over a 10-year period was not associated with increased risk of mortality. In addition, high antipsychotic continuity in the year prior to death was associated with a decreased risk of mortality, even after adjusting for medical comorbidities and mental health visits.
Studies examining antipsychotic adherence among persons with schizophrenia have shown that compared with persons with high adherence, persons with low adherence have more positive symptoms, less insight, higher rates of comorbid substance abuse, and worse therapeutic alliance with a psychiatrist or counselor.24,25 Although our study controls for substance abuse, the increased risk of mortality associated with low antipsychotic continuity may be related to these other risk factors.
High antipsychotic continuity may contribute to reduced risk of mortality in several ways. Persons with schizophrenia experience higher burden of chronic diseases, such as hypertension and diabetes, than persons without schizophrenia.11,14 Controlling psychiatric symptoms with continuous antipsychotic treatment, therefore, may lead to improvements in organized thinking that facilitate management of comorbid conditions.26 High antipsychotic continuity may also contribute to reduced risk of mortality by improving social support among persons with schizophrenia. Persons with high continuity and controlled symptoms may be more likely to engage in the positive social relationships with caregivers, family members, and friends. Prior work has shown these positive relationships to be associated with improved health outcomes.27 The direction of this relationship is difficult to discern, however, as persons with good prognoses and high social competence may be more likely to both engage in social support and adhere to antipsychotic medications.27
Our results support the results of Tiihonen et al.20, who found that both current and long-term (7–11 years) use of antipsychotics was associated with reduced risk of death compared with no antipsychotic use. Consistent with Tiihonen’s result, we found that the proximal use of antipsychotics in the year prior to death reduced risk of mortality among persons with high (90% or higher) vs low (less than 10%) annual antipsychotic continuity.20 In contrast to Tiihonen’s findings, in our study, the HR comparing mortality among persons with high vs low average antipsychotic continuity across the 10-year study period was less than 1 but not statistically significant, possibly because we compared mortality among persons with high and low continuity rather than high continuity vs no use.
Given evidence suggesting that antipsychotic adherence may increase risk of cardiovascular mortality due to side effects related to weight gain10 but decrease risk of suicide by effectively controlling the positive symptoms of schizophrenia,28 Tiihonen et al.20 examined risk of death from ischemic heart disease and suicide, respectively. The authors found that none of the antipsychotics studied were associated with risk of heart disease and that clozapine was associated with reduced risk of suicide.20 Although we described causes of death among our study cohort, due to small sample sizes within each cause-of-death category, we were unable to test the association between either overall antipsychotic adherence or adherence to specific antipsychotic drugs and mortality from cardiovascular disease or suicide. The associations between specific drugs and causes of death warrant further study, particularly as De Hert et al.29 raise important methodological concerns regarding Tiihonen’s findings on clozapine and suicide.
In our study population, antipsychotic continuity fluctuated over the 10-year study period. Forty-seven percent of annual MPRs were 90% or higher and 25% were less than 10%. Over the 10-year study period, however, the proportion of cohort members with very high or very low continuity decreased; 29% of cohort members had an average MPR greater than or equal to 90% and 10% had average continuity lower than 10% across the entire 1994–2004 period. In addition to showing that antipsychotic continuity varies over time among persons with schizophrenia, our results suggest that long-term adherence to the PORT guideline recommending continuous antipsychotic use is low among the Maryland Medicaid population with schizophrenia. A 2008 study using Maryland Medicaid data also found low antipsychotic continuity among this population; the average duration of antipsychotic use among persons with schizophrenia was 6 months per year and 4.5 years during the 7-year study period (1993–2000).30 At least one prior study has demonstrated that adherence to the PORT guidelines is low in the Medicaid setting. A 2009 study examined receipt of antipsychotic medication, antipsychotic continuity, dosing consistent with PORT recommendations, and mental health visit continuity among Florida Medicaid beneficiaries with schizophrenia.31 The authors found that less than 20% of study participants in the acute phase of schizophrenia and less than 10% in the maintenance phase received care meeting all 4 guidelines.31
To our knowledge, our study is one of the first to show that excessive CPZ equivalent doses of first-generation antipsychotics are associated with increased risk of mortality. The 2009 PORT guidelines recommend a daily dose of first-generation antipsychotics between 300–1000 CPZs for acute exacerbations and 300–600 CPZs for maintenance doses.9 This recommendation is based on the results of clinical trials demonstrating that these doses are both safe and efficacious.9 Consistent with our results, a study by Bralet et al.32 found increased risk of mortality among 150 persons with schizophrenia who were on high CPZ equivalent doses of first-generation antipsychotics, although the authors did not specifically examine the effect of dosing on mortality.32 A 2009 review by Liu and Haan33 concluded that there was no evidence of any benefit of prescribing a CPZ equivalent dose higher than the limits recommended by the PORT guidelines and found that persons receiving high CPZ doses experienced more adverse side effects, including dystonia and other extrapyramidal side effects compared with persons taking low doses. High CPZ equivalent dosing has also been associated with electrocardiographic QTc interval prolongation, and thus could lead to increased cardiac arrhythmias.34,35
Importantly, more than half of study participants (53%) who took 1500 or more CPZ equivalents of a first-generation antipsychotic in the year of death died due to cardiovascular disease, possibly due to increased risk of sudden cardiac death; 2 studies of Tennessee Medicaid beneficiaries found increased risk of sudden cardiac death associated with high vs low dose antipsychotic use.36,37
The third PORT recommendation we studied was the concurrent use of anti-Parkinson medication among persons using first-generation antipsychotics. Similar to the results of a 10-year prospective study that found reduced survival rates among persons with schizophrenia using antipsychotic medications alone compared with those using antipsychotic medications and concurrent anti-Parkinson medications,38 our study found decreased risk of mortality associated with concurrent use of first-generation antipsychotics and anti-Parkinson medications. Anti-Parkinson medications may be a proxy for adherence; persons who take these medications may be more likely to adhere to antipsychotics because they experience fewer side effects. Anti-Parkinson medications’ ability to decrease extrapyramidal symptoms could reduce risk of mortality by protecting against falls or other injuries or by allowing increased physical activity, although this has not been studied. Although the majority (84%) of cohort members taking any first-generation antipsychotic also took an anti-Parkinson medication during the study period, the PORT guidelines call for prescription of these medications on a case-by-case basis.9 Cohort members not taking anti-Parkinson medication may comprise a group at higher risk for mortality due to disease severity or other unknown reason.
Although we did not measure whether cohort members had outpatient mental visits with the same providers over time, the finding that both antipsychotic continuity and mental health visits are associated with reduced risk of mortality in our models suggests that continuity of care for persons with schizophrenia is multidimensional. This finding supports prior work showing that both visit continuity39 and antipsychotic continuity30,39,40 are associated with positive outcomes among persons with schizophrenia, including increased outpatient visits,39 decreased hospital admissions,30,39,40 and lower costs. To our knowledge, this is one of the first studies to show that mental health visits are associated with decreased mortality among persons with schizophrenia independent of antipsychotic continuity. Although high utilizers of mental health care may be sicker than low utilizers, outpatient mental health care was associated with reduced mortality among Maryland Medicaid beneficiaries with schizophrenia.
Our study had several limitations related to use of Medicaid administrative claims data. First, use of claims data did not allow us to identify severity of schizophrenia, which could affect patients’ ability to adhere to an antipsychotic regimen as well as increase risk of mortality, making it a potentially important unmeasured confounder. Critically, claims data allow for identification of prescriptions filled but not actual medications taken; however, MPRs using pharmacy claims data have been shown by prior studies to predict admission rates in patients with schizophrenia41 as well as outcomes in patients with chronic illnesses.42 Our data set also did not allow us to identify cohort members whose antipsychotic use was discontinued due to symptom reduction and may have resulted in imperfect measurement precision leading to residual confounding. However, only a minority of patients with schizophrenia have a sustained remission without medication and prior studies have used Medicaid claims data to evaluate the association between quality of care measures and mortality.43 Furthermore, use of claims data affects generalizability of findings; our study sample included Maryland Medicaid beneficiaries, who may not be representative of all Maryland residents with schizophrenia. A schizophrenia diagnosis qualifies for Medicaid eligibility in Maryland, although we were unable to determine the actual proportion of Maryland residents with Medicaid covered by schizophrenia. A 2010 study found that nationwide, nearly 70% of persons with schizophrenia are covered by Medicaid,44 suggesting that our results are generalizable to the majority of persons with schizophrenia in Maryland. Finally, in spite of the high proportion of study participants using second-generation antipsychotics, due to the fact that guidelines for effective upper dose limits have not been established for the majority of second-generation drugs, our findings on the association between high antipsychotic dosing and risk of mortality are limited to first-generation antipsychotics. Other studies suggest that polypharmacy, which was common in our study sample (in 2004, 42% of participants took a mix of first- and second-generation antipsychotics) may contribute to excessive dosing,45 however, we were unable to examine this due to lack of established upper limits for dosing of second-generation antipsychotics. Nonetheless, our findings in regards to dosing are relevant given that many persons with schizophrenia are still treated with first-generation antipsychotics alone or in combination with second-generation antipsychotics. In spite of these limitations, to our knowledge, our study is the first to demonstrate an association between adherence to the PORT guidelines for schizophrenia and reduced risk of mortality. Importantly, our 10-year cohort study controlled for medical comorbidities and mental health visits over the entire study period, demonstrating that high antipsychotic continuity is associated with decreased mortality even when differences in comorbid conditions and mental health visits among patients are accounted for.
Our study results suggest that it is important to consider methods to increase adherence to the PORT pharmacological recommendations. In addition to patient and provider education, innovative service delivery systems such as the Assertive Community Treatment (ACT) program, in which a multidisciplinary team works with persons with serious mental illness to coordinate medical care, medication management, and social services have been shown to improve adherence to antipsychotic medication.46 The ACT program, which is recommended by the schizophrenia PORT psychosocial guidelines,47 targets persons with high illness severity and could benefit persons with schizophrenia who are at high risk for poor antipsychotic continuity. In addition, adoption of routine outcomes monitoring systems could facilitate dissemination of guideline-based care. Prior studies of the Veteran’s Health System (VA) suggest that valid and feasible measures exist to measure quality of care related to psychotic symptoms and medication side effects among persons with schizophrenia48 and that electronic medical systems can be used to integrate patient information with clinical guidelines in order to aid clinical decision making regarding care for persons with schizophrenia. As electronic health technology continues to develop, health systems administrators could potentially use real-time information on antipsychotic adherence and dosing to communicate with providers about continuity of treatment. Evaluation of initiatives like these could assess if information technology interventions designed to improve adherence to the PORT pharmacological guidelines improve patient outcomes.
In conclusion, we found that annual antipsychotic continuity, CPZ equivalent dosing, anti-Parkinson medication use, and outpatient mental health visits are associated with risk of mortality among persons with schizophrenia. Given this association between guideline adherence and reduced risk of mortality, further research should address why long-term adherence to PORT pharmacological guidelines is low among Medicaid beneficiaries with schizophrenia. Innovative health service delivery programs targeting persons at high risk for low antipsychotic continuity due to illness severity or other factors could reduce the risk of mortality associated with low antipsychotic continuity. In order to improve adherence to PORT guidelines, quality improvement programs should consider implementation of systems to routinely monitor outcomes among patients with schizophrenia.
Funding
National Institute of Mental Health (grant R01MH074070).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Davis JM, Barner JT, Kane JM. Antipsychotic Drugs. In: Kaplan HI, Saddock BJ, eds. Comprehensive Textbook of Psychiatry. Vol 5 Baltimore, MD: Williams and Wilkins; 1989:1591–1626 [Google Scholar]
- 2. Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;12:1–9 [DOI] [PubMed] [Google Scholar]
- 4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223 [DOI] [PubMed] [Google Scholar]
- 5. Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second vs first generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antispychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Pscyhiatry. 2006;63:209–1223 [DOI] [PubMed] [Google Scholar]
- 6. Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10 [DOI] [PubMed] [Google Scholar]
- 7. Lehman A, Kreyenbuhl J, Buchanan R, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull. 2004;30:193–217 [DOI] [PubMed] [Google Scholar]
- 8. Dixon LB, Lehman AF, Levine J. Conventional antipsychotic medications for schizophrenia. Schizophr Bull. 1995;21:567–577 [DOI] [PubMed] [Google Scholar]
- 9. Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113:1–11 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell AJ, Malone D. Physical health and schizophrenia. Curr Opin Psychiatry. 2006;19:432–437 [DOI] [PubMed] [Google Scholar]
- 12. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93 [DOI] [PubMed] [Google Scholar]
- 13. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131 [DOI] [PubMed] [Google Scholar]
- 14. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796 [DOI] [PubMed] [Google Scholar]
- 15. Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192:19–27 [DOI] [PubMed] [Google Scholar]
- 16. Osborn DP, Nazareth I, King MB. Risk for coronary heart disease in people with severe mental illness: cross-sectional comparative study in primary care. Br J Psychiatry. 2006;188:271–277 [DOI] [PubMed] [Google Scholar]
- 17. Haukka J, Tiihonen J, Härkänen T, Lönnqvist J. Association between medication and risk of suicide, attempted suicide and death in nationwide cohort of suicidal patients with schizophrenia. Pharmacoepidemiol Drug Saf. 2008;17:686–696 [DOI] [PubMed] [Google Scholar]
- 18. Loas G, Azi A, Noisette C, Yon V. Mortality among chronic schizophrenic patients: a prospective 14-year follow-up study of 150 schizophrenic patients.[French] Encephale. 2008;34:54–60 [DOI] [PubMed] [Google Scholar]
- 19. Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157:975–981 [DOI] [PubMed] [Google Scholar]
- 20. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). The Lancet. 2009;374:1–12 [DOI] [PubMed] [Google Scholar]
- 21. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019 [DOI] [PubMed] [Google Scholar]
- 22. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Schizophrenia. Am J Psychiatry. 1997;154:1–63 [DOI] [PubMed] [Google Scholar]
- 23. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 24. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909 [DOI] [PubMed] [Google Scholar]
- 25. Liu-Sefert H, Osuntokum OO, Feldman PD. Factors associated with adherence to treatment with olanzapine and other atypical antipsychotic medications in patients with schizophrenia. J Clin Psychiatry. 2012;53:107–115 [DOI] [PubMed] [Google Scholar]
- 26. Auquier P, Lançon C, Rouillon F, Lader M. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2007;16:1308–1312 [DOI] [PubMed] [Google Scholar]
- 27. Buchanan J. Social support and schizophrenia: a review of the literature. Arch Psychiatr Nurs. 1995;9:68–76 [DOI] [PubMed] [Google Scholar]
- 28. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf. 2003;12:423–424 [DOI] [PubMed] [Google Scholar]
- 29. De Hert M, Correll CU, Cohen D. Do antipsychotic medications reduce or increase mortality in schizophrenia? A critical appraisal of the FIN-11 study. Schizophr Res. 2010;117:68–74 [DOI] [PubMed] [Google Scholar]
- 30. dosReis S, Johnson E, Steinwachs D, et al. Antipsychotic treatment patterns and hospitalizations among adults with schizophrenia. Schizophr Res. 2008;101:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busch AB, Lehman AF, Goldman H, Frank RG. Changes over time and disparities in schizophrenia treatment quality. Med Care. 2009;47:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bralet MC, Yon V, Loas G, Noisette C. Cause of mortality in schizophrenic patients: prospective study of years of a cohort of 150 chronic schizophrenic patients. Encephale. 2000;26:32–41 [PubMed] [Google Scholar]
- 33. Liu X, De Haan S. Chlorpromazine dose for people with schizophrenia. Cochrane Database Syst Rev. 2009;15:CD007778. [DOI] [PubMed] [Google Scholar]
- 34. Ozeki Y, Fujii K, Kurimoto N, et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:401–405 [DOI] [PubMed] [Google Scholar]
- 35. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052 [DOI] [PubMed] [Google Scholar]
- 36. Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58:1161–1167 [DOI] [PubMed] [Google Scholar]
- 37. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waddington JL, Youssef HA, Kinsella A. Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry. 1998;173:325–329 [DOI] [PubMed] [Google Scholar]
- 39. Olfson M, Marcus SC, Doshi JA. Continuity of care after inpatient discharge of patients with schizophrenia in the Medicaid program: a retrospective longitudinal cohort analysis. J Clin Psychiatry. 2010;71:831–838 [DOI] [PubMed] [Google Scholar]
- 40. Eaddy M, Grogg A, Locklear J. Assessment of compliance with antipsychotic treatment and resource utilization in a Medicaid population. Clin Ther. 2005;27:263–272 [DOI] [PubMed] [Google Scholar]
- 41. Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40:630–639 [DOI] [PubMed] [Google Scholar]
- 42. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116 [DOI] [PubMed] [Google Scholar]
- 43. Blecker S, Zhang Y, Ford DE, et al. Quality of care for heart failure among disabled Medicaid recipients with and without severe mental illness. Gen Hosp Psychiatry. 2010;32:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khaykin E, Eaton WW, Ford DE, Anthony CB, Daumit GL. Health insurance coverage among persons with schizophrenia in the United States. Psychiatr Serv. 2010;61:830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lelliott P, Paton C, Harrington M, Konsolaki M, Sensky T, Okocha T. The influence of patient variables on polypharmacy and combined high dose of antipsychotic drugs prescribed for inpatients. Psychiatrist. 2002;26:411–414 [Google Scholar]
- 46. Stein LI, Test MA. Alternative to mental hospital treatment. I. Conceptual model, treatment program, and clinical evaluation. Arch Gen Psychiatry. 1980;37:392–397 [DOI] [PubMed] [Google Scholar]
- 47. Dixon LB, Dickerson F, Bellack AS, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Young AS, Niv N, Chinman M, et al. Routine outcomes monitoring to support improving care for schizophrenia: report from the VA Mental Health QUERI. Community Ment Health J. 2011;47:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]